Research Progress of Neutrophil-Mediated Drug Delivery Strategies for Inflammation-Related Disease
Abstract
:1. Introduction
2. Role of Neutrophils in Diseases
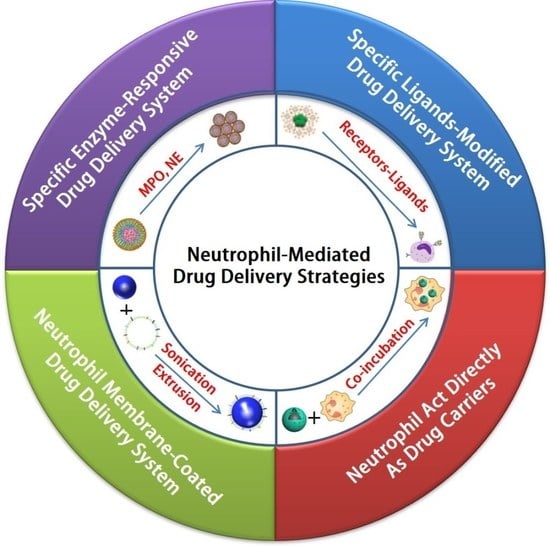
3. Neutrophil-Mediated Drug Delivery Strategies
3.1. Specific Enzyme-Responsive Drug Delivery System Targeting Neutrophils
3.2. Specific Ligand-Modified Drug Delivery System Targeting Neutrophils
3.3. Neutrophils Act Directly as Drug Carriers That Target Diseases
3.4. Neutrophil-Membrane-Coated Drug Delivery System for Disease Targeting
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petros, R.; DeSimone, J. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, S.; Yu, Y.; Zhu, Y.; Tong, R. A Mini-Review of Diagnostic and Therapeutic Nano-Tools for Pancreatitis. Int. J. Nanomed. 2022, 17, 4367–4381. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, J.; Yang, M.; Du, R.; Pontrelli, G.; McGinty, S.; Wang, G.; Yin, T.; Wang, Y. Penetration of the blood-brain barrier and the anti-tumour effect of a novel PLGA-lysoGM1/DOX micelle drug delivery system. Nanoscale 2020, 12, 2946–2960. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Qiu, J.; Liu, H.; Wang, Y.; Cui, Y.; Humphry, R.; Wang, N.; DurKan, C.; Chen, Y.; et al. Nanoparticles retard immune cells recruitment in vivo by inhibiting chemokine expression. Biomaterials 2021, 265, 120392. [Google Scholar] [CrossRef]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application—ScienceDirect. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Morachis, J.; Mahmoud, E.; Almutairi, A. Physical and chemical strategies for therapeutic delivery by using polymeric nanoparticles. Pharmacol. Rev. 2012, 64, 505–519. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Du, X.; Yang, J.; Shen, S.; Li, H.; Luo, Y.; Iqbal, S.; Xu, C.; Ye, X.; Cao, J.; et al. The effect of surface poly(ethylene glycol) length on in vivo drug delivery behaviors of polymeric nanoparticles. Biomaterials 2018, 182, 104–113. [Google Scholar] [CrossRef]
- Suk, J.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, S.; He, L.; Feng, X. A brief review of polysialic acid-based drug delivery systems. Int. J. Biol. Macromol. 2023, 230, 123151. [Google Scholar] [CrossRef]
- Shiraishi, K.; Hamano, M.; Ma, H.; Kawano, K.; Maitani, Y.; Aoshi, T.; Ishii, K.; Yokoyama, M. Hydrophobic blocks of PEG-conjugates play a significant role in the accelerated blood clearance (ABC) phenomenon. J. Control. Release Off. J. Control. Release Soc. 2013, 165, 183–190. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, J.; Yu, W.; Shen, L.; Ji, S.; Hu, T. Effect of protein immunogenicity and PEG size and branching on the anti-PEG immune response to PEGylated proteins. Process Biochem. 2016, 52, 183–191. [Google Scholar] [CrossRef]
- Jin, K.; Luo, Z.; Zhang, B.; Pang, Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm. Sin. B 2018, 8, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, S.; Guo, K.; Yin, X.; Tong, R. Polysialylated nanoinducer for precisely enhancing apoptosis and anti-tumor immune response in B-cell lymphoma. Acta Biomater. 2022, 149, 321–333. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Ren, J.; He, X.; Shi, H.; Zhang, F.; Li, H.; Tong, R. ROS-triggered nanoinducer based on dermatan sulfate enhances immunogenic cell death in melanoma. J. Control. Release 2022, 348, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Agrahari, V.; Mitra, A. Next generation drug delivery: Circulatory cells-mediated nanotherapeutic approaches. Expert Opin. Drug Deliv. 2017, 14, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Luo, J.; Chen, X.; Liu, W.; Chen, T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications. Nano-Micro Lett. 2019, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Oroojalian, F.; Beygi, M.; Baradaran, B.; Mokhtarzadeh, A.; Shahbazi, M. Immune Cell Membrane-Coated Biomimetic Nanoparticles for Targeted Cancer Therapy. Small 2021, 17, e2006484. [Google Scholar] [CrossRef]
- Chu, D.; Dong, X.; Shi, X.; Zhang, C.; Wang, Z. Neutrophil-Based Drug Delivery Systems. Adv. Mater. 2018, 30, e1706245. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Yee, L.W.; Xuan, K.Y.; Kunalan, K.; Rou, L.C.; Jean, L.S.; Ying, L.Y.; Wie, L.X.; Chellian, J.; Mehta, M.; et al. Targeting neutrophils using novel drug delivery systems in chronic respiratory diseases. Drug Dev. Res. 2020, 81, 419–436. [Google Scholar] [CrossRef]
- Hosseinalizadeh, H.; Mahmoodpour, M.; Razaghi Bahabadi, Z.; Hamblin, M.R.; Mirzaei, H. Neutrophil mediated drug delivery for targeted glioblastoma therapy: A comprehensive review. Biomed. Pharmacother. 2022, 156, 113841. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, R.; Xu, F. Neutrophil-Based Delivery Systems for Nanotherapeutics. Small 2018, 14, e1801674. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Guo, J.; Ma, Y.; Zhao, Y.; Jin, T.; Gu, L.; Dou, Y.; Liu, J.; Hu, H.; Xiong, X.; et al. Luminescence Imaging of Acute Liver Injury by Biodegradable and Biocompatible Nanoprobes. ACS Nano 2020, 14, 11083–11099. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, Z.; Mu, Q.; Yu, Z.; Chen, X. Targeting Neutrophils for Enhanced Cancer Theranostics. Adv. Mater. 2020, 32, 2002739. [Google Scholar] [CrossRef]
- Liu, S.Y.; Yan, A.M.; Guo, W.Y.; Fang, Y.Y.; Dong, Q.J.; Li, R.R.; Ni, S.N.; Sun, Y.; Yang, W.C.; Yang, G.F. Human Neutrophil Elastase Activated Fluorescent Probe for Pulmonary Diseases Based on Fluorescence Resonance Energy Transfer Using CdSe/ZnS Quantum Dots. ACS Nano 2020, 14, 4244–4254. [Google Scholar] [CrossRef]
- Cruz, M.A.; Bohinc, D.; Andraska, E.A.; Alvikas, J.; Raghunathan, S.; Masters, N.A.; van Kleef, N.D.; Bane, K.L.; Hart, K.; Medrow, K.; et al. Nanomedicine platform for targeting activated neutrophils and neutrophil-platelet complexes using an alpha(1)-antitrypsin-derived peptide motif. Nat. Nanotechnol. 2022, 17, 1004–1014. [Google Scholar] [CrossRef]
- Zhang, C.; Ling, C.L.; Pang, L.; Wang, Q.; Liu, J.X.; Wang, B.S.; Liang, J.M.; Guo, Y.Z.; Qin, J.; Wang, J.X. Direct Macromolecular Drug Delivery to Cerebral Ischemia Area using Neutrophil-Mediated Nanoparticles. Theranostics 2017, 7, 3260–3275. [Google Scholar]
- Vij, N.; Min, T.; Bodas, M.; Gorde, A.; Roy, I. Neutrophil targeted nano-drug delivery system for chronic obstructive lung diseases. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wu, Y.; Wang, Q.; Xue, L.; Su, Z.; Zhang, C. Cellular Vehicles Based on Neutrophils Enable Targeting of Atherosclerosis. Mol. Pharm. 2019, 16, 3109–3120. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, H.; Tie, C.; Yan, C.; Deng, Z.; Wan, Q.; Liu, X.; Yan, F.; Zheng, H. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat. Commun. 2018, 9, 4777. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.H.; Duan, W.X.; Chen, J.; You, T.; Li, S.Y.; Jiang, W.; Li, M.; Wang, G.; Pan, X.Y.; Wu, J.; et al. Neutrophil Decoys with Anti-Inflammatory and Anti-Oxidative Properties Reduce Secondary Spinal Cord Injury and Improve Neurological Functional Recovery. Adv. Funct. Mater. 2021, 31, 2102912. [Google Scholar] [CrossRef]
- Wang, K.; Lei, Y.; Xia, D.; Xu, P.; Ma, Y. Neutrophil membranes coated, antibiotic agent loaded nanoparticles targeting to the lung inflammation. Colloids Surf. B Biointerfaces 2019, 188, 110755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.; Gao, W.; Zhang, L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Henson, P. Dampening inflammation. Nat. Immunol. 2005, 6, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.M.; Corriden, R.; Nizet, V. The Ontogeny of a Neutrophil: Mechanisms of Granulopoiesis and Homeostasis. Microbiol. Mol. Biol. Rev. 2018, 82, e00057-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Summers, C.; Rankin, S.; Condliffe, A.; Singh, N.; Peters, A.; Chilvers, E. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Tao, H.; Dou, Y.; Li, L.; Xu, X.; Zhang, Q.; Cheng, J.; Han, S.; Huang, J.; Li, X. A myeloperoxidase-responsive and biodegradable luminescent material for real-time imaging of inflammatory diseases. Mater. Today 2017, 20, 493–500. [Google Scholar] [CrossRef]
- Han, G.; Songmin, Y.; Yuanrong, D. Pathological Roles of Neutrophil-Mediated Inflammation in Asthma and Its Potential for Therapy as a Target. J. Immunol. Res. 2017, 2017, 3743048. [Google Scholar]
- Wright, H.L.; Moots, R.J.; Edwards, S.W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 593–601. [Google Scholar] [CrossRef]
- Soehnlein, O. Multiple Roles for Neutrophils in Atherosclerosis. Circ. Res. 2012, 110, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Swierczak, A.; Mouchemore, K.A.; Hamilton, J.A.; Anderson, R.L. Neutrophils: Important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 2015, 34, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiao, Y.; Zheng, P.; Zhou, W.; Wang, Y.; Huang, G.; Xu, A.; Zhou, Z. Distinct neutrophil counts and functions in newly diagnosed type 1 diabetes, latent autoimmune diabetes in adults, and type 2 diabetes. Diabetes Metab. Res. Rev. 2019, 35, e3064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzemaekers, M.; Gouwy, M.; Proost, P. Neutrophil chemoattractant receptors in health and disease: Double-edged swords. Cell. Mol. Immunol. 2020, 17, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Burn, G.; Foti, A.; Marsman, G.; Patel, D.; Zychlinsky, A. The Neutrophil. Immunity 2021, 54, 1377–1391. [Google Scholar] [CrossRef]
- Hidalgo, A.; Libby, P.; Soehnlein, O.; Aramburu, I.V.; Papayannopoulos, V.; Silvestre-Roig, C. Neutrophil extracellular traps: From physiology to pathology. Cardiovasc. Res. 2022, 118, 2737–2753. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.; Douda, D.; Brüggemann, T.; Ricklefs, I.; Duvall, M.; Abdulnour, R.; Martinod, K.; Tavares, L.; Wang, X.; Cernadas, M.; et al. Neutrophil cytoplasts induce T17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci. Immunol. 2018, 3, eaao4747. [Google Scholar] [CrossRef] [Green Version]
- Ionita, M.; van den Borne, P.; Catanzariti, L.; Moll, F.; de Vries, J.; Pasterkamp, G.; Vink, A.; de Kleijn, D. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1842–1848. [Google Scholar] [CrossRef]
- Drechsler, M.; Megens, R.; van Zandvoort, M.; Weber, C.; Soehnlein, O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 2010, 122, 1837–1845. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Braster, Q.; Wichapong, K.; Lee, E.; Teulon, J.; Berrebeh, N.; Winter, J.; Adrover, J.; Santos, G.; Froese, A.; et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 2019, 569, 236–240. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.; Friday, S.; Li, S.; Patel, R.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrengues, J.; Shields, M.; Ng, D.; Park, C.; Ambrico, A.; Poindexter, M.; Upadhyay, P.; Uyeminami, D.; Pommier, A.; Küttner, V.; et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018, 361, eaao4227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odobasic, D.; Kitching, A.; Holdsworth, S. Neutrophil-Mediated Regulation of Innate and Adaptive Immunity: The Role of Myeloperoxidase. J. Immunol. Res. 2016, 2016, 2349817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017, 17, 248–261. [Google Scholar] [CrossRef]
- Grieshaber-Bouyer, R.; Nigrovic, P. Neutrophil Heterogeneity as Therapeutic Opportunity in Immune-Mediated Disease. Front. Immunol. 2019, 10, 346. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Szabo, G. Two Faces of Neutrophils in Liver Disease Development and Progression. Hepatology 2021, 74, 503–512. [Google Scholar] [CrossRef]
- Gross, S.; Gammon, S.; Moss, B.; Rauch, D.; Harding, J.; Heinecke, J.; Ratner, L.; Piwnica-Worms, D. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 2009, 15, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Panizzi, P.; Nahrendorf, M.; Wildgruber, M.; Waterman, P.; Figueiredo, J.L.; Aikawa, E.; McCarthy, J.; Weissleder, R.; Hilderbrand, S.A. Oxazine conjugated nanoparticle detects in vivo hypochlorous acid and peroxynitrite generation. J. Am. Chem. Soc. 2009, 131, 15739–15744. [Google Scholar] [CrossRef] [Green Version]
- Weiss, F.; Lauffenburger, D.; Friedl, P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat. Rev. Cancer 2022, 22, 157–173. [Google Scholar] [CrossRef]
- McFarlane, A.; Fercoq, F.; Coffelt, S.; Carlin, L. Neutrophil dynamics in the tumor microenvironment. J. Clin. Investig. 2021, 131, e143759. [Google Scholar] [CrossRef]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef]
- Tüting, T.; de Visser, K.E. How neutrophils promote metastasis. Science 2016, 352, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.T. Neutrophil serine proteases: Specific regulators of inflammation. Nat. Rev. Immunol. 2006, 6, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Bisso, P.; Gaglione, S.; Guimarães, P.; Mitchell, M.; Langer, R. Nanomaterial Interactions with Human Neutrophils. ACS Biomater. Sci. Eng. 2018, 4, 4255–4265. [Google Scholar] [CrossRef]
- Chu, D.; Dong, X.; Zhao, Q.; Gu, J.; Wang, Z. Photosensitization Priming of Tumor Microenvironments Improves Delivery of Nanotherapeutics via Neutrophil Infiltration. Adv. Mater. 2017, 29, 1701021. [Google Scholar] [CrossRef] [PubMed]
- Brasnjevic, I.; Steinbusch, H.; Schmitz, C.; Martinez-Martinez, P. Delivery of peptide and protein drugs over the blood-brain barrier. Prog. Neurobiol. 2009, 87, 212–251. [Google Scholar] [CrossRef]
- Courties, G.; Herisson, F.; Sager, H.B.; Heidt, T.; Ye, Y. Ischemic Stroke Activates Hematopoietic Bone Marrow Stem Cells. Circ. Res. 2014, 116, 407–417. [Google Scholar] [CrossRef]
- Grønberg, N.; Johansen, F.F.; Kristiansen, U.; Hasseldam, H. Leukocyte infiltration in experimental stroke. J. Neuroinflamm. 2013, 10, 115. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P. Chronic obstructive pulmonary disease: A growing but neglected global epidemic. PLoS Med. 2007, 4, e112. [Google Scholar] [CrossRef] [Green Version]
- Brightling, C.; Greening, N. Airway inflammation in COPD: Progress to precision medicine. Eur. Respir. J. 2019, 54, 1900651. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.; Badimon, L.; Hansson, G.; Deanfield, J.; Bittencourt, M.; Tokgözoğlu, L.; Lewis, E. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Braster, Q.; Ortega-Gomez, A.; Soehnlein, O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 2020, 17, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Azcutia, V.; Newton, G.; Alcaide, P.; Luscinskas, F. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011, 32, 461–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baetta, R.; Corsini, A. Role of polymorphonuclear neutrophils in atherosclerosis: Current state and future perspectives. Atherosclerosis 2009, 210, 1–13. [Google Scholar] [CrossRef]
- Xue, J.; Zhao, Z.; Zhang, L.; Xue, L.; Shen, S.; Wen, Y.; Wei, Z.; Wang, L.; Kong, L.; Sun, H.; et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017, 12, 692–700. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant Gliomas in Adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [Green Version]
- Giese, A.; Bjerkvig, R.; Berens, M.; Westphal, M. Cost of migration: Invasion of malignant gliomas and implications for treatment. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 1624–1636. [Google Scholar] [CrossRef]
- Groothuis, D. The blood-brain and blood-tumor barriers: A review of strategies for increasing drug delivery. Neuro-Oncol. 2000, 2, 45–59. [Google Scholar] [CrossRef]
- Li, J.; Cai, P.; Shalviri, A.; Henderson, J.; He, C.; Foltz, W.; Prasad, P.; Brodersen, P.; Chen, Y.; DaCosta, R.; et al. A multifunctional polymeric nanotheranostic system delivers doxorubicin and imaging agents across the blood-brain barrier targeting brain metastases of breast cancer. ACS Nano 2014, 8, 9925–9940. [Google Scholar] [CrossRef]
- Xie, Z.; Su, Y.; Kim, G.; Selvi, E.; Ma, C.; Aragon-Sanabria, V.; Hsieh, J.; Dong, C.; Yang, J. Immune Cell-Mediated Biodegradable Theranostic Nanoparticles for Melanoma Targeting and Drug Delivery. Small 2017, 13, 1603121. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Cao, H.; Wang, H.; Tan, T.; Yu, H.; Zhang, P.; Yin, Q.; Zhang, Z.; Li, Y. Inflammatory Monocytes Loading Protease-Sensitive Nanoparticles Enable Lung Metastasis Targeting and Intelligent Drug Release for Anti-Metastasis Therapy. Nano Lett. 2017, 17, 5546–5554. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed]
- Witcher, K.; Eiferman, D.; Godbout, J. Priming the inflammatory pump of the CNS after traumatic brain injury. Trends Neurosci. 2015, 38, 609–620. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Velagapudi, R.; Terrando, N. Neuroinflammation after surgery: From mechanisms to therapeutic targets. Nat. Immunol. 2020, 21, 1319–1326. [Google Scholar] [CrossRef]
- McCreedy, D.; Lee, S.; Sontag, C.; Weinstein, P.; Olivas, A.; Martinez, A.; Fandel, T.; Trivedi, A.; Lowell, C.; Rosen, S.; et al. Early Targeting of L-Selectin on Leukocytes Promotes Recovery after Spinal Cord Injury, Implicating Novel Mechanisms of Pathogenesis. eNeuro 2018, 5, ENEURO.0101-0118.2018. [Google Scholar] [CrossRef] [Green Version]
- Gris, D.; Marsh, D.; Oatway, M.; Chen, Y.; Hamilton, E.; Dekaban, G.; Weaver, L. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 4043–4051. [Google Scholar] [CrossRef] [Green Version]
- Ghasemlou, N.; Bouhy, D.; Yang, J.; López-Vales, R.; Haber, M.; Thuraisingam, T.; He, G.; Radzioch, D.; Ding, A.; David, S. Beneficial effects of secretory leukocyte protease inhibitor after spinal cord injury. Brain J. Neurol. 2010, 133, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Dou, C.; Xia, Y.; Li, B.; Zhao, M.; Yu, P.; Zheng, Y.; El-Toni, A.M.; Atta, N.F.; Galal, A.; et al. Neutrophil-like Cell-Membrane-Coated Nanozyme Therapy for Ischemic Brain Damage and Long-Term Neurological Functional Recovery. ACS Nano 2021, 15, 2263–2280. [Google Scholar] [CrossRef]
- Smolen, J.; Aletaha, D. Rheumatoid arthritis therapy reappraisal: Strategies, opportunities and challenges. Nat. Rev. Rheumatol. 2015, 11, 276–289. [Google Scholar] [CrossRef]
- Firestein, G.; McInnes, I. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burmester, G.; Feist, E.; Dörner, T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Wipke, B.; Allen, P. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol. 2001, 167, 1601–1608. [Google Scholar] [CrossRef]
- Kang, T.; Zhu, Q.; Wei, D.; Feng, J.; Yao, J.; Jiang, T.; Song, Q.; Wei, X.; Chen, H.; Gao, X.; et al. Nanoparticles Coated with Neutrophil Membranes Can Effectively Treat Cancer Metastasis. ACS Nano 2017, 11, 1397–1411. [Google Scholar] [CrossRef]
- Jie, Z.; Zhang, Y.; Wang, C.; Shen, B.; Guan, X.; Ren, Z.; Ding, X.; Dai, W.; Jiang, Y. Large-scale ex vivo generation of human neutrophils from cord blood CD34+ cells. PLoS ONE 2017, 12, e0180832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Q.; Shabrani, N.; Thon, J.; Huo, H.; Thiel, A.; Machlus, K.; Kim, K.; Brooks, J.; Li, F.; Luo, C.; et al. Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Rep. 2014, 3, 817–831. [Google Scholar] [CrossRef] [Green Version]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef] [Green Version]
- Brusini, R.; Varna, M.; Couvreur, P. Advanced nanomedicines for the treatment of inflammatory diseases. Adv. Drug Deliv. Rev. 2020, 157, 161–178. [Google Scholar] [CrossRef]
















| Targeting Strategies | Mechanisms | Applications | Ref. |
|---|---|---|---|
| Specific enzyme-responsive drug delivery system | MPO and NE are specific enzymes abundantly expressed in neutrophils and mediate inflammation | Acute liver injury, lung metastasis, venous thrombosis | [22,23,24,25] |
| Specific ligand-modified drug delivery system | Specific molecular recognition ligands (antibodies, aptamers, and peptides) can be bound to neutrophils | Cerebral ischemia, COPD | [26,27] |
| Neutrophils act directly as drug carriers | Neutrophils can cross biological barriers and be recruited to inflammatory tissues | Atherosclerosis, gliomas | [28,29] |
| Neutrophil-membrane-coated drug delivery system | Neutrophil membranes are capable of mimicking the source cells and transporting to the inflamed focus | Traumatic spinal cord injury, pneumonia, rheumatoid arthritis | [30,31,32] |
| Nanodrug | Delivery Strategies | Targeting | Challenges | Ref. |
|---|---|---|---|---|
| HZ-5 NPs | Specific enzyme-responsive drug delivery system | Specifically target neutrophils through MPO-catalyzed aggregation | The concentrations of enzymes should be considered | [23] |
| PINPNIMP | Specific ligand-modified drug delivery system | Anti-NIMP-R14 antibodies can effectively target neutrophils | Cannot mimic the complex intercellular interactions | [27] |
| ND-MMSNs | Neutrophils act directly as drug carriers | Neutrophils can cross biological barriers and be recruited to inflammatory tissues | Living neutrophils as drug delivery carriers are limited by their poor viability after isolation | [29] |
| NM-NP-SPX | Neutrophil-membrane-coated drug delivery system | The resulting membrane-coated nanoparticles are capable of mimicking the source cells | Complicated, careful manufacturing processes and should be stored for a prolonged time with excellent stability | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhang, H.; Zhang, Q.; Tao, H. Research Progress of Neutrophil-Mediated Drug Delivery Strategies for Inflammation-Related Disease. Pharmaceutics 2023, 15, 1881. https://doi.org/10.3390/pharmaceutics15071881
Zhao Y, Zhang H, Zhang Q, Tao H. Research Progress of Neutrophil-Mediated Drug Delivery Strategies for Inflammation-Related Disease. Pharmaceutics. 2023; 15(7):1881. https://doi.org/10.3390/pharmaceutics15071881
Chicago/Turabian StyleZhao, Yang, Haigang Zhang, Qixiong Zhang, and Hui Tao. 2023. "Research Progress of Neutrophil-Mediated Drug Delivery Strategies for Inflammation-Related Disease" Pharmaceutics 15, no. 7: 1881. https://doi.org/10.3390/pharmaceutics15071881