Recent Advances of Ocular Drug Delivery Systems: Prominence of Ocular Implants for Chronic Eye Diseases
Abstract
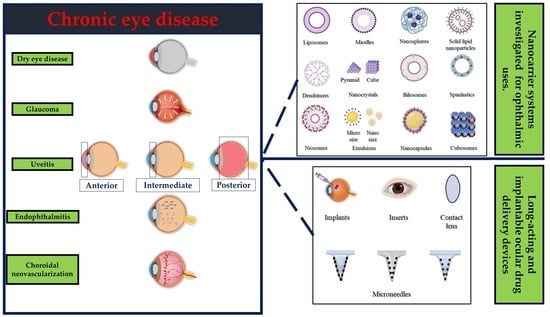
:1. Introduction
2. Chronic Eye Diseases That Require Long-Acting Therapy
2.1. Dry Eye Diseases
2.2. Glaucoma
2.3. Uveitis
2.4. Endophthalmitis
2.5. Cytomegalovirus Retinitis
2.6. Retinal Diseases
2.6.1. Age-Related Macular Degeneration
2.6.2. Diabetic Retinopathy
| Disease | Treatment | Drug | Delivery System Platform | Advantages of Delivery Systems In Vivo | Refs. | |
|---|---|---|---|---|---|---|
| Dry eye syndrome | Tear substitutes | Hypromellose | Solution | [126] | ||
| Methylcellulose and derivatives | Solution | [127] | ||||
| hyaluronic acid | Solution | [128] | ||||
| Aqueous secretagogues | Diquafosol sodium | Solution | [129] | |||
| Punctal plugs | Collagen and atelocollagen | In situ hydrogel | Prolonged activity | [49,50,130] | ||
| methacrylate-modified silk fibroin | In situ hydrogel | Prolonged activity | [54] | |||
| Mucin secretagogues | Rebamipide | Nanoparticles | Sustained release | [131] | ||
| Liposomes | Improved activity | [132] | ||||
| Micelles | Improved penetration | [133] | ||||
| Anti-inflammatory and immunomodulatory drugs | Cyclosporine | Micelles | Improved activity | [134] | ||
| Self-nanoemulsifying | Improved efficacy | [135] | ||||
| Liposomes | Improved activity | [136] | ||||
| Nanoparticles | Improved activity | [137] | ||||
| Nano-emulsion | Improved penetration | [138] | ||||
| Solid lipid nanoparticles | Controlled release | [139] | ||||
| In situ hydrogel | Improved activity | [134] | ||||
| Epigallocatechin gallate | Nanoparticles | Extended activity | [140] | |||
| In situ gels | Enhanced efficacy | [141] | ||||
| Lactoferrin | Nanoparticles | Enhanced efficacy | [142] | |||
| Nanocapsules | Controlled release | [143] | ||||
| Liposomes | Reduced irritation | [144] | ||||
| Nanostructured lipid carriers | Controlled release | [145] | ||||
| Vitamin A | Liposomes | Improved activity | [146] | |||
| Tacrolimus | Nanoparticles | Improved penetration | [147] | |||
| Progylcosomes | Improved activity | [148] | ||||
| Microcrystals | Improved efficacy | [149] | ||||
| Liposomes | Improved retention time | [150] | ||||
| Micelles | Prolonged activity | [151] | ||||
| Nanocapsules | Improved activity | [152] | ||||
| Corticosteroids | Dexamethasone | Dendrimer | Improved activity | [153] | ||
| Nano-wafer | Improved activity | [154] | ||||
| Nanostructured lipid carriers | Improved activity | [155] | ||||
| Nanoparticles | Improved penetration | [156] | ||||
| Micelles | Release modulation | [157] | ||||
| Nanosuspension | Prolonged activity | [158] | ||||
| Nano emulsion | Improved activity | [159] | ||||
| Nanosponges | Improved permeability | [160] | ||||
| Fluorometholone | Nanoparticles | Improved activity | [161] | |||
| Triamcinolone acetonide | Micelles | Release modulation | [60] | |||
| Nanoparticles | Improved activity | [162] | ||||
| Hydrocortisone | Nanosuspension | Prolonged activity | [158] | |||
| Micelles | Improved targeting | [163] | ||||
| Nanoparticles | Improved penetration | [163] | ||||
| Nanosuspension | Prolonged activity | [158] | ||||
| Prednisolone | Nanoparticles | Prolonged activity | [164] | |||
| Nano capsules | Reduced toxicity | [165] | ||||
| Lotep rednol etabonate | Nanoparticles | Improved penetration | [166] | |||
| Non-steroidal anti-inflammatory drugs | Diclofenac sodium | Nanoparticles | Improved bioavailability | [167] | ||
| Nanosuspension | Prolonged activity | [168] | ||||
| Pranoprofen | Nanosuspension | Improved activity | [169] | |||
| Nanoparticles | Improved activity | [169,170] | ||||
| Bromfenac sodium | Liposomes | Extended release | [171] | |||
| Nanoparticles | Improved permeation | [172] | ||||
| Cubosomes | Improved bioavailability | [61] | ||||
| Ketorolac | Nanoparticles | Improved delivery | [173] | |||
| lymphocyte function-associated antigen-1 antagonists | Lifitegrast | Solution | [174,175] | |||
| Glaucoma | Prostaglandin analogues | Latanoprost | Nanoparticles | Controlled release | [176] | |
| PEGylated solid lipid | Improved permeability | [177] | ||||
| Micelles | Extended release | [178] | ||||
| Cubosomes | Sustained release | [179] | ||||
| Nanoparticles | Improved permeability | [180] | ||||
| Travoprost | Gold nanoparticles | Improved stability | [181] | |||
| Liposomes | Sustained release | [182] | ||||
| Spanlastics | Prolonged activity | [183] | ||||
| Nanoemulsion | Improved pharmacokinetics | [75] | ||||
| Implant | Controlled release | [184] | ||||
| Bimatoprost | Nanoparticles | Improved therapeutic activity | [185] | |||
| Gold nanoparticles | Controlled release | [186] | ||||
| Nanoparticle hydrogel | Controlled release | [187] | ||||
| Microemulsion | Improved permeability | [188] | ||||
| Graphene oxide-laden | Controlled release | [189] | ||||
| Implants | Sustained release | [190] | ||||
| Nanovesicular systems | Sustained release | [191] | ||||
| Inserts | Extended release | [192] | ||||
| Unoprostone | Transscleral device | Sustained release | [193] | |||
| Rho kinase inhibitors | Fasudil | Liposomes | Enhanced bioavailability | [194] | ||
| Microspheres | Sustained release | [195] | ||||
| Ripasudil | Solution | [196] | ||||
| Netarsudil | Solution | [197] | ||||
| β-adrenergic blockers | Timolol | Nanoparticles | Extended release | [198] | ||
| Micelles | Extended release | [178] | ||||
| Cubosomes | Improved bioavailability | [199] | ||||
| Nanogel | Sustained release | [200] | ||||
| Gelatinized core liposomes | Improved encapsulation | [201] | ||||
| Microemulsion | Improved bioavailability | [202] | ||||
| Levobunolol | Nanoparticles | Extended release | [203] | |||
| Microparticles | Sustained release | [76] | ||||
| Carteolol | Nanocapsules | Improved activity | [204] | |||
| Nanoparticles | Improved activity | [205] | ||||
| Chitosomes | Improved penetration | [206] | ||||
| Metipranolol | Nanocapsules | Reduced systemic side effects | [207] | |||
| Betaxolol | Liposomes | Extended activity | [208] | |||
| Nanoparticles | Controlled release | [209] | ||||
| Niosomes | Improved bioavailability | [210] | ||||
| Bilosomes | Improved transcorneal permeation | [211] | ||||
| α-adrenergic agonists | Brimonidine | Nanoparticles | Sustained release | [212] | ||
| Inserts | Controlled release | [213] | ||||
| Niosomes | Sustained release | [214] | ||||
| Microspheres | Sustained release | [215] | ||||
| Liposomes | Improved effectiveness | [216] | ||||
| Implant | Sustained release | [217] | ||||
| Gelatin-core liposomes | Improved drug loading | [77] | ||||
| Carbonic anhydrase inhibitors | Dorzolamide | Nanoparticles | Improved activity | [218] | ||
| Nanoemulsion | Enhanced ocular delivery | [219] | ||||
| Liposomes | Prolonged action | [78] | ||||
| Microparticles | Sustained release | [220] | ||||
| Niosomes | Improved activity | [221] | ||||
| Implant | Extended drug delivery | [222] | ||||
| Inserts | Improved activity | [223] | ||||
| Brinzolamide | Nanoparticles | Improved therapeutic activity | [224] | |||
| Nanocrystals | Improved penetration | [225] | ||||
| Liposomes | Sustained release | [226] | ||||
| Nanocapsules | Improved bioavailability | [227] | ||||
| Nanoemulsion | Improved therapeutic efficacy | [228] | ||||
| Nanofibers | Improved patient compliance | [229] | ||||
| Implant | Sustained release | [230] | ||||
| Acetazolamide | Cubosomes | Improved therapeutic efficacy | [231] | |||
| Spanlastics | Enhanced ocular delivery | [232] | ||||
| Transgelosomes | Enhanced ocular delivery | [233] | ||||
| Implants | Sustained release | [234] | ||||
| Niosomes | Improved permeability | [235] | ||||
| Bilosomes | Improved permeability | [236] | ||||
| Microsponges | Improved therapeutic efficacy | [237] | ||||
| Dendrimers | Sustained release | [238] | ||||
| Cholinergic agonists | Pilocarpine | Nanoparticles | Sustained release | [239] | ||
| Nanocapsules | Improved bioavailability | [240] | ||||
| Dendrimers | Prolonged residence time | [241] | ||||
| Uveitis | Corticosteroids | Fluocinolone acetonide | Implant (Retisert®) | Sustained release | [242] | |
| Nanoparticles | Improved bioavailability | [243] | ||||
| Difluprednate | Microneedles | Sustained release | [244] | |||
| Fluormetholone | Nanoparticles | Improved penetration | [245] | |||
| Nanocrystals | Improved sustained activity | [246] | ||||
| Triamcinolone acetonide | Nano lipid carriers | Improved penetration | [247] | |||
| Immunomodulator drugs | Adalimumab | Hydrogel | Improved permeability | [248] | ||
| Infliximab | Liposomes | Prolonged activity | [249] | |||
| Methotrexate | Implant | Sustained release | [250] | |||
| Sirolimus (Rapamycin) | Implant | Extended release | [251] | |||
| Micelles | Sustained release | [252] | ||||
| Exosomes | Improved therapeutic activity | [253] | ||||
| Liposomes | Improved therapeutic activity | [86] | ||||
| Endophthalmitis | Antimicrobials | Daptomycin | Nanoparticles | Noninvasive and improved activity | [254] | |
| Vancomycin | Nanostructured lipid carriers | Improved permeability and activity | [255] | |||
| Nanoparticles | Sustained release | [256] | ||||
| Thermoresponsive hydrogels | Controlled release | [257] | ||||
| Liposomes | Improved permeability | [258] | ||||
| Implant | Controlled release | [259] | ||||
| Niosomes | Improved permeability | [260] | ||||
| Ceftazidime | Nanoparticles | Improved activity and permeability | [261] | |||
| Antifungals | Amphotericin B | Liposomes | Improved activity-reduce toxicity | [262] | ||
| Voriconazole | Thermo-sensitive in situ gel | Sustained release | [263] | |||
| Nanoparticles | Improved permeability | [264] | ||||
| Microemulsion | Controlled release | [265] | ||||
| Elastosomes | Improved activity and reduced toxicity | [266] | ||||
| Micelles | Improved stability | [266] | ||||
| Liposomes | Improved permeability | [267] | ||||
| Antivirals | Cidofovir | Micelles | Prolonged activity | [268] | ||
| Liposomes | Prolonged activity | [269] | ||||
| Foscarnet | Liposomes | Improved activity and permeability | [270] | |||
| Ganciclovir | Nanoparticles | Sustained release | [271] | |||
| Glycerosomes | Sustained release | [272] | ||||
| Microemulsion | Improved permeability | [273] | ||||
| Vitrasert | Prolonged activity | [274] | ||||
| Minitablets | Sustained release | [275] | ||||
| Retinal diseases | Age-related macular degeneration | Anti-VEGF Agents | Ranibizumab | Nanoparticles | Improved activity | [276] |
| (Antibody fragment) | Microparticles | Improved intravitreal delivery | [277] | |||
| Liposomes | Increased encapsulation-release | [278] | ||||
| Quantum dots | Sustained release | [279] | ||||
| Implant | Sustained release | [280] | ||||
| Bevacizumab | Nanoparticles | Sustained delivery | [281] | |||
| (Monoclonal antibody) | Bi-layered capsule | Sustained delivery | [282] | |||
| Nanocapsules | Improved bioavailability | [283] | ||||
| Implant | Sustained release | [284] | ||||
| Microparticles | Sustained release | [285] | ||||
| Liposomes | Sustained release | [286] | ||||
| Aflibercept (VEGF-Trap) | Nanoparticles | Sustained drug release | [287] | |||
| Microspheres | Extended release | [288] | ||||
| Sunitinib | Nanoparticles | Superior prolonged activity | [289] | |||
| Micelles | Extended release | [290] | ||||
| Axitinib | Nanoparticles | Superior activity | [291] | |||
| Pegaptanib | PEGylated aptamer | Prolonged activity | [113] | |||
| Gene therapy | VEGF-siRNA | Liposomes | Improved activity-stability | [292] | ||
| Nanoball | Improved activity-targeting | [293] | ||||
| Nanoparticles | Improved therapeutic activity | [294] | ||||
| Integrin antagonists | C16Y peptide | Nanoparticles | Sustained release | [295] | ||
| Antioxidants | Serine-threonine-tyrosine peptide | Nanoparticles | Targeting | [296] | ||
| Resveratrol | Nanoparticles | Sustained release | [297] | |||
| Curcumin | Liposomes | Improved activity | [298] | |||
| Astragaloside | Nanocapsules | Improved activity | [299] | |||
| Diabetic retinopathy | Antiangiogenics | Anti-Flt1 peptide | Nanoparticles | Sustained release | [300] | |
| Micropump implant | On-demand targeting | [301] | ||||
| Fenofibrate | Nanoparticles | Controlled release | [302] | |||
| Pioglitazone | Nanoparticles | Controlled/improved activity | [303] | |||
| Apatinib | Nanoparticles | Improved activity | [304] | |||
| Silicate | Nanoparticles | Improved activity | [305] | |||
| Tacrolimus | Nanoparticles | Improved activity | [306] | |||
| Sorafenib tosylate | Nanoparticles | Improved activity | [307] | |||
| Octreotide | Nanoparticles | Improved activity-targeting | [308] | |||
| Anti-inflammatory and antioxidants | p-Coumaric acid | Nanoparticles | Improved activity | [309] | ||
| Connexin43 mimetic peptide | Nanoparticles | Targeting | [310] | |||
| Inulin D α-tocopherol succinate | Nanomicelles | Improved activity | [311] | |||
| Citicoline | Liposomes | Improved permeation | [312] | |||
| Melatonin | Nanoparticles | Controlled release and enhanced tolerability | [312] | |||
3. Overview of Ocular Delivery Systems
3.1. Liposomes
3.2. Polymeric Micelles
3.3. Polymeric Nanoparticles
3.4. Solid Lipid Nanoparticles
3.5. Hydrogels
3.6. Dendrimers
3.7. Nanocrystals
3.8. Cubosomes
3.9. Niosomes
3.10. Emulsions
3.11. Bilosomes
3.12. Nanocapsules
3.13. Spanlastics
4. Long-Acting Ocular Drug Delivery Devices
4.1. Solid Devices
4.2. Microneedles
4.3. Three-Dimensional Printable Systems
4.4. In Situ Gelling Implants
5. Implantable Systems/Devices for Drug Delivery
5.1. Polymers Used to Formulate IDDS
5.1.1. Nonbiodegradable Polymers
5.1.2. Biodegradable Polymers
5.2. Techniques for the Preparation of IDDS
5.2.1. Solvent Casting
5.2.2. Extrusion
5.2.3. Electrospinning
5.2.4. Other Techniques
5.3. Characterization and Evaluation of IDDS
5.4. Sites for Delivery and Implantation
5.5. Regulatory Aspects of IDDS
6. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e888–e897. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Suri, R.; Beg, S.; Kohli, K. Target strategies for drug delivery bypassing ocular barriers. J. Drug Deliv. Sci. Technol. 2020, 55, 101389. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Abdelkader, H.; G Alany, R. Controlled and Continuous Release Ocular Drug Delivery Systems: Pros and Cons. Curr. Drug Deliv. 2012, 9, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Gumbiner, B. Structure, biochemistry, and assembly of epithelial tight junctions. Am. J. Physiol.-Cell Physiol. 1987, 253, C749–C758. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Hovanesian, J.; Singh, I.P.; Bauskar, A.; Vantipalli, S.; Ozden, R.G.; Goldstein, M.H. Identifying and addressing common contributors to nonadherence with ophthalmic medical therapy. Curr. Opin. Ophthalmol. 2023, 34, S1–S13. [Google Scholar] [CrossRef] [PubMed]
- Constable, P.A.; Lawrenson, J.G.; Dolman, D.E.M.; Arden, G.B.; Abbott, N.J. P-Glycoprotein expression in human retinal pigment epithelium cell lines. Exp. Eye Res. 2006, 83, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, T.; Mishima, H.; Mishima, H.; Murakami, T.; Takano, M. Role of P-glycoprotein in distribution of rhodamine 123 into aqueous humor in rabbits. Curr. Eye Res. 1999, 18, 240–246. [Google Scholar] [CrossRef]
- Pelis, R.M.; Shahidullah, M.; Ghosh, S.; Coca-Prados, M.; Wright, S.H.; Delamere, N.A. Localization of Multidrug Resistance-Associated Protein 2 in the Nonpigmented Ciliary Epithelium of the Eye. J. Pharmacol. Exp. Ther. 2009, 329, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Freddo, T.F. Shifting the Paradigm of the Blood–Aqueous Barrier. Exp. Eye Res. 2001, 73, 581–592. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Shetty, K.H.; Desai, D.T.; Shah, D.O.; Willcox, M.D.P. Recent advances in ophthalmic preparations: Ocular barriers, dosage forms and routes of administration. Int. J. Pharm. 2021, 608, 121105. [Google Scholar] [CrossRef] [PubMed]
- Das, N.D.; Shichi, H. Enzymes of mercapturate synthesis and other drug-metabolizing reactions-specific localization in the eye. Exp. Eye Res. 1981, 33, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Thrimawithana, T.R.; Young, S.; Bunt, C.R.; Green, C.; Alany, R.G. Drug delivery to the posterior segment of the eye. Drug Discov. Today 2011, 16, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Dalkara, D.; Kolstad, K.D.; Caporale, N.; Visel, M.; Klimczak, R.R.; Schaffer, D.V.; Flannery, J.G. Inner Limiting Membrane Barriers to AAV-mediated Retinal Transduction From the Vitreous. Mol. Ther. 2009, 17, 2096–2102. [Google Scholar] [CrossRef] [Green Version]
- Mains, J.; Wilson, C.G. The vitreous humor as a barrier to nanoparticle distribution. J. Ocul. Pharmacol. Ther. 2013, 29, 143–150. [Google Scholar] [CrossRef]
- Halfter, W.; Winzen, U.; Bishop, P.N.; Eller, A. Regulation of Eye Size by the Retinal Basement Membrane and Vitreous Body. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3586–3594. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, T.; Ueno, H.; Goto, Y.; Oshima, Y.; Ishibashi, T.; Inomata, H. A vitrectomy improves the transfection efficiency of adenoviral vector-mediated gene transfer to Müller cells. Gene Ther. 1998, 5, 1088–1097. [Google Scholar] [CrossRef] [Green Version]
- Jackson, T.L.; Antcliff, R.J.; Hillenkamp, J.; Marshall, J. Human Retinal Molecular Weight Exclusion Limit and Estimate of Species Variation. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2141–2146. [Google Scholar] [CrossRef] [Green Version]
- Matet, A.; Behar-Cohen, F.; Cassoux, N.; Declèves, X.; Cisternino, S. Chapter 10-Retinal and choroidal cancers: Blood-retinal barriers considerations in ocular chemotherapy. In Drug Efflux Pumps in Cancer Resistance Pathways: From Molecular Recognition and Characterization to Possible Inhibition Strategies in Chemotherapy; Sosnik, A., Bendayan, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 7, pp. 303–335. [Google Scholar]
- Verjee, M.A.; Brissette, A.R.; Starr, C.E. Dry Eye Disease: Early Recognition with Guidance on Management and Treatment for Primary Care Family Physicians. Ophthalmol. Ther. 2020, 9, 877–888. [Google Scholar] [CrossRef] [PubMed]
- The Definition and Classification of Dry Eye Disease: Report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul. Surf. 2007, 5, 75–92. [CrossRef] [PubMed]
- Navarro-Lopez, S.; Moya-Ramón, M.; Gallar, J.; Carracedo, G.; Aracil-Marco, A. Effects of physical activity/exercise on tear film characteristics and dry eye associated symptoms: A literature review. Contact Lens Anterior Eye 2023, 43, 101854. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.E.; Murphy, P.J. Changes in the tear film and ocular surface from dry eye syndrome. Prog. Retin. Eye Res. 2004, 23, 449–474. [Google Scholar] [CrossRef]
- Messmer, E.M. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Arztebl. Int. 2015, 112, 71–81, quiz 82. [Google Scholar] [CrossRef] [Green Version]
- Vehof, J.; Snieder, H.; Jansonius, N.; Hammond, C.J. Prevalence and risk factors of dry eye in 79,866 participants of the population-based Lifelines cohort study in the Netherlands. Ocul. Surf. 2021, 19, 83–93. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Periman, L.M.; Perez, V.L.; Saban, D.R.; Lin, M.C.; Neri, P. The Immunological Basis of Dry Eye Disease and Current Topical Treatment Options. J. Ocul. Pharmacol. Ther. 2020, 36, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Shu, H.-Y.; Wu, J.-L.; Su, T.; Liu, Y.-J.; Zhang, L.-J.; Li, Q.-Y.; Pan, Y.-C.; Ge, Q.-M.; Shao, Y. Investigation of changes in the activity and function of dry eye-associated brain regions using the amplitude of low-frequency fluctuations method. Biosci. Rep. 2022, 42, BSR20210941. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.F.; Wang, M.Y.; Dou, X.Y. Gastrointestinal microbiome and primary Sjögren’s syndrome: A review of the literature and conclusions. Int. J. Ophthalmol. 2022, 15, 1864–1872. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- Management and Therapy of Dry Eye Disease: Report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 163–178. [CrossRef] [PubMed]
- Thacker, M.; Singh, V.; Basu, S.; Singh, S. Biomaterials for dry eye disease treatment: Current overview and future perspectives. Exp. Eye Res. 2023, 226, 109339. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cheng, W.; Liu, C.; Jin, X.; Ming, S.; Zhao, D.; Feng, X. Evaluation of effects of 3% diquafosol ophthalmic solution on preocular tear film stability after trabeculectomy. Int. Ophthalmol. 2023, 43, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Awwad, S.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Brocchini, S.; Gaisford, S.; Goyanes, A.; Basit, A.W. 3D Printed Punctal Plugs for Controlled Ocular Drug Delivery. Pharmaceutics 2021, 13, 1421. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Corrales, R.M.; Villarreal, A.L.; Farley, W.J.; Li, D.-Q.; Stern, M.E.; Pflugfelder, S.C. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp. Eye Res. 2006, 83, 526–535. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Corrales, R.M.; Villarreal, A.L.; Farley, W.; Li, D.-Q.; Stern, M.E.; Pflugfelder, S.C. Apical Corneal Barrier Disruption in Experimental Murine Dry Eye Is Abrogated by Methylprednisolone and Doxycycline. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2847–2856. [Google Scholar] [CrossRef]
- Prinz, J.; Maffulli, N.; Fuest, M.; Walter, P.; Bell, A.; Migliorini, F. Efficacy of Topical Administration of Corticosteroids for the Management of Dry Eye Disease: Systematic Review and Meta-Analysis. Life 2022, 12, 1932. [Google Scholar] [CrossRef]
- Lekhanont, K.; Leyngold, I.M.; Suwan-Apichon, O.; Rangsin, R.; Chuck, R.S. Comparison of Topical Dry Eye Medications for the Treatment of Keratoconjunctivitis Sicca in a Botulinum Toxin B-Induced Mouse Model. J. Cornea Extern. Dis. 2007, 26, 84–89. [Google Scholar] [CrossRef]
- Avunduk, A.M.; Avunduk, M.C.; Varnell, E.D.; Kaufman, H.E. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: A clinical and immunocytochemical study. Am. J. Ophthalmol. 2003, 136, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Ryu, I.H.; Seo, K.Y.; Hong, S.; Kim, H.C.; Kim, E.K. Topical 0.1% Prednisolone Lowers Nerve Growth Factor Expression in Keratoconjunctivitis Sicca Patients. Ophthalmology 2006, 113, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Schechter, B.A. Ketorolac During the Induction Phase of Cyclosporin-A Therapy. J. Ocul. Pharmacol. Ther. 2006, 22, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Liu, L.-p.; Chen, Z.; Ma, Q. Cyclosporine (0.05%) Combined with Diclofenac Sodium Eye Drops for the Treatment of Dry Eye Disease. J. Ophthalmol. 2022, 2022, 2334077. [Google Scholar] [CrossRef]
- Kunert, K.S.; Tisdale, A.S.; Stern, M.E.; Smith, J.A.; Gipson, I.K. Analysis of Topical Cyclosporine Treatment of Patients with Dry Eye Syndrome: Effect on Conjunctival Lymphocytes. Arch. Ophthalmol. 2000, 118, 1489–1496. [Google Scholar] [CrossRef]
- Turner, K.; Pflugfelder, S.C.; Ji, Z.; Feuer, W.J.; Stern, M.; Reis, B.L. Interleukin-6 Levels in the Conjunctival Epithelium of Patients with Dry Eye Disease Treated with Cyclosporine Ophthalmic Emulsion. J. Cornea Extern. Dis. 2000, 19, 492–496. [Google Scholar] [CrossRef]
- Gao, J.; Sana, R.; Calder, V.; Calonge, M.; Lee, W.; Wheeler, L.A.; Stern, M.E. Mitochondrial Permeability Transition Pore in Inflammatory Apoptosis of Human Conjunctival Epithelial Cells and T Cells: Effect of Cyclosporin A. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4717–4733. [Google Scholar] [CrossRef]
- Modi, D.; Nirmal, J.; Warsi, M.H.; Bhatia, M.; Hasan, N.; Kesharwani, P.; Jain, G.K. Formulation and development of tacrolimus-gellan gum nanoformulation for treatment of dry eye disease. Colloids Surf. B Biointerfaces 2022, 211, 112255. [Google Scholar] [CrossRef]
- Pearce, E.I.; Dorman, M.; Wilkinson, B.C.; Oliver, K.M. Effect of Blink Frequency on Tear Turnover Rate. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3726. [Google Scholar]
- Saarinen-Savolainen, P.; Järvinen, T.; Araki-Sasaki, K.; Watanabe, H.; Urtti, A. Evaluation of Cytotoxicity of Various Ophthalmic Drugs, Eye Drop Excipients and Cyclodextrins in an Immortalized Human Corneal Epithelial Cell Line. Pharm. Res. 1998, 15, 1275–1280. [Google Scholar] [CrossRef]
- Kojima, T.; Matsumoto, Y.; Ibrahim, O.M.A.; Wakamatsu, T.H.; Dogru, M.; Tsubota, K. Evaluation of a Thermosensitive Atelocollagen Punctal Plug Treatment for Dry Eye Disease. Am. J. Ophthalmol. 2014, 157, 311–317.e311. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.D.; Surbeck, J.W.; Marsh, H.R.; Ding, K.; Kingsley, R.M.; Riaz, K.M.; Park, S.S.K.; Shah, V.A. The effect of punctal plugs in reducing ocular surface irritation after povidone-iodine preparation of intravitreal injection—A randomized trial. Eye 2022, 36, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Xu, K.; Xiao, D.; Zheng, Y.; Zheng, Q.; Shen, J.; Qian, Y.; Chen, W. In Situ Forming Hydrogel as a Tracer and Degradable Lacrimal Plug for Dry Eye Treatment. Adv. Healthc. Mater. 2022, 11, 2200678. [Google Scholar] [CrossRef] [PubMed]
- Mithani, S.D.; Bakatselou, V.; TenHoor, C.N.; Dressman, J.B. Estimation of the Increase in Solubility of Drugs as a Function of Bile Salt Concentration. Pharm. Res. 1996, 13, 163–167. [Google Scholar] [CrossRef] [PubMed]
- El Tayar, N.; Mark, A.E.; Vallat, P.; Brunne, R.M.; Testa, B.; van Gunsteren, W.F. Solvent-dependent conformation and hydrogen-bonding capacity of cyclosporin A: Evidence from partition coefficients and molecular dynamics simulations. J. Med. Chem. 1993, 36, 3757–3764. [Google Scholar] [CrossRef]
- Mandal, A.; Gote, V.; Pal, D.; Ogundele, A.; Mitra, A.K. Ocular Pharmacokinetics of a Topical Ophthalmic Nanomicellar Solution of Cyclosporine (Cequa®) for Dry Eye Disease. Pharm. Res. 2019, 36, 36. [Google Scholar] [CrossRef]
- Perry, H.D.; Solomon, R.; Donnenfeld, E.D.; Perry, A.R.; Wittpenn, J.R.; Greenman, H.E.; Savage, H.E. Evaluation of Topical Cyclosporine for the Treatment of Dry Eye Disease. Arch. Ophthalmol. 2008, 126, 1046–1050. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, A.; Labetoulle, M.; Ismail, D.; Garrigue, J.S.; Rancho, L.; Brignole-Baudouin, F.; Amrane, M.; Baudouin, C. The Effect of Ikervis® (1 mg/mL Ciclosporin cationic emulsion) on severe keratitis in patients with dry eye disease participating in a phase III study. Acta Pharmacol. 2015, 93. [Google Scholar] [CrossRef]
- Safwat, M.A.; Mansour, H.F.; Hussein, A.K.; Abdelwahab, S.; Soliman, G.M. Polymeric micelles for the ocular delivery of triamcinolone acetonide: Preparation and in vivo evaluation in a rabbit ocular inflammatory model. Drug Deliv. 2020, 27, 1115–1124. [Google Scholar] [CrossRef]
- Shoman, N.A.; Gebreel, R.M.; El-Nabarawi, M.A.; Attia, A. Optimization of hyaluronan-enriched cubosomes for bromfenac delivery enhancing corneal permeation: Characterization, ex vivo, and in vivo evaluation. Drug Deliv. 2023, 30, 2162162. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razeghinejad, R.; Lin, M.M.; Lee, D.; Katz, L.J.; Myers, J.S. Pathophysiology and management of glaucoma and ocular hypertension related to trauma. Surv. Ophthalmol. 2020, 65, 530–547. [Google Scholar] [CrossRef]
- Khatib, T.Z.; Martin, K.R. Protecting retinal ganglion cells. Eye 2017, 31, 218–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jindal, V. Glaucoma: An Extension of Various Chronic Neurodegenerative Disorders. Mol. Neurobiol. 2013, 48, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, J.; Cassidy, P.S.; Humphries, P. Open-angle glaucoma: Therapeutically targeting the extracellular matrix of the conventional outflow pathway. Expert Opin. Ther. Targets 2017, 21, 1037–1050. [Google Scholar] [CrossRef] [Green Version]
- Braunger, B.M.; Fuchshofer, R.; Tamm, E.R. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur. J. Pharm. Biopharm. 2015, 95, 173–181. [Google Scholar] [CrossRef]
- Pattabiraman, P.P.; Toris, C.B. The exit strategy: Pharmacological modulation of extracellular matrix production and deposition for better aqueous humor drainage. Eur. J. Pharmacol. 2016, 787, 32–42. [Google Scholar] [CrossRef]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef]
- Szigiato, A.-A.; Podbielski, D.W.; Ahmed, I.I.K. Sustained drug delivery for the management of glaucoma. Expert Rev. Ophthalmol. 2017, 12, 173–186. [Google Scholar] [CrossRef]
- Pita-Thomas, D.W.; Goldberg, J.L. Nanotechnology and glaucoma: Little particles for a big disease. Curr. Opin. Ophthalmol. 2013, 24, 130–135. [Google Scholar] [CrossRef]
- Alviset, G.; Corvis, Y.; Hammad, K.; Lemut, J.; Maury, M.; Mignet, N.; Boudy, V. New Preservative-Free Formulation for the Enhanced Ocular Bioavailability of Prostaglandin Analogues in Glaucoma. Pharmaceutics 2022, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Nasr, M.; Sammour, O. Nanoemulsion as a feasible and biocompatible carrier for ocular delivery of travoprost: Improved pharmacokinetic/pharmacodynamic properties. Int. J. Pharm. 2020, 583, 119402. [Google Scholar] [CrossRef] [PubMed]
- Karataş, A.; Sonakin, Ö.; KiliÇarslan, M.; Baykara, T. Poly (ε-caprolactone) microparticles containing Levobunolol HCl prepared by a multiple emulsion (W/O/W) solvent evaporation technique: Effects of some formulation parameters on microparticle characteristics. J. Microencapsul. 2009, 26, 63–74. [Google Scholar] [CrossRef]
- Abdel Azim, E.A.; Elkheshen, S.A.; Hathout, R.M.; Fouly, M.A.; El Hoffy, N.M. Augmented in vitro and in vivo Profiles of Brimonidine Tartrate Using Gelatinized-Core Liposomes. Int. J. Nanomed. 2022, 17, 2753–2776. [Google Scholar] [CrossRef] [PubMed]
- Kouchak, M.; Malekahmadi, M.; Bavarsad, N.; Saki Malehi, A.; Andishmand, L. Dorzolamide nanoliposome as a long action ophthalmic delivery system in open angle glaucoma and ocular hypertension patients. Drug Dev. Ind. Pharm. 2018, 44, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.T.; Asquith, M. The microbiome and HLA-B27-associated acute anterior uveitis. Nat. Rev. Rheumatol. 2018, 14, 704–713. [Google Scholar] [CrossRef]
- Nussenblatt, R.B. The natural history of uveitis. Int. Ophthalmol. 1990, 14, 303–308. [Google Scholar] [CrossRef]
- Suttorp-Schulten, M.S.; Rothova, A. The possible impact of uveitis in blindness: A literature survey. Br. J. Ophthalmol. 1996, 80, 844–848. [Google Scholar] [CrossRef] [Green Version]
- Mérida, S.; Palacios, E.; Navea, A.; Bosch-Morell, F. New Immunosuppressive Therapies in Uveitis Treatment. Int. J. Mol. Sci. 2015, 16, 18778–18795. [Google Scholar] [CrossRef] [Green Version]
- Oh, B.-L.; Lee, J.S.; Lee, E.Y.; Lee, H.Y.; Yu, H.G. Incidence and Risk Factors for Blindness in Uveitis: A Nationwide Cohort Study from 2002 to 2013. Ocul. Immunol. Inflamm. 2021, 29, 1040–1044. [Google Scholar] [CrossRef]
- Gritz, D.C.; Wong, I.G. Incidence and prevalence of uveitis in Northern California: The Northern California Epidemiology of Uveitis Study. Ophthalmology 2004, 111, 491–500. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yasueda, S.-i.; Isowaki, A.; Yamamoto, M.; Kimura, M.; Inada, K.; Ohtori, A. Formulation of an ophthalmic lipid emulsion containing an anti-inflammatory steroidal drug, difluprednate. Int. J. Pharm. 2005, 301, 121–128. [Google Scholar] [CrossRef]
- Suri, R.; Neupane, Y.R.; Mehra, N.; Jain, G.K.; Kohli, K. Sirolimus loaded polyol modified liposomes for the treatment of Posterior Segment Eye Diseases. Med. Hypotheses 2020, 136, 109518. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.L. Endophthalmitis. Clin. Microbiol. Infect. 2013, 19, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Callegan, M.C.; Engelbert, M.; Parke, D.W.; Jett, B.D.; Gilmore, M.S. Bacterial Endophthalmitis: Epidemiology, Therapeutics, and Bacterium-Host Interactions. Clin. Microbiol. Rev. 2002, 15, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Asencio-Egea, M.A.; Huertas-Vaquero, M.; Carranza-González, R.; Cells-Sánchez, J.; González-del Valle, F.; Tenías-Burillo, J.M.; Barberá-Farré, J.R. Endogenous endophthalmitis: Case report and brief review of a serious ocular disease. Rev. Chil. Infectol. 2013, 30, 516–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.-S.; Chan, T.-K.; Lee, H.-M.; Chee, S.-P. Endogenous bacterial endophthalmitis: An East Asian experience and a reappraisal of a severe ocular affliction. Ophthalmology 2000, 107, 1483–1491. [Google Scholar] [CrossRef]
- Cam, D.; Saatci, A.O.; Micili, S.C.; Ergur, B.U.; Karabag, R.Y.; Durak, I.; Berk, A.T. The Effect of Intravitreal Azithromycin on the Albino Newborn Rabbit Retina. Open Ophthalmol. J. 2016, 10, 12–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citirik, M.; Dilsiz, N.; Batman, C.; Zilelioglu, O. Comparative toxicity of 4 commonly used intravitreal corticosteroids on rat retina. Can. J. Ophthalmol. 2009, 44, e3–e8. [Google Scholar] [CrossRef]
- del Amo, E.M.; Urtti, A. Current and future ophthalmic drug delivery systems: A shift to the posterior segment. Drug Discov. Today 2008, 13, 135–143. [Google Scholar] [CrossRef]
- D’Amico, D.J.; Caspers-Velu, L.; Libert, J.; Shanks, E.; Schrooyen, M.; Hanninen, L.A.; Kenyon, K.R. Comparative Toxicity of Intravitreal Aminoglycoside Antibiotics. Am. J. Ophthalmol. 1985, 100, 264–275. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Damico, F.M.; Viola, F.; D’Amico, D.J.; Young, L.H. Retinal Toxicity of intravitreal triamcinolone Acetonide: A Morphological Study. RETINA 2006, 26, 531–536. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, Y.; Ke, W.; Li, Y.; Wang, K.; Chen, M. Overview and update on cytomegalovirus-associated anterior uveitis and glaucoma. Front. Public Health 2023, 11, 1117412. [Google Scholar] [CrossRef] [PubMed]
- Melancia, D.; Fernandes, A.; Manita, M.; Cordeiro, I.M. Cytomegalovirus optic neuropathy in a young immunocompetent patient. J. NeuroVirology 2021, 27, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.N.; Vaudaux, J.D.; Shiramizu, K.M.; Yu, F.; Goldenberg, D.T.; Gupta, A.; Carlson, M.; Read, R.W.; Novack, R.D.; Kuppermann, B.D. Characteristics of Untreated AIDS-related Cytomegalovirus Retinitis. II. Findings in the Era of Highly Active Antiretroviral Therapy (1997 to 2000). Am. J. Ophthalmol. 2008, 145, 12–22.e10. [Google Scholar] [CrossRef]
- Jabs, D.A.; Ahuja, A.; Van Natta, M.; Lyon, A.; Srivastava, S.; Gangaputra, S. Course of Cytomegalovirus Retinitis in the Era of Highly Active Antiretroviral Therapy: Five-Year Outcomes. Ophthalmology 2010, 117, 2152–2161.e2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Port, A.D.; Orlin, A.; Kiss, S.; Patel, S.; D’Amico, D.J.; Gupta, M.P. Cytomegalovirus Retinitis: A Review. J. Ocul. Pharmacol. Ther. 2017, 33, 224–234. [Google Scholar] [CrossRef]
- Gupta, M.P.; Koenig, L.R.; Doubrovina, E.; Hasan, A.; Dahi, P.B.; O’Reilly, R.J.; Koehne, G.; Orlin, A.; Chan, R.V.P.; D’Amico, D.J.; et al. Ocular Outcomes after Treatment of Cytomegalovirus Retinitis Using Adoptive Immunotherapy with Cytomegalovirus-Specific Cytotoxic T Lymphocytes. Ophthalmol. Retin. 2021, 5, 838–849. [Google Scholar] [CrossRef]
- Coleman, H.R.; Chan, C.-C.; Ferris, F.L.; Chew, E.Y. Age-related macular degeneration. Lancet 2008, 372, 1835–1845. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- de Jong, S.; Tang, J.; Clark, S.J. Age-related macular degeneration: A disease of extracellular complement amplification. Immunol. Rev. 2023, 313, 279–297. [Google Scholar] [CrossRef]
- Friedman, D.S.; O’Colmain, B.J.; Munoz, B.; Tomany, S.C.; McCarty, C.; De Jong, P.T.; Nemesure, B.; Mitchell, P.; Kempen, J. Prevalence of Age-Related Macular Degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.; Chakravarthy, U.; Klein, R.; Mitchell, P.; Zlateva, G.; Buggage, R.; Fahrbach, K.; Probst, C.; Sledge, I. The Natural History and Prognosis of Neovascular Age-Related Macular Degeneration: A Systematic Review of the Literature and Meta-analysis. Ophthalmology 2008, 115, 116–126.e111. [Google Scholar] [CrossRef]
- Guyer, D.R.; Fine, S.L.; Maguire, M.G.; Hawkins, B.S.; Owens, S.L.; Murphy, R.P. Subfoveal Choroidal Neovascular Membranes in Age-Related Macular Degeneration: Visual Prognosis in Eyes with Relatively Good Initial Visual Acuity. Arch. Ophthalmol. 1986, 104, 702–705. [Google Scholar] [CrossRef]
- Hageman, G.S.; Luthert, P.J.; Victor Chong, N.H.; Johnson, L.V.; Anderson, D.H.; Mullins, R.F. An Integrated Hypothesis That Considers Drusen as Biomarkers of Immune-Mediated Processes at the RPE-Bruch’s Membrane Interface in Aging and Age-Related Macular Degeneration. Prog. Retin. Eye Res. 2001, 20, 705–732. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, M.L.; Ferris, F.L.; Armstrong, J.; Hwang, T.S.; Chew, E.Y.; Bressler, S.B.; Chandra, S.R. Retinal Precursors and the Development of Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmology 2008, 115, 1026–1031. [Google Scholar] [CrossRef]
- Ambati, J.; Fowler, B.J. Mechanisms of Age-Related Macular Degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Jensen, E.G.; Jakobsen, T.S.; Thiel, S.; Askou, A.L.; Corydon, T.J. Associations between the Complement System and Choroidal Neovascularization in Wet Age-Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 9752. [Google Scholar] [CrossRef]
- Eandi, C.M.; Alovisi, C.; De Sanctis, U.; Grignolo, F.M. Treatment for neovascular age related macular degeneration: The state of the art. Eur. J. Pharmacol. 2016, 787, 78–83. [Google Scholar] [CrossRef]
- Parravano, M.; Costanzo, E.; Scondotto, G.; Trifirò, G.; Virgili, G. Anti-VEGF and Other Novel Therapies for Neovascular Age-Related Macular Degeneration: An Update. BioDrugs 2021, 35, 673–692. [Google Scholar] [CrossRef]
- Muñoz-Ramón, P.V.; Hernández Martínez, P.; Muñoz-Negrete, F.J. New therapeutic targets in the treatment of age-related macular degeneration. Arch. Soc. Española Oftalmol. (Engl. Ed.) 2020, 95, 75–83. [Google Scholar] [CrossRef]
- Yafai, Y.; Yang, X.M.; Niemeyer, M.; Nishiwaki, A.; Lange, J.; Wiedemann, P.; King, A.G.; Yasukawa, T.; Eichler, W. Anti-angiogenic effects of the receptor tyrosine kinase inhibitor, pazopanib, on choroidal neovascularization in rats. Eur. J. Pharmacol. 2011, 666, 12–18. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation with Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss: AREDS Report No. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef] [Green Version]
- Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration: The Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef] [Green Version]
- Ng, E.W.M.; Shima, D.T.; Calias, P.; Cunningham, E.T.; Guyer, D.R.; Adamis, A.P. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov. 2006, 5, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, N.; Hjortdal, J.; Schielke, K.C.; Bek, T.; Grauslund, J.; Laugesen, C.S.; Lund-Andersen, H.; Cerqueira, C.; Andresen, J. The Danish Registry of Diabetic Retinopathy. Clin. Epidemiol. 2016, 8, 613–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, M.L.; Pérez, S.; Mena-Mollá, S.; Desco, M.C.; Ortega, Á.L. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxidative Med. Cell Longev. 2019, 2019, 4940825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Aroca, P.; Baget-Bernaldiz, M.; Pareja-Rios, A.; Lopez-Galvez, M.; Navarro-Gil, R.; Verges, R. Diabetic Macular Edema Pathophysiology: Vasogenic versus Inflammatory. J. Diabetes Res. 2016, 2016, 2156273. [Google Scholar] [CrossRef] [Green Version]
- Tauber, J. Efficacy, tolerability and comfort of a 0.3% hypromellose gel ophthalmic lubricant in the treatment of patients with moderate to severe dry eye syndrome. Curr. Med. Res. Opin. 2007, 23, 2629–2636. [Google Scholar] [CrossRef]
- Rajendraprasad, R.M.; Kwatra, G.; Batra, N. Carboxymethyl Cellulose versus Hydroxypropyl Methylcellulose Tear Substitutes for Dry Eye Due to Computer Vision Syndrome: Comparison of Efficacy and Safety. Int. J. Appl. Basic Med. Res. 2021, 11, 4–8. [Google Scholar] [CrossRef]
- Hynnekleiv, L.; Magno, M.; Vernhardsdottir, R.R.; Moschowits, E.; Tønseth, K.A.; Dartt, D.A.; Vehof, J.; Utheim, T.P. Hyaluronic acid in the treatment of dry eye disease. Acta Ophthalmol. 2022, 100, 844–860. [Google Scholar] [CrossRef]
- Sun, X.; Liu, L.; Liu, C. Topical diquafosol versus hyaluronic acid for the treatment of dry eye disease: A meta-analysis of randomized controlled trials. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 1–13. [Google Scholar] [CrossRef]
- Chen, M.; Yung Choi, S. Preliminary Outcomes of Temporary Collagen Punctal Plugs for Patients with Dry Eye and Glaucoma. Med. Hypothesis Discov. Innov. Ophthalmol. J. 2020, 9, 56–60. [Google Scholar]
- Nagai, N.; Ishii, M.; Seiriki, R.; Ogata, F.; Otake, H.; Nakazawa, Y.; Okamoto, N.; Kanai, K.; Kawasaki, N. Novel Sustained-Release Drug Delivery System for Dry Eye Therapy by Rebamipide Nanoparticles. Pharmaceutics 2020, 12, 155. [Google Scholar] [CrossRef] [Green Version]
- Qiao, H.; Xu, Z.; Sun, M.; Fu, S.; Zhao, F.; Wang, D.; He, Z.; Zhai, Y.; Sun, J. Rebamipide liposome as an effective ocular delivery system for the management of dry eye disease. J. Drug Deliv. Sci. Technol. 2022, 75, 103654. [Google Scholar] [CrossRef]
- Li, Q.; Wu, X.; Xin, M. Strengthened rebamipide ocular nanoformulation to effectively treat corneal alkali burns in mice through the HMGB1 signaling pathway. Exp. Eye Res. 2021, 213, 108824. [Google Scholar] [CrossRef]
- Terreni, E.; Zucchetti, E.; Tampucci, S.; Burgalassi, S.; Monti, D.; Chetoni, P. Combination of Nanomicellar Technology and In Situ Gelling Polymer as Ocular Drug Delivery System (ODDS) for Cyclosporine-A. Pharmaceutics 2021, 13, 192. [Google Scholar] [CrossRef]
- Bang, S.P.; Yeon, C.Y.; Adhikari, N.; Neupane, S.; Kim, H.; Lee, D.C.; Son, M.J.; Lee, H.G.; Kim, J.-Y.; Jun, J.H. Cyclosporine A eyedrops with self-nanoemulsifying drug delivery systems have improved physicochemical properties and efficacy against dry eye disease in a murine dry eye model. PLoS ONE 2019, 14, e0224805. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.-Y.; Liu, Y.; Zhou, L.; Wong, M.-S.; Liu, J. Mucin-targeting-aptamer functionalized liposomes for delivery of cyclosporin A for dry eye diseases. J. Mater. Chem. B 2023, 11. [Google Scholar] [CrossRef]
- Chhowala, I.; Patel, A.; Patel, R.; Bhavsar, V.; Dharamsi, A. Optimisation of PCL-HA laden biodegradable nanoparticles containing cyclosporine-A for the treatment of dry eye syndrome: In vitro-in vivo evaluation. Int. J. Nanoparticles 2021, 13, 106–120. [Google Scholar] [CrossRef]
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.-S.J.J.O.D.D. Successfully improving ocular drug delivery using the cationic nanoemulsion, novasorb. J. Drug Deliv. 2012, 2012, 604204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gökçe, E.H.; Sandri, G.; Eğrilmez, S.; Bonferoni, M.C.; Güneri, T.; Caramella, C. Cyclosporine A-Loaded Solid Lipid Nanoparticles: Ocular Tolerance and In Vivo Drug Release in Rabbit Eyes. Curr. Eye Res. 2009, 34, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Wang, M.C.; Chen, Z.Y.; Chiu, W.Y.; Chen, K.H.; Lin, I.C.; Yang, W.V.; Wu, C.C.; Tseng, C.L. Gelatin-epigallocatechin gallate nanoparticles with hyaluronic acid decoration as eye drops can treat rabbit dry-eye syndrome effectively via inflammatory relief. Int. J. Nanomed. 2018, 13, 7251–7273. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.-J.; Lai, J.-Y. Epigallocatechin Gallate-Loaded Gelatin-g-Poly(N-Isopropylacrylamide) as a New Ophthalmic Pharmaceutical Formulation for Topical Use in the Treatment of Dry Eye Syndrome. Sci. Rep. 2017, 7, 9380. [Google Scholar] [CrossRef] [Green Version]
- López-Machado, A.; Díaz, N.; Cano, A.; Espina, M.; Badía, J.; Baldomà, L.; Calpena, A.C.; Biancardi, M.; Souto, E.B.; García, M.L.; et al. Development of topical eye-drops of lactoferrin-loaded biodegradable nanoparticles for the treatment of anterior segment inflammatory processes. Int. J. Pharm. 2021, 609, 121188. [Google Scholar] [CrossRef] [PubMed]
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Isabel Lema, M.; Otero-Espinar, F.J. Mucoadhesive PLGA Nanospheres and Nanocapsules for Lactoferrin Controlled Ocular Delivery. Pharmaceutics 2022, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- López-Machado, A.; Díaz-Garrido, N.; Cano, A.; Espina, M.; Badia, J.; Baldomà, L.; Calpena, A.C.; Souto, E.B.; García, M.L.; Sánchez-López, E. Development of Lactoferrin-Loaded Liposomes for the Management of Dry Eye Disease and Ocular Inflammation. Pharmaceutics 2021, 13, 1698. [Google Scholar] [CrossRef] [PubMed]
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Isabel Lema, M.; Javier Otero-Espinar, F. Lactoferrin-loaded nanostructured lipid carriers (NLCs) as a new formulation for optimized ocular drug delivery. Eur. J. Pharm. Biopharm. 2022, 172, 144–156. [Google Scholar] [CrossRef]
- He, W.; Guo, X.; Feng, M.; Mao, N. In vitro and in vivo studies on ocular vitamin A palmitate cationic liposomal in situ gels. Int. J. Pharm. 2013, 458, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.F.M.; Fulgêncio, G.d.O.; Domingos, L.C.; Cotta, O.A.L.; Silva-Cunha, A.; Fialho, S.L. Positively charged polymeric nanoparticles improve ocular penetration of tacrolimus after topical administration. J. Drug Deliv. Sci. Technol. 2020, 60, 101912. [Google Scholar] [CrossRef]
- Mohammad; Garg, V.; Nirmal, J.; Warsi, M.H.; Pandita, D.; Kesharwani, P.; Jain, G.K. Topical Tacrolimus Progylcosomes Nano-Vesicles As a Potential Therapy for Experimental Dry Eye Syndrome. J. Pharm. Sci. 2022, 111, 479–484. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, Y.; Li, M.; Zhang, Z.; Chang, L.; Ai, M.; Wang, J.; Zhao, S.; Li, C.; Zhou, Z. Carboxymethyl Cellulose-Coated Tacrolimus Nonspherical Microcrystals for Improved Therapeutic Efficacy of Dry Eye. Macromol. Biosci. 2020, 20, 2000079. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Lin, X.; Wu, X.; Yu, X.; Wang, B.; Xu, W. Tacrolimus Loaded Cationic Liposomes for Dry Eye Treatment. Front. Pharmacol. 2022, 13, 838168. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Q.; Chen, W.; Lin, H.; Zhu, Y.; Liu, Y.; Liang, H.; Zhu, F. A novel FK506 loaded nanomicelles consisting of amino-terminated poly(ethylene glycol)-block-poly(D,L)-lactic acid and hydroxypropyl methylcellulose for ocular drug delivery. Int. J. Pharm. 2019, 562, 1–10. [Google Scholar] [CrossRef]
- Rebibo, L.; Tam, C.; Sun, Y.; Shoshani, E.; Badihi, A.; Nassar, T.; Benita, S. Topical tacrolimus nanocapsules eye drops for therapeutic effect enhancement in both anterior and posterior ocular inflammation models. J. Control. Release 2021, 333, 283–297. [Google Scholar] [CrossRef]
- Soiberman, U.; Kambhampati, S.P.; Wu, T.; Mishra, M.K.; Oh, Y.; Sharma, R.; Wang, J.; Al Towerki, A.E.; Yiu, S.; Stark, W.J.; et al. Subconjunctival injectable dendrimer-dexamethasone gel for the treatment of corneal inflammation. Biomaterials 2017, 125, 38–53. [Google Scholar] [CrossRef] [Green Version]
- Bian, F.; Shin, C.S.; Wang, C.; Pflugfelder, S.C.; Acharya, G.; De Paiva, C.S. Dexamethasone Drug Eluting Nanowafers Control Inflammation in Alkali-Burned Corneas Associated with Dry Eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3222–3230. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.; Yu, S.; Li, J.; Pan, W. Development and characterization of nanostructured lipid carriers based chitosan thermosensitive hydrogel for delivery of dexamethasone. Int. J. Biol. Macromol. 2017, 103, 941–947. [Google Scholar] [CrossRef]
- Taheri, S.L.; Rezazadeh, M.; Hassanzadeh, F.; Akbari, V.; Dehghani, A.; Talebi, A.; Mostafavi, S.A. Preparation, physicochemical, and retinal anti-angiogenic evaluation of poloxamer hydrogel containing dexamethasone/avastin-loaded chitosan-N-acetyl-L-cysteine nanoparticles. Int. J. Biol. Macromol. 2022, 220, 1605–1618. [Google Scholar] [CrossRef]
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Sattari, S.; Salatin, S.; Jelvehgari, M. Evaluation of anti-inflammatory impact of dexamethasone-loaded PCL-PEG-PCL micelles on endotoxin-induced uveitis in rabbits. Pharm. Dev. Technol. 2019, 24, 680–688. [Google Scholar] [CrossRef]
- Kassem, M.A.; Abdel Rahman, A.A.; Ghorab, M.M.; Ahmed, M.B.; Khalil, R.M. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int. J. Pharm. 2007, 340, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Fialho, S.L.; Da Silva-Cunha, A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin. Exp. Ophthalmol. 2004, 32, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Vavia, P.R.; Trotta, F.; Cavalli, R. Nanosponges Encapsulating Dexamethasone for Ocular Delivery: Formulation Design, Physicochemical Characterization, Safety and Corneal Permeability Assessment. J. Biomed. Nanotechnol. 2013, 9, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-Z.; Guan, B.; Liu, X.-X.; Ke, L.-N.; Wang, J.-J.; Nan, K.-H. A topical fluorometholone nanoformulation fabricated under aqueous condition for the treatment of dry eye. Colloids Surf. B Biointerfaces 2022, 212, 112351. [Google Scholar] [CrossRef] [PubMed]
- Sabzevari, A.; Adibkia, K.; Hashemi, H.; De Geest, B.G.; Mohsenzadeh, N.; Atyabi, F.; Ghahremani, M.H.; Khoshayand, M.-R.; Dinarvand, R. Improved Anti-Inflammatory Effects in Rabbit Eye Model Using Biodegradable Poly Beta-Amino Ester Nanoparticles of Triamcinolone Acetonide. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5520–5526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, A.K.; Maincent, P.; Thouvenot, P.; Kreuter, J. Hydrocortisone delivery to healthy and inflamed eyes using a micellar polysorbate 80 solution or albumin nanoparticles. Int. J. Pharm. 1994, 110, 211–222. [Google Scholar] [CrossRef]
- Ibrahim, H.K.; El-Leithy, I.S.; Makky, A.A. Mucoadhesive Nanoparticles as Carrier Systems for Prolonged Ocular Delivery of Gatifloxacin/Prednisolone Bitherapy. Mol. Pharm. 2010, 7, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Katzer, T.; Chaves, P.; Bernardi, A.; Pohlmann, A.; Guterres, S.S.; Ruver Beck, R.C. Prednisolone-loaded nanocapsules as ocular drug delivery system: Development, in vitro drug release and eye toxicity. J. Microencapsul. 2014, 31, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Schopf, L.; Enlow, E.; Popov, A.; Bourassa, J.; Chen, H. Ocular Pharmacokinetics of a Novel Loteprednol Etabonate 0.4% Ophthalmic Formulation. Ophthalmol. Ther. 2014, 3, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asasutjarit, R.; Theerachayanan, T.; Kewsuwan, P.; Veeranodha, S.; Fuongfuchat, A.; Ritthidej, G.C. Development and Evaluation of Diclofenac Sodium Loaded-N-Trimethyl Chitosan Nanoparticles for Ophthalmic Use. AAPS PharmSciTech 2015, 16, 1013–1024. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, S.M.; Vavia, P.R. Diclofenac-loaded biopolymeric nanosuspensions for ophthalmic application. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 90–95. [Google Scholar] [CrossRef]
- Abrego, G.; Alvarado, H.L.; Egea, M.A.; Gonzalez-Mira, E.; Calpena, A.C.; Garcia, M.L. Design of Nanosuspensions and Freeze-Dried PLGA Nanoparticles as a Novel Approach for Ophthalmic Delivery of Pranoprofen. J. Pharm. Sci. 2014, 103, 3153–3164. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, L.; Feng, P.; Qiu, H.; Wu, X.; Lu, S.; Zhou, M.; Xu, L.; Zhu, Y. Pranoprofen Nanoparticles with Poly(L-Lactide)-b-Poly(Ethylene Glycol)-b-Poly(L-Lactide) as the Matrix Toward Improving Ocular Anti-inflammation. Front. Bioeng. Biotechnol. 2020, 8, 581621. [Google Scholar] [CrossRef]
- Sánchez-Santos, I.; García-Sánchez, G.A.; Gonzalez-Salinas, R.; Linares-Alba, M.A.; Rodríguez-Reyes, A.A.; García-Santisteban, R.; Tirado-González, V.; Hernández-Piñamora, E.; García-Arzate, D.; Morales-Cantón, V.; et al. Intravitreal bromfenac liposomal suspension (100 μg/0.1 mL). A safety study in rabbit eyes. Exp. Eye Res. 2020, 194, 108020. [Google Scholar] [CrossRef]
- Otake, H.; Goto, R.; Ogata, F.; Isaka, T.; Kawasaki, N.; Kobayakawa, S.; Matsunaga, T.; Nagai, N. Fixed-Combination Eye Drops Based on Fluorometholone Nanoparticles and Bromfenac/Levofloxacin Solution Improve Drug Corneal Penetration. Int. J. Nanomed. 2021, 16, 5343–5356. [Google Scholar] [CrossRef]
- Warsi, M.H. Development and optimization of vitamin E TPGS based PLGA nanoparticles for improved and safe ocular delivery of ketorolac. J. Drug Deliv. Sci. Technol. 2021, 61, 102121. [Google Scholar] [CrossRef]
- Tauber, J.; Karpecki, P.; Latkany, R.; Luchs, J.; Martel, J.; Sall, K.; Raychaudhuri, A.; Smith, V.; Semba, C.P. Lifitegrast Ophthalmic Solution 5.0% versus Placebo for Treatment of Dry Eye Disease: Results of the Randomized Phase III OPUS-2 Study. Ophthalmology 2015, 122, 2423–2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hovanesian, J.; Epitropoulos, A.; Donnenfeld, E.D.; Holladay, J.T. The Effect of Lifitegrast on Refractive Accuracy and Symptoms in Dry Eye Patients Undergoing Cataract Surgery. Clin. Ophthalmol. (Auckl. N.Z.) 2020, 14, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Ko, Y.-C.; Chang, Y.-F.; Huang, S.-H.; Liu, C.J.-l. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187. [Google Scholar] [CrossRef]
- Dang, H.; Dong, C.; Zhang, L. Sustained latanoprost release from PEGylated solid lipid nanoparticle-laden soft contact lens to treat glaucoma. Pharm. Dev. Technol. 2022, 27, 127–133. [Google Scholar] [CrossRef]
- Xu, J.; Ge, Y.; Bu, R.; Zhang, A.; Feng, S.; Wang, J.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; et al. Co-delivery of latanoprost and timolol from micelles-laden contact lenses for the treatment of glaucoma. J. Control. Release 2019, 305, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Bessone, C.D.V.; Akhlaghi, S.P.; Tártara, L.I.; Quinteros, D.A.; Loh, W.; Allemandi, D.A. Latanoprost-loaded phytantriol cubosomes for the treatment of glaucoma. Eur. J. Pharm. Sci. 2021, 160, 105748. [Google Scholar] [CrossRef] [PubMed]
- Schnichels, S.; Hurst, J.; de Vries, J.W.; Ullah, S.; Gruszka, A.; Kwak, M.; Löscher, M.; Dammeier, S.; Bartz-Schmidt, K.-U.; Spitzer, M.S.; et al. Self-assembled DNA nanoparticles loaded with travoprost for glaucoma-treatment. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102260. [Google Scholar] [CrossRef]
- Masse, F.; Ouellette, M.; Boisselier, E. Ultrastable gold nanoparticles as a new drug vector for glaucoma therapy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3512. [Google Scholar]
- Shukr, M.H.; Ismail, S.; El-Hossary, G.G.; El-Shazly, A.H. Design and evaluation of mucoadhesive in situ liposomal gel for sustained ocular delivery of travoprost using two steps factorial design. J. Drug Deliv. Sci. Technol. 2021, 61, 102333. [Google Scholar] [CrossRef]
- Shukr, M.H.; Ismail, S.; El-Hossary, G.G.; El-Shazly, A.H. Spanlastics nanovesicular ocular insert as a novel ocular delivery of travoprost: Optimization using Box–Behnken design and in vivo evaluation. J. Liposome Res. 2022, 32, 354–364. [Google Scholar] [CrossRef]
- Goldstein, M.H.; Goldberg, D.; Walters, T.R.; Vantipalli, S.; Braun, E.; Metzinger, J.L. Evaluating Safety, Tolerability and Efficacy of an Intracameral Hydrogel-Based Travoprost Implant in Subjects with Glaucoma-Phase 1 Trial. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4266. [Google Scholar]
- Wadetwar, R.N.; Agrawal, A.R.; Kanojiya, P.S. In situ gel containing Bimatoprost solid lipid nanoparticles for ocular delivery: In-vitro and ex-vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 56, 101575. [Google Scholar] [CrossRef]
- Li, Q.; Ma, C.; Ma, Y.; Ma, Y.; Mao, Y.; Meng, Z. Sustained bimatoprost release using gold nanoparticles laden contact lenses. J. Biomater. Sci. Polym. Ed. 2021, 32, 1618–1634. [Google Scholar] [CrossRef]
- Meany, E.L.; Andaya, R.; Tang, S.; Kasse, C.M.; Fuji, R.N.; Grosskopf, A.K.; d’Aquino, A.L.; Bartoe, J.T.; Ybarra, R.; Shelton, A.; et al. Injectable Polymer-Nanoparticle Hydrogel for the Sustained Intravitreal Delivery of Bimatoprost. Adv. Ther. 2023, 6, 2200207. [Google Scholar] [CrossRef]
- Xu, W.; Jiao, W.; Li, S.; Tao, X.; Mu, G. Bimatoprost loaded microemulsion laden contact lens to treat glaucoma. J. Drug Deliv. Sci. Technol. 2019, 54, 101330. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, P.D.; Patel, P.J.; Desai, A.R.; Desai, D.T.; Shukla, M.R.; Shah, S.A.; Shah, D.O.; Willcox, M.D.P. Controlled bimatoprost release from graphene oxide laden contact lenses: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2021, 208, 112096. [Google Scholar] [CrossRef]
- Seal, J.R.; Robinson, M.R.; Burke, J.; Bejanian, M.; Coote, M.; Attar, M. Intracameral Sustained-Release Bimatoprost Implant Delivers Bimatoprost to Target Tissues with Reduced Drug Exposure to Off-Target Tissues. J. Ocul. Pharmacol. Ther. 2019, 35, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.; Guzman-Aranguez, A.; Perez de Lara, M.J.; Singh, M.; Singh, J.; Kaur, I.P. Bimatoprost loaded nanovesicular long-acting sub-conjunctival in-situ gelling implant: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2019, 103, 109730. [Google Scholar] [CrossRef] [PubMed]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Rodrigues, L.B.; Bravo, R.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.J.P.O. Bimatoprost-loaded ocular inserts as sustained release drug delivery systems for glaucoma treatment: In vitro and in vivo evaluation. PLoS ONE 2014, 9, e95461. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yamada, S.; Kawasaki, J.; Koyanagi, E.; Saijo, S.; Kaji, H.; Nishizawa, M.; Nakazawa, T.; Abe, T. Pharmacokinetic and Safety Evaluation of a Transscleral Sustained Unoprostone Release Device in Monkey Eyes. Investig. Ophthalmol. Vis. Sci. 2018, 59, 644–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khallaf, A.M.; El-Moslemany, R.M.; Ahmed, M.F.; Morsi, M.H.; Khalafallah, N.M. Exploring a Novel Fasudil-Phospholipid Complex Formulated as Liposomal Thermosensitive in situ Gel for Glaucoma. Int. J. Nanomed. 2022, 17, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Mietzner, R.; Kade, C.; Froemel, F.; Pauly, D.; Stamer, W.D.; Ohlmann, A.; Wegener, J.; Fuchshofer, R.; Breunig, M. Fasudil Loaded PLGA Microspheres as Potential Intravitreal Depot Formulation for Glaucoma Therapy. Pharmaceutics 2020, 12, 706. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, S.; Nakamura, M. Ripasudil Hydrochloride Hydrate in the Treatment of Glaucoma: Safety, Efficacy, and Patient Selection. Clin. Ophthalmol. 2020, 14, 1229–1236. [Google Scholar] [CrossRef]
- Mehran, N.A.; Sinha, S.; Razeghinejad, R. New glaucoma medications: Latanoprostene bunod, netarsudil, and fixed combination netarsudil-latanoprost. Eye 2020, 34, 72–88. [Google Scholar] [CrossRef]
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J. Control. Release 2013, 165, 82–89. [Google Scholar] [CrossRef]
- Huang, J.; Peng, T.; Li, Y.; Zhan, Z.; Zeng, Y.; Huang, Y.; Pan, X.; Wu, C.-Y.; Wu, C. Ocular Cubosome Drug Delivery System for Timolol Maleate: Preparation, Characterization, Cytotoxicity, Ex Vivo, and In Vivo Evaluation. AAPS PharmSciTech 2017, 18, 2919–2926. [Google Scholar] [CrossRef]
- Cuggino, J.C.; Tártara, L.I.; Gugliotta, L.M.; Palma, S.D.; Alvarez Igarzabal, C.I. Mucoadhesive and responsive nanogels as carriers for sustainable delivery of timolol for glaucoma therapy. Mater. Sci. Eng. C 2021, 118, 111383. [Google Scholar] [CrossRef]
- Hathout, R.M.; Gad, H.A.; Abdel-Hafez, S.M.; Nasser, N.; Khalil, N.; Ateyya, T.; Amr, A.; Yasser, N.; Nasr, S.; Metwally, A.A. Gelatinized core liposomes: A new Trojan horse for the development of a novel timolol maleate glaucoma medication. Int. J. Pharm. 2019, 556, 192–199. [Google Scholar] [CrossRef]
- Wei, N.; Dang, H.; Huang, C.; Sheng, Y. Timolol loaded microemulsion laden silicone contact lens to manage glaucoma: In vitro and in vivo studies. J. Dispers. Sci. Technol. 2021, 42, 742–750. [Google Scholar] [CrossRef]
- Kumar, N.; Aggarwal, R.; Chauhan, M.K. Extended levobunolol release from Eudragit nanoparticle-laden contact lenses for glaucoma therapy. Future J. Pharm. Sci. 2020, 6, 109. [Google Scholar] [CrossRef]
- Marchal-Heussler, L.; Sirbat, D.; Hoffman, M.; Maincent, P. Poly(ε-Caprolactone) Nanocapsules in Carteolol Ophthalmic Delivery. Pharm. Res. 1993, 10, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yamaoka, S.; Fukuoka, Y.; Ishii, M.; Otake, H.; Kanai, K.; Okamoto, N.; Shimomura, Y. Enhancement in Corneal Permeability of Dissolved Carteolol by Its Combination with Magnesium Hydroxide Nanoparticles. Int. J. Mol. Sci. 2018, 19, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Alharbi, K.S.; Yasir, M.; Elmowafy, M.; Ansari, M.J.; Salahuddin, M.; Alshehri, S. Formulation of carteolol chitosomes for ocular delivery: Formulation optimization, ex-vivo permeation, and ocular toxicity examination. Cutan. Ocul. Toxicol. 2021, 40, 338–349. [Google Scholar] [CrossRef]
- Losa, C.; Marchal-Heussler, L.; Orallo, F.; Jato, J.L.V.; Alonso, M.J. Design of New Formulations for Topical Ocular Administration: Polymeric Nanocapsules Containing Metipranolol. Pharm. Res. 1993, 10, 80–87. [Google Scholar] [CrossRef]
- Huang, Y.; Tao, Q.; Hou, D.; Hu, S.; Tian, S.; Chen, Y.; Gui, R.; Yang, L.; Wang, Y. A novel ion-exchange carrier based upon liposome-encapsulated montmorillonite for ophthalmic delivery of betaxolol hydrochloride. Int. J. Nanomed. 2017, 12, 1731–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Li, J.; Han, X.; Tao, Q.; Liu, S.; Jiang, G.; Zhu, G.; Yang, F.; Lv, Z.; Chen, Y.; et al. Dual controlled release effect of montmorillonite loaded polymer nanoparticles for ophthalmic drug delivery. Appl. Clay Sci. 2019, 180, 105167. [Google Scholar] [CrossRef]
- Allam, A.; Elsabahy, M.; El Badry, M.; Eleraky, N.E. Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma. Int. J. Pharm. 2021, 598, 120380. [Google Scholar] [CrossRef]
- Sakr, M.G.; El-Zahaby, S.A.; Al-Mahallawi, A.M.; Ghorab, D.M. Fabrication of betaxolol hydrochloride-loaded highly permeable ocular bilosomes (HPOBs) to combat glaucoma: In vitro, ex vivo & in vivo characterizations. J. Drug Deliv. Sci. Technol. 2023, 82, 104363. [Google Scholar] [CrossRef]
- Sun, J.; Lei, Y.; Dai, Z.; Liu, X.; Huang, T.; Wu, J.; Xu, Z.P.; Sun, X. Sustained Release of Brimonidine from a New Composite Drug Delivery System for Treatment of Glaucoma. ACS Appl. Mater. Interfaces 2017, 9, 7990–7999. [Google Scholar] [CrossRef]
- Shivakumar, H.N.; Desai, B.G.; Subhash, P.G.; Ashok, P.; Hulakoti, B. Design of ocular inserts of brimonidine tartrate by response surface methodology. J. Drug Deliv. Sci. Technol. 2007, 17, 421–430. [Google Scholar] [CrossRef]
- Emad Eldeeb, A.; Salah, S.; Ghorab, M. Proniosomal gel-derived niosomes: An approach to sustain and improve the ocular delivery of brimonidine tartrate; formulation, in-vitro characterization, and in-vivo pharmacodynamic study. Drug Deliv. 2019, 26, 509–521. [Google Scholar] [CrossRef] [Green Version]
- Chiang, B.; Kim, Y.C.; Doty, A.C.; Grossniklaus, H.E.; Schwendeman, S.P.; Prausnitz, M.R. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. J. Control. Release 2016, 228, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigdeli, A.; Makhmalzadeh, B.S.; Feghhi, M.; SoleimaniBiatiani, E. Cationic liposomes as promising vehicles for timolol/brimonidine combination ocular delivery in glaucoma: Formulation development and in vitro/in vivo evaluation. Drug Deliv. Transl. Res. 2022, 13, 1035–1047. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, C.; Zhang, Z.; Hong, J.; Xu, J.; Sun, X.; Sun, J. Sustained release of brimonidine from BRI@SR@TPU implant for treatment of glaucoma. Drug Deliv. 2022, 29, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Shahab, M.S.; Rizwanullah, M.; Alshehri, S.; Imam, S.S. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: In vitro, ex vivo and toxicity assessments. Int. J. Biol. Macromol. 2020, 163, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.A.; Salama, A.; Mohsen, A.M. Formulation and optimization of cationic nanoemulsions for enhanced ocular delivery of dorzolamide hydrochloride using Box-Behnken design: In vitro and in vivo assessments. J. Drug Deliv. Sci. Technol. 2022, 68, 103047. [Google Scholar] [CrossRef]
- Fu, J.; Sun, F.; Liu, W.; Liu, Y.; Gedam, M.; Hu, Q.; Fridley, C.; Quigley, H.A.; Hanes, J.; Pitha, I. Subconjunctival Delivery of Dorzolamide-Loaded Poly(ether-anhydride) Microparticles Produces Sustained Lowering of Intraocular Pressure in Rabbits. Mol. Pharm. 2016, 13, 2987–2995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouda, N.H.; Abdelrehim, R.T.; Hegazy, D.A.; Habib, B.A. Sustained ocular delivery of Dorzolamide-HCl via proniosomal gel formulation: In-vitro characterization, statistical optimization, and in-vivo pharmacodynamic evaluation in rabbits. Drug Deliv. 2018, 25, 1340–1349. [Google Scholar] [CrossRef] [Green Version]
- Özdemir, S.; Çakırlı, E.; Sürücü, B.; Aygüler, C.İ.; Üner, B.; Çelebi, A.R.C. Preparation and characterization studies of dorzolamide loaded ophthalmic implants for the treatment of glaucoma. Turk. J. Pharm. Sci. 2022. [Google Scholar] [CrossRef]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.A.; Cronemberger, S.; Nogueira, J.C.; et al. Chitosan/hydroxyethyl cellulose inserts for sustained-release of dorzolamide for glaucoma treatment: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 570, 118662. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, J.; Wu, L.; Wang, B.; Wang, Z.; Xu, Q.; Xin, H. Ophthalmic Delivery of Brinzolamide by Liquid Crystalline Nanoparticles: In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2013, 14, 1063–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuomela, A.; Liu, P.; Puranen, J.; Rönkkö, S.; Laaksonen, T.; Kalesnykas, G.; Oksala, O.; Ilkka, J.; Laru, J.; Järvinen, K.; et al. Brinzolamide nanocrystal formulations for ophthalmic delivery: Reduction of elevated intraocular pressure in vivo. Int. J. Pharm. 2014, 467, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Zhang, Y.; Fang, D.; Xu, B.; Zhang, L.; Chen, T.; Ren, K.; Nie, Y.; Yao, S.; et al. Liposomes as a Novel Ocular Delivery System for Brinzolamide: In Vitro and In Vivo Studies. AAPS PharmSciTech 2016, 17, 710–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, V.; Mohan, P.; Dangi, J.S.; Kesavan, K. Brinzolamide loaded chitosan-pectin mucoadhesive nanocapsules for management of glaucoma: Formulation, characterization and pharmacodynamic study. Int. J. Biol. Macromol. 2020, 152, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Bhalerao, H.; Koteshwara, K.B.; Chandran, S. Design, optimisation and evaluation of in situ gelling nanoemulsion formulations of brinzolamide. Drug Deliv. Transl. Res. 2020, 10, 529–547. [Google Scholar] [CrossRef]
- Cegielska, O.; Sierakowski, M.; Sajkiewicz, P.; Lorenz, K.; Kogermann, K. Mucoadhesive brinzolamide-loaded nanofibers for alternative glaucoma treatment. Eur. J. Pharm. Biopharm. 2022, 180, 48–62. [Google Scholar] [CrossRef]
- Smith, S.M.; Salmon, J.H.; Abbaraju, S.; Amin, R.; Gilger, B.C. Tolerability, pharmacokinetics, and pharmacodynamics of a brinzolamide episcleral sustained release implant in normotensive New Zealand white rabbits. J. Drug Deliv. Sci. Technol. 2021, 61, 102123. [Google Scholar] [CrossRef]
- Teba, H.E.; Khalil, I.A.; El Sorogy, H.M. Novel cubosome based system for ocular delivery of acetazolamide. Drug Deliv. 2021, 28, 2177–2186. [Google Scholar] [CrossRef]
- Abdel-Rashid, R.S.; Helal, D.A.; Omar, M.M.; El Sisi, A.M. Nanogel loaded with surfactant based nanovesicles for enhanced ocular delivery of acetazolamide. Int. J. Nanomed. 2019, 14, 2973–2983. [Google Scholar] [CrossRef] [Green Version]
- Mazyed, E.A.; Abdelaziz, A.E. Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study. Pharmaceutics 2020, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Coimbra, P.; Pina, M.E. Comparative Analysis of Morphological and Release Profiles in Ocular Implants of Acetazolamide Prepared by Electrospinning. Pharmaceutics 2021, 13, 260. [Google Scholar] [CrossRef] [PubMed]
- El-Menshawe, S.F. A novel approach to topical acetazolamide/PEG 400 ocular niosomes. J. Drug Deliv. Sci. Technol. 2012, 22, 295–299. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Salama, A.; Kassem, A.A. Development of acetazolamide loaded bilosomes for improved ocular delivery: Preparation, characterization and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101910. [Google Scholar] [CrossRef]
- Obiedallah, M.M.; Abdel-Mageed, A.M.; Elfaham, T.H. Ocular administration of acetazolamide microsponges in situ gel formulations. Saudi Pharm. J. 2018, 26, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Jain, N.K. Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int. J. Pharm. 2014, 461, 380–390. [Google Scholar] [CrossRef]
- Lin, H.-R.; Yu, S.-P.; Kuo, C.-J.; Kao, H.-J.; Lo, Y.-L.; Lin, Y.-J. Pilocarpine-loaded chitosan-PAA nanosuspension for ophthalmic delivery. J. Biomater. Sci. Polym. Ed. 2007, 18, 205–221. [Google Scholar] [CrossRef]
- Suketu, D.; Desai, J.B. Pluronic® F127-Based Ocular Delivery System Containing Biodegradable Polyisobutylcyanoacrylate Nanocapsules of Pilocarpine. Drug Deliv. 2000, 7, 201–207. [Google Scholar] [CrossRef]
- Vandamme, T.F.; Brobeck, L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J. Control. Release 2005, 102, 23–38. [Google Scholar] [CrossRef]
- Patel, C.C.; Mandava, N.; Oliver, S.C.N.; Braverman, R.; Quiroz-Mercado, H.; Olson, J.L. Treatment of Intractable Posterior Uveitis in Pediatric Patients with the Fluocinolone Acetonide Intravitreal Implant (Retisert). J. Retin. Vitr. Dis. 2012, 32, 537–542. [Google Scholar] [CrossRef]
- Salama, A.H.; Mahmoud, A.A.; Kamel, R. A Novel Method for Preparing Surface-Modified Fluocinolone Acetonide Loaded PLGA Nanoparticles for Ocular Use: In Vitro and In Vivo Evaluations. AAPS PharmSciTech 2016, 17, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Shelley, H.; Annaji, M.; Grant, M.; Fasina, O.; Babu, R.J. Sustained Release Biodegradable Microneedles of Difluprednate for Delivery to Posterior Eye. J. Ocul. Pharmacol. Ther. 2022, 38, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pizarro, R.; Parrotta, G.; Vera, R.; Sánchez-López, E.; Galindo, R.; Kjeldsen, F.; Badia, J.; Baldoma, L.; Espina, M.; García, M.L. Ocular penetration of fluorometholone-loaded PEG-PLGA nanoparticles functionalized with cell-penetrating peptides. Nanomedicine 2019, 14, 3089–3104. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Hashida, N.; Tujikawa, M.; Quantock, A.J.; Nishida, K. The generation of fluorometholone nanocrystal eye drops, their metabolization to dihydrofluorometholone and penetration into rabbit eyes. Int. J. Pharm. 2021, 592, 120067. [Google Scholar] [CrossRef]
- Nirbhavane, P.; Sharma, G.; Singh, B.; Begum, G.; Jones, M.-C.; Rauz, S.; Vincent, R.; Denniston, A.K.; Hill, L.J.; Katare, O.P. Triamcinolone acetonide loaded-cationic nano-lipoidal formulation for uveitis: Evidences of improved biopharmaceutical performance and anti-inflammatory activity. Colloids Surf. B Biointerfaces 2020, 190, 110902. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, M.; Wang, Q.; Bai, J.; McAlinden, C.; Skiadaresi, E.; Zhang, J.; Pan, L.; Mei, C.; Zeng, Z.; et al. Hydrogel eye drops as a non-invasive drug carrier for topical enhanced Adalimumab permeation and highly efficient uveitis treatment. Carbohydr. Polym. 2021, 253, 117216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qian, J.; Li, X.; Yuan, Y. Treatment of experimental autoimmune uveoretinitis with intravitreal injection of infliximab encapsulated in liposomes. Br. J. Ophthalmol. 2017, 101, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Donnell, A.M.; Faraj, R.Q.C.; Riemann, B.I.; Riemann, C.D.; Augsburger, J.J.; Correa, Z.M.; Banerjee, R.K. Pharmacokinetics and Toxicity Evaluation of a PLGA and Chitosan-Based Micro-Implant for Sustained Release of Methotrexate in Rabbit Vitreous. Pharmaceutics 2021, 13, 1227. [Google Scholar] [CrossRef]
- Paiva, M.R.B.D.; Vasconcelos-Santos, D.V.; Vieira, L.C.; Fialho, S.L.; Silva-Cunha, A. Sirolimus-Loaded Intravitreal Implant for Effective Treatment of Experimental Uveitis. AAPS PharmSciTech 2021, 22, 35. [Google Scholar] [CrossRef]
- Wu, W.; He, Z.; Zhang, Z.; Yu, X.; Song, Z.; Li, X. Intravitreal injection of rapamycin-loaded polymeric micelles for inhibition of ocular inflammation in rat model. Int. J. Pharm. 2016, 513, 238–246. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Li, Y.; Su, L.; Duan, Y.; Zhang, H.; An, J.; Ni, T.; Li, X.; Zhang, X. Therapeutic Effect of Rapamycin-Loaded Small Extracellular Vesicles Derived from Mesenchymal Stem Cells on Experimental Autoimmune Uveitis. Front. Immunol. 2022, 13, 864956. [Google Scholar] [CrossRef]
- Silva, N.C.; Silva, S.; Sarmento, B.; Pintado, M. Chitosan nanoparticles for daptomycin delivery in ocular treatment of bacterial endophthalmitis. Drug Deliv. 2015, 22, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Jounaki, K.; Makhmalzadeh, B.S.; Feghhi, M.; Heidarian, A. Topical ocular delivery of vancomycin loaded cationic lipid nanocarriers as a promising and non-invasive alternative approach to intravitreal injection for enhanced bacterial endophthalmitis management. Eur. J. Pharm. Sci. 2021, 167, 105991. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.F.; Perasoli, F.B.; Almeida, T.C.; Marques, M.B.D.F.; Toledo, C.R.; Gil, P.O.; Tavares, H.D.S.; Da Paz, M.C.; Mussel, W.D.N.; Magalhães, J.T.; et al. Vancomycin-loaded N,N-dodecyl,methyl-polyethylenimine nanoparticles coated with hyaluronic acid to treat bacterial endophthalmitis: Development, characterization, and ocular biocompatibility. Int. J. Biol. Macromol. 2021, 169, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Dosmar, E.; Liu, W.; Patel, G.; Rogozinski, A.; Mieler, W.F.; Kang-Mieler, J.J. Controlled Release of Vancomycin From a Thermoresponsive Hydrogel System for the Prophylactic Treatment of Postoperative Acute Endophthalmitis. Transl. Vis. Sci. Technol. 2019, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Abrishami, M.; Motamed Shariati, M.; Malaekeh-Nikouei, B.; Tajani, A.S.; Mahmoudi, A.; Abrishami, M.; Khameneh, B. Preparation and in vivo evaluation of nanoliposomes containing vancomycin after intravitreal injection in albino rabbits. Iran. J. Basic Med. Sci. 2020, 23, 551–555. [Google Scholar] [CrossRef]
- Resende, A.F.C.; Pereira, A.F.; Moreira, T.P.; Patrício, P.S.O.; Fialho, S.L.; Cunha, G.M.F.; Silva-Cunha, A.; Magalhães, J.T.; Silva, G.R. PLGA Implants containing vancomycin and dexamethasone: Development, characterization and bactericidal effects. Pharm.-Int. J. Pharm. Sci. 2016, 71, 439–446. [Google Scholar] [CrossRef]
- Allam, A.; El-Mokhtar, M.A.; Elsabahy, M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J. Pharm. Pharmacol. 2019, 71, 1209–1221. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Jabbarvand, M.; Karimi, N. Therapeutic possibilities of ceftazidime nanoparticles in devastating pseudomonas ophthalmic infections; keratitis and endophthalmitis. Med. Hypothesis Discov. Innov. Ophthalmol. J. 2012, 1, 6–9. [Google Scholar]
- Bae, J.H.; Lee, S.C. Intravitreal liposomal amphotericin B for treatment of endogenous candida endophthalmitis. Jpn. J. Ophthalmol. 2015, 59, 346–352. [Google Scholar] [CrossRef]
- Üstündağ Okur, N.; Yozgatlı, V.; Okur, M.E.; Yoltaş, A.; Siafaka, P.I. Improving therapeutic efficacy of voriconazole against fungal keratitis: Thermo-sensitive in situ gels as ophthalmic drug carriers. J. Drug Deliv. Sci. Technol. 2019, 49, 323–333. [Google Scholar] [CrossRef]
- Kumar, R.; Sinha, V.R. Solid lipid nanoparticle: An efficient carrier for improved ocular permeation of voriconazole. Drug Dev. Ind. Pharm. 2016, 42, 1956–1967. [Google Scholar] [CrossRef]
- Bhosale, R.; Bhandwalkar, O.; Duduskar, A.; Jadhav, R.; Pawar, P. Water soluble chitosan mediated voriconazole microemulsion as sustained carrier for ophthalmic application: In vitro/ex vivo/in vivo evaluations. Open Pharm. Sci. J. 2016, 3, 215–234. [Google Scholar] [CrossRef] [Green Version]
- Fahmy, A.M.; Hassan, M.; El-Setouhy, D.A.; Tayel, S.A.; Al-mahallawi, A.M. Voriconazole Ternary Micellar Systems for the Treatment of Ocular Mycosis: Statistical Optimization and In Vivo Evaluation. J. Pharm. Sci. 2021, 110, 2130–2138. [Google Scholar] [CrossRef] [PubMed]
- de Sá, F.A.P.; Taveira, S.F.; Gelfuso, G.M.; Lima, E.M.; Gratieri, T. Liposomal voriconazole (VOR) formulation for improved ocular delivery. Colloids Surf. B Biointerfaces 2015, 133, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Nan, K.; Lee, S.; Beadle, J.R.; Hou, H.; Freeman, W.R.; Hostetler, K.Y.; Cheng, L. Micelle formulation of hexadecyloxypropyl-cidofovir (HDP-CDV) as an intravitreal long-lasting delivery system. Eur. J. Pharm. Biopharm. 2015, 89, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuppermann, B.D.; Assil, K.K.; Vuong, C.; Besen, G.; Wiley, C.A.; De Clercq, E.; Bergeron-Lynn, G.; Connor, J.D.; Pursley, M.; Munguia, D.; et al. Liposome-Encapsulated (S)-1-(3-Hydroxy-2-Phosphonylmethoxypropyl)cytosine for Long-Acting Therapy of Viral Retinitis. J. Infect. Dis. 1996, 173, 18–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claro, C.; Ruiz, R.; Cordero, E.; Pastor, M.T.; López-Cortés, L.F.; Jiménez-Castellanos, M.R.; Lucero, M.J. Determination and pharmacokinetic profile of liposomal foscarnet in rabbit ocular tissues after intravitreal administration. Exp. Eye Res. 2009, 88, 528–534. [Google Scholar] [CrossRef]
- Peter, S.; Mathews, M.M.; Saju, F.; Paul, S. Development, Optimization and In Vitro Characterization of Eudragit-Ganciclovir Nanosuspension or Treating Herpes Simplex Keratitis. J. Pharm. Innov. 2023, 1–10. [Google Scholar] [CrossRef]
- Naguib, M.J.; Hassan, Y.R.; Abd-Elsalam, W.H. 3D printed ocusert laden with ultra-fluidic glycerosomes of ganciclovir for the management of ocular cytomegalovirus retinitis. Int. J. Pharm. 2021, 607, 121010. [Google Scholar] [CrossRef]
- Choudhari, M.; Nayak, K.; Nagai, N.; Nakazawa, Y.; Khunt, D.; Misra, M. Role of mucoadhesive agent in ocular delivery of ganciclovir microemulsion: Cytotoxicity evaluation in vitro and ex vivo. Int. Ophthalmol. 2022, 43, 1153–1167. [Google Scholar] [CrossRef]
- Yasukawa, T.; Ogura, Y.; Kimura, H.; Sakurai, E.; Tabata, Y. Drug delivery from ocular implants. Expert Opin. Drug Deliv. 2006, 3, 261–273. [Google Scholar] [CrossRef]
- Choonara, Y.E.; Pillay, V.; Carmichael, T.; Danckwerts, M.P. An in vitro study of the design and development of a novel doughnut-shaped minitablet for intraocular implantation. Int. J. Pharm. 2006, 310, 15–24. [Google Scholar] [CrossRef]
- Yan, J.; Peng, X.; Cai, Y.; Cong, W. Development of facile drug delivery platform of ranibizumab fabricated PLGA-PEGylated magnetic nanoparticles for age-related macular degeneration therapy. J. Photochem. Photobiol. B Biol. 2018, 183, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, N.; Jackson, T.L.; Elsaid, Z.; Alqathama, A.; Somavarapu, S. PLGA Microparticles Entrapping Chitosan-Based Nanoparticles for the Ocular Delivery of Ranibizumab. Mol. Pharm. 2016, 13, 2923–2940. [Google Scholar] [CrossRef]
- Joseph, R.R.; Tan, D.W.N.; Ramon, M.R.M.; Natarajan, J.V.; Agrawal, R.; Wong, T.T.; Venkatraman, S.S. Characterization of liposomal carriers for the trans-scleral transport of Ranibizumab. Sci. Rep. 2017, 7, 16803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, C.; Yan, P.; Wan, G.; Liang, S.; Dong, Y.; Wang, J. Facile synthetic Photoluminescent Graphene Quantum dots encapsulated β-cyclodextrin drug carrier system for the management of macular degeneration: Detailed analytical and biological investigations. J. Photochem. Photobiol. B Biol. 2018, 189, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Gune, S.; Maia, M.; Ding, H.T.; Maass, K. Pharmacokinetic profile of the Port Delivery System with ranibizumab (PDS) in the phase 3 Archway trial. Investig. Ophthalmol. Vis. Sci. 2021, 62, 350. [Google Scholar]
- Yandrapu, S.K.; Upadhyay, A.K.; Petrash, J.M.; Kompella, U.B. Nanoparticles in Porous Microparticles Prepared by Supercritical Infusion and Pressure Quench Technology for Sustained Delivery of Bevacizumab. Mol. Pharm. 2013, 10, 4676–4686. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Chaparro, F.J.; Cuddington, C.T.; Palmer, A.F.; Ohr, M.P.; Lannutti, J.J.; Swindle-Reilly, K.E. Injectable biodegradable bi-layered capsule for sustained delivery of bevacizumab in treating wet age-related macular degeneration. J. Control. Release 2020, 320, 442–456. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Sun, J.-G.; Yao, J.; Shan, K.; Liu, B.-H.; Yao, M.-D.; Ge, H.-M.; Jiang, Q.; Zhao, C.; Yan, B. Effect of nanoencapsulation using poly (lactide-co-glycolide) (PLGA) on anti-angiogenic activity of bevacizumab for ocular angiogenesis therapy. Biomed. Pharmacother. 2018, 107, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Badiee, P.; Varshochian, R.; Rafiee-Tehrani, M.; Abedin Dorkoosh, F.; Khoshayand, M.R.; Dinarvand, R. Ocular implant containing bevacizumab-loaded chitosan nanoparticles intended for choroidal neovascularization treatment. J. Biomed. Mater. Res. 2018, 106, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Jacobs, K.M.; Ohr, M.P.; Swindle-Reilly, K.E. Chitosan–Polycaprolactone Core–Shell Microparticles for Sustained Delivery of Bevacizumab. Mol. Pharm. 2020, 17, 2570–2584. [Google Scholar] [CrossRef]
- Karumanchi, D.K.; Skrypai, Y.; Thomas, A.; Gaillard, E.R. Rational design of liposomes for sustained release drug delivery of bevacizumab to treat ocular angiogenesis. J. Drug Deliv. Sci. Technol. 2018, 47, 275–282. [Google Scholar] [CrossRef]
- Kelly, S.J.; Hirani, A.; Shahidadpury, V.; Solanki, A.; Halasz, K.; Varghese Gupta, S.; Madow, B.; Sutariya, V. Aflibercept Nanoformulation Inhibits VEGF Expression in Ocular In Vitro Model: A Preliminary Report. Biomedicines 2018, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Lee, B.-S.; Mieler, W.F.; Kang-Mieler, J.J. Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Bioactive Aflibercept In Vitro. Curr. Eye Res. 2019, 44, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Narvekar, P.; Lalani, R.; Chougule, M.B.; Pathak, Y.; Sutariya, V. An in vitro Assessment of Thermo-Reversible Gel Formulation Containing Sunitinib Nanoparticles for Neovascular Age-Related Macular Degeneration. AAPS PharmSciTech 2019, 20, 281. [Google Scholar] [CrossRef]
- Streets, J.; Bhatt, P.; Bhatia, D.; Sutariya, V. Sunitinib-Loaded MPEG-PCL Micelles for the Treatment of Age-Related Macular Degeneration. Sci. Pharm. 2020, 88, 30. [Google Scholar] [CrossRef]
- Narvekar, P.; Bhatt, P.; Fnu, G.; Sutariya, V.J.A.; technologies, d.d. Axitinib-loaded poly (lactic-co-glycolic acid) nanoparticles for age-related macular degeneration: Formulation development and in vitro characterization. ASSAY Drug Dev. Technol. 2019, 17, 167–177. [Google Scholar] [CrossRef]
- Chen, C.W.; Yeh, M.K.; Shiau, C.Y.; Chiang, C.H.; Lu, D.W. Efficient downregulation of VEGF in retinal pigment epithelial cells by integrin ligand-labeled liposome-mediated siRNA delivery. Int. J. Nanomed. 2013, 8, 2613–2627. [Google Scholar] [CrossRef] [Green Version]
- Ryoo, N.K.; Lee, J.; Lee, H.; Hong, H.K.; Kim, H.; Lee, J.B.; Woo, S.J.; Park, K.H.; Kim, H. Therapeutic effects of a novel siRNA-based anti-VEGF (siVEGF) nanoball for the treatment of choroidal neovascularization. Nanoscale 2017, 9, 15461–15469. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, A.; Zhang, H.; Wang, M.; Tang, Q.; Huang, Y.; Wang, L. Inhibition of retinal neovascularization by VEGF siRNA delivered via bioreducible lipid-like nanoparticles. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Csaky, K.G. Nanoparticle–integrin antagonist C16Y peptide treatment of choroidal neovascularization in rats. J. Control. Release 2010, 142, 286–293. [Google Scholar] [CrossRef]
- Jayaraman, M.S.; Bharali, D.J.; Sudha, T.; Mousa, S.A. Nano chitosan peptide as a potential therapeutic carrier for retinal delivery to treat age-related macular degeneration. Mol. Vis. 2012, 18, 2300–2308. [Google Scholar] [PubMed]
- Nguyen, D.D.; Luo, L.-J.; Yang, C.-J.; Lai, J.-Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183. [Google Scholar] [CrossRef]
- Ayyagari, S.; Dar, H.; Morton, V.; Moy, K.; Patel, C.; Ramasubramanian, L.; Ravi, N.; Wood, S.; Zhao, A.; Zheng, M. Evaluation of Curcumin-Loaded Nanoliposomes for the Treatment and Prevention of Age-Related Macular Degeneration. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2017. [Google Scholar]
- Sun, R.; Zhang, A.; Ge, Y.; Gou, J.; Yin, T.; He, H.; Wang, Y.; Zhang, G.; Kong, J.; Shang, L.; et al. Ultra-small-size Astragaloside-IV loaded lipid nanocapsules eye drops for the effective management of dry age-related macular degeneration. Expert Opin. Drug Deliv. 2020, 17, 1305–1320. [Google Scholar] [CrossRef]
- Oh, E.J.; Choi, J.-S.; Kim, H.; Joo, C.-K.; Hahn, S.K. Anti-Flt1 peptide–Hyaluronate conjugate for the treatment of retinal neovascularization and diabetic retinopathy. Biomaterials 2011, 32, 3115–3123. [Google Scholar] [CrossRef]
- Wang, C.; Seo, S.-J.; Kim, J.-S.; Lee, S.-H.; Jeon, J.-K.; Kim, J.-W.; Kim, K.-H.; Kim, J.-K.; Park, J. Intravitreal implantable magnetic micropump for on-demand VEGFR-targeted drug delivery. J. Control. Release 2018, 283, 105–112. [Google Scholar] [CrossRef]
- Qiu, F.; Meng, T.; Chen, Q.; Zhou, K.; Shao, Y.; Matlock, G.; Ma, X.; Wu, W.; Du, Y.; Wang, X.; et al. Fenofibrate-Loaded Biodegradable Nanoparticles for the Treatment of Experimental Diabetic Retinopathy and Neovascular Age-Related Macular Degeneration. Mol. Pharm. 2019, 16, 1958–1970. [Google Scholar] [CrossRef]
- Laddha, U.D.; Kshirsagar, S.J. Formulation of PPAR-gamma agonist as surface modified PLGA nanoparticles for non-invasive treatment of diabetic retinopathy: In vitro and in vivo evidences. Heliyon 2020, 6, e04589. [Google Scholar] [CrossRef]
- Radwan, S.E.; El-Kamel, A.; Zaki, E.I.; Burgalassi, S.; Zucchetti, E.; El-Moslemany, R.M. Hyaluronic-Coated Albumin Nanoparticles for the Non-Invasive Delivery of Apatinib in Diabetic Retinopathy. Int. J. Nanomed. 2021, 16, 4481–4494. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kim, J.H.; Yu, Y.S.; Lee, T.G.; Kim, J.H. Antiangiogenic effect of silicate nanoparticle on retinal neovascularization induced by vascular endothelial growth factor. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Paiva, M.R.B.; Andrade, G.F.; Dourado, L.F.N.; Castro, B.F.M.; Fialho, S.L.; Sousa, E.M.B.; Silva-Cunha, A. Surface functionalized mesoporous silica nanoparticles for intravitreal application of tacrolimus. J. Biomater. Appl. 2021, 35, 1019–1033. [Google Scholar] [CrossRef]
- Dave, V.; Sharma, R.; Gupta, C.; Sur, S. Folic acid modified gold nanoparticle for targeted delivery of Sorafenib tosylate towards the treatment of diabetic retinopathy. Colloids Surf. B Biointerfaces 2020, 194, 111151. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Giannaccini, M.; Dal Monte, M.; Cammalleri, M.; Pini, A.; Raffa, V.; Lulli, M.; Casini, G. Association of the Somatostatin Analog Octreotide with Magnetic Nanoparticles for Intraocular Delivery: A Possible Approach for the Treatment of Diabetic Retinopathy. Front. Bioeng. Biotechnol. 2020, 8, 144. [Google Scholar] [CrossRef]
- Venkatesan, A.; Roy, A.; Kulandaivel, S.; Natesan, V.; Kim, S.-J. p-Coumaric Acid Nanoparticles Ameliorate Diabetic Nephropathy via Regulating mRNA Expression of KIM-1 and GLUT-2 in Streptozotocin-Induced Diabetic Rats. Metabolites 2022, 12, 1166. [Google Scholar] [CrossRef]
- Huang, D.; Chen, Y.-S.; Thakur, S.S.; Rupenthal, I.D. Ultrasound-mediated nanoparticle delivery across ex vivo bovine retina after intravitreal injection. Eur. J. Pharm. Biopharm. 2017, 119, 125–136. [Google Scholar] [CrossRef]
- Rassu, G.; Pavan, B.; Mandracchia, D.; Tripodo, G.; Botti, G.; Dalpiaz, A.; Gavini, E.; Giunchedi, P. Polymeric nanomicelles based on inulin D α-tocopherol succinate for the treatment of diabetic retinopathy. J. Drug Deliv. Sci. Technol. 2021, 61, 102286. [Google Scholar] [CrossRef]
- Bogdanov, P.; Sampedro, J.; Solà-Adell, C.; Simó-Servat, O.; Russo, C.; Varela-Sende, L.; Simó, R.; Hernández, C. Effects of Liposomal Formulation of Citicoline in Experimental Diabetes-Induced Retinal Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 2458. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, M.; Alaaeldin, E.; Aly, U.F.; Sarhan, H.A. Optimization and Characterization of Thymoquinone-Loaded Liposomes with Enhanced Topical Anti-inflammatory Activity. AAPS PharmSciTech 2018, 19, 3490–3500. [Google Scholar] [CrossRef]
- Zheng, C.; Luo, W.; Liu, Y.; Chen, J.; Deng, H.; Zhou, Z.; Shen, J. Killing three birds with one stone: Multi-stage metabolic regulation mediated by clinically usable berberine liposome to overcome photodynamic immunotherapy resistance. Chem. Eng. J. 2023, 454, 140164. [Google Scholar] [CrossRef]
- Huang, X.; Li, M.; Bruni, R.; Messa, P.; Cellesi, F. The effect of thermosensitive liposomal formulations on loading and release of high molecular weight biomolecules. Int. J. Pharm. 2017, 524, 279–289. [Google Scholar] [CrossRef]
- López-Cano, J.J.; González-Cela-Casamayor, M.A.; Andrés-Guerrero, V.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Liposomes as vehicles for topical ophthalmic drug delivery and ocular surface protection. Expert Opin. Drug Deliv. 2021, 18, 819–847. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.; Barut, L.; Urimi, D.; Schipper, N.; Paquet-Durand, F. Investigating Ex Vivo Animal Models to Test the Performance of Intravitreal Liposomal Drug Delivery Systems. Pharmaceutics 2021, 13, 1013. [Google Scholar] [CrossRef]
- Salas-Ambrosio, P.J.; Bernad-Bernad, M.J.; Linares-Alba, M.A.; García-Santisteban, R.; Tonix-Aburto, L.A.; Ornelas-Lobato, G.J.; Gracia-Mora, I.; Rivera-Huerta, M.; Sánchez-Bartez, F.; Rico-Morales, H.; et al. Toxicity Evaluation of a Novel Rapamycin Liposomal Formulation After Subconjunctival and Intravitreal Injection. J. Ocul. Pharmacol. Ther. 2021, 37, 261–276. [Google Scholar] [CrossRef]
- Blazaki, S.; Pachis, K.; Tzatzarakis, M.; Tsilimbaris, M.; Antimisiaris, S.G. Novel Liposome Aggregate Platform (LAP) system for sustained retention of drugs in the posterior ocular segment following intravitreal injection. Int. J. Pharm. 2020, 576, 118987. [Google Scholar] [CrossRef] [PubMed]
- Terreni, E.; Chetoni, P.; Tampucci, S.; Burgalassi, S.; Al-kinani, A.A.; Alany, R.G.; Monti, D. Assembling Surfactants-Mucoadhesive Polymer Nanomicelles (ASMP-Nano) for Ocular Delivery of Cyclosporine-A. Pharmaceutics 2020, 12, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binkhathlan, Z.; Alomrani, A.H.; Hoxha, O.; Ali, R.; Kalam, M.A.; Alshamsan, A. Development and Characterization of PEGylated Fatty Acid-Block-Poly(ε-caprolactone) Novel Block Copolymers and Their Self-Assembled Nanostructures for Ocular Delivery of Cyclosporine A. Polymers 2022, 14, 1635. [Google Scholar]
- Lynch, C.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Advances in Biodegradable Nano-Sized Polymer-Based Ocular Drug Delivery. Polymers 2019, 11, 1371. [Google Scholar] [CrossRef] [Green Version]
- Suri, R.; Neupane, Y.R.; Mehra, N.; Nematullah, M.; Khan, F.; Alam, O.; Iqubal, A.; Jain, G.K.; Kohli, K. Sirolimus loaded chitosan functionalized poly (lactic-co-glycolic acid) (PLGA) nanoparticles for potential treatment of age-related macular degeneration. Int. J. Biol. Macromol. 2021, 191, 548–559. [Google Scholar] [CrossRef]
- Romeo, A.; Musumeci, T.; Carbone, C.; Bonaccorso, A.; Corvo, S.; Lupo, G.; Anfuso, C.D.; Puglisi, G.; Pignatello, R. Ferulic Acid-Loaded Polymeric Nanoparticles for Potential Ocular Delivery. Pharmaceutics 2021, 13, 687. [Google Scholar] [CrossRef]
- Bhatt, P.; Fnu, G.; Bhatia, D.; Shahid, A.; Sutariya, V. Nanodelivery of Resveratrol-Loaded PLGA Nanoparticles for Age-Related Macular Degeneration. AAPS PharmSciTech 2020, 21, 291. [Google Scholar] [CrossRef]
- Mahaling, B.; Baruah, N.; Ahamad, N.; Maisha, N.; Lavik, E.; Katti, D.S. A non-invasive nanoparticle-based sustained dual-drug delivery system as an eyedrop for endophthalmitis. Int. J. Pharm. 2021, 606, 120900. [Google Scholar] [CrossRef]
- Bu, H.-Z.; Gukasyan, H.J.; Goulet, L.; Lou, X.-J.; Xiang, C.; Koudriakova, T. Ocular Disposition, Pharmacokinetics, Efficacy and Safety of Nanoparticle-Formulated Ophthalmic Drugs. Curr. Drug Metab. 2007, 8, 91–107. [Google Scholar] [CrossRef]
- Yan, R.; Xu, L.; Wang, Q.; Wu, Z.; Zhang, H.; Gan, L. Cyclosporine A Nanosuspensions for Ophthalmic Delivery: A Comparative Study between Cationic Nanoparticles and Drug-Core Mucus Penetrating Nanoparticles. Mol. Pharm. 2021, 18, 4290–4298. [Google Scholar] [CrossRef]
- Shah, S.; Bhanderi, B.; Soniwala, M.; Chavda, J. Lutein-Loaded Solid Lipid Nanoparticles for Ocular Delivery: Statistical Optimization and Ex Vivo Evaluation. J. Pharm. Innov. 2022, 17, 584–598. [Google Scholar] [CrossRef]
- Onugwu, A.L.; Attama, A.A.; Nnamani, P.O.; Onugwu, S.O.; Onuigbo, E.B.; Khutoryanskiy, V.V. Development and optimization of solid lipid nanoparticles coated with chitosan and poly(2-ethyl-2-oxazoline) for ocular drug delivery of ciprofloxacin. J. Drug Deliv. Sci. Technol. 2022, 74, 103527. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Hosny, K.M.; Aldryhim, A.Y.; Rizg, W.Y.; Eshmawi, B.A.; Bukhary, H.A.; Murshid, S.S.A.; Khallaf, R.A. Development and optimization of ofloxacin as solid lipid nanoparticles for enhancement of its ocular activity. J. Drug Deliv. Sci. Technol. 2022, 72, 103373. [Google Scholar] [CrossRef]
- Eid, H.M.; Elkomy, M.H.; El Menshawe, S.F.; Salem, H.F. Development, Optimization, and In Vitro/In Vivo Characterization of Enhanced Lipid Nanoparticles for Ocular Delivery of Ofloxacin: The Influence of Pegylation and Chitosan Coating. AAPS PharmSciTech 2019, 20, 183. [Google Scholar] [CrossRef] [PubMed]
- Shahab, M.S.; Rizwanullah, M.; Sarim Imam, S. Formulation, optimization and evaluation of vitamin E TPGS emulsified dorzolamide solid lipid nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 68, 103062. [Google Scholar] [CrossRef]
- Fathi, M.; Barar, J.; Aghanejad, A.; Omidi, Y. Hydrogels for ocular drug delivery and tissue engineering. BioImpacts BI 2015, 5, 159–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annala, A.; Ilochonwu, B.C.; Wilbie, D.; Sadeghi, A.; Hennink, W.E.; Vermonden, T. Self-Healing Thermosensitive Hydrogel for Sustained Release of Dexamethasone for Ocular Therapy. ACS Polym. Au 2023, 3, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Ilochonwu, B.C.; Mihajlovic, M.; Maas-Bakker, R.F.; Rousou, C.; Tang, M.; Chen, M.; Hennink, W.E.; Vermonden, T. Hyaluronic Acid-PEG-Based Diels–Alder In Situ Forming Hydrogels for Sustained Intraocular Delivery of Bevacizumab. Biomacromolecules 2022, 23, 2914–2929. [Google Scholar] [CrossRef]
- Pakzad, Y.; Fathi, M.; Omidi, Y.; Mozafari, M.; Zamanian, A. Synthesis and characterization of timolol maleate-loaded quaternized chitosan-based thermosensitive hydrogel: A transparent topical ocular delivery system for the treatment of glaucoma. Int. J. Biol. Macromol. 2020, 159, 117–128. [Google Scholar] [CrossRef]
- Xu, Q.; Kambhampati, S.P.; Kannan, R.M. Nanotechnology approaches for ocular drug delivery. Middle East Afr. J. Ophthalmol. 2013, 20, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Honda, M.; Asai, T.; Oku, N.; Araki, Y.; Tanaka, M.; Ebihara, N. Liposomes and nanotechnology in drug development: Focus on ocular targets. Int. J. Nanomed. 2013, 8, 495–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez Villanueva, J.; Navarro, M.G.; Rodríguez Villanueva, L. Dendrimers as a promising tool in ocular therapeutics: Latest advances and perspectives. Int. J. Pharm. 2016, 511, 359–366. [Google Scholar] [CrossRef]
- Yavuz, B.; Pehlivan, S.B.; Vural, İ.; Ünlü, N. In Vitro/In Vivo Evaluation of Dexamethasone—PAMAM Dendrimer Complexes for Retinal Drug Delivery. J. Pharm. Sci. 2015, 104, 3814–3823. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Huang, D.; Norat, P.; Grannonico, M.; Cooper, R.C.; Gui, Q.; Nam Chow, W.; Liu, X.; Yang, H. Nano-in-Nano dendrimer gel particles for efficient topical delivery of antiglaucoma drugs into the eye. Chem. Eng. J. 2021, 425, 130498. [Google Scholar] [CrossRef]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X.; Wang, F. Application of Drug Nanocrystal Technologies on Oral Drug Delivery of Poorly Soluble Drugs. Pharm. Res. 2013, 30, 307–324. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.B.; Wang, J.; Ghanma, R.; Qin, N.; Palma, S.D.; Donnelly, R.F.; Paredes, A.J. Nanocrystals as a master key to deliver hydrophobic drugs via multiple administration routes. J. Control. Release 2022, 345, 334–353. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Patel, V.; Mehta, T. Nanocrystal for ocular drug delivery: Hope or hype. Drug Deliv. Transl. Res. 2016, 6, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Romero, G.B.; Keck, C.M.; Müller, R.H.; Bou-Chacra, N.A. Development of cationic nanocrystals for ocular delivery. Eur. J. Pharm. Biopharm. 2016, 107, 215–222. [Google Scholar] [CrossRef]
- Tetyczka, C.; Brisberger, K.; Reiser, M.; Zettl, M.; Jeitler, R.; Winter, C.; Kolb, D.; Leitinger, G.; Spoerk, M.; Roblegg, E. Itraconazole Nanocrystals on Hydrogel Contact Lenses via Inkjet Printing: Implications for Ophthalmic Drug Delivery. ACS Appl. Nano Mater. 2022, 5, 9435–9446. [Google Scholar] [CrossRef]
- Kalam, M.A.; Iqbal, M.; Alshememry, A.; Alkholief, M.; Alshamsan, A. Fabrication and Characterization of Tedizolid Phosphate Nanocrystals for Topical Ocular Application: Improved Solubilization and In Vitro Drug Release. Pharmaceutics 2022, 14, 1328. [Google Scholar] [CrossRef]
- Tan, C.; Hosseini, S.F.; Jafari, S.M. Cubosomes and Hexosomes as Novel Nanocarriers for Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 1423–1437. [Google Scholar] [CrossRef]
- Gaballa, S.A.; El Garhy, O.H.; Moharram, H.; Abdelkader, H. Preparation and Evaluation of Cubosomes/Cubosomal Gels for Ocular Delivery of Beclomethasone Dipropionate for Management of Uveitis. Pharm. Res. 2020, 37, 198. [Google Scholar] [CrossRef]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef]
- Han, S.; Shen, J.-Q.; Gan, Y.; Geng, H.-M.; Zhang, X.-X.; Zhu, C.-L.; Gan, L. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol. Sin. 2010, 31, 990–998. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Ghorab, M. Formulation and evaluation of cubosomes drug delivery system for treatment of glaucoma: Ex-vivo permeation and in-vivo pharmacodynamic study. J. Drug Deliv. Sci. Technol. 2019, 52, 236–247. [Google Scholar] [CrossRef]
- Yasamineh, S.; Yasamineh, P.; Ghafouri Kalajahi, H.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Hossein Kheirkhah, A.; Taghizadeh, M.; Yazdani, Y.; et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef]
- Saettone, M.F.; Perini, G.; Carafa, M.; Santucci, E.; Alhaique, F. Nonionic surfactant vesicles as ophthalmic carriers for cyclopentolate: Preliminary evaluation. STP Pharma. Sci. 1996, 6, 94–98. [Google Scholar]
- Shukr, M.H. Novel in situ gelling ocular inserts for voriconazole-loaded niosomes: Design, in vitro characterisation and in vivo evaluation of the ocular irritation and drug pharmacokinetics. J. Microencapsul. 2016, 33, 71–79. [Google Scholar] [CrossRef]
- Aggarwal, D.; Pal, D.; Mitra, A.K.; Kaur, I.P. Study of the extent of ocular absorption of acetazolamide from a developed niosomal formulation, by microdialysis sampling of aqueous humor. Int. J. Pharm. 2007, 338, 21–26. [Google Scholar] [CrossRef]
- Abdelbary, G.; El-gendy, N. Niosome-Encapsulated Gentamicin for Ophthalmic Controlled Delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [CrossRef]
- Gupta, P.; Yadav, K.S. Formulation and evaluation of brinzolamide encapsulated niosomal in-situ gel for sustained reduction of IOP in rabbits. J. Drug Deliv. Sci. Technol. 2022, 67, 103004. [Google Scholar] [CrossRef]
- Owodeha-Ashaka, K.; Ilomuanya, M.O.; Iyire, A. Evaluation of sonication on stability-indicating properties of optimized pilocarpine hydrochloride-loaded niosomes in ocular drug delivery. Prog. Biomater. 2021, 10, 207–220. [Google Scholar] [CrossRef]
- Zeng, W.; Li, Q.; Wan, T.; Liu, C.; Pan, W.; Wu, Z.; Zhang, G.; Pan, J.; Qin, M.; Lin, Y.; et al. Hyaluronic acid-coated niosomes facilitate tacrolimus ocular delivery: Mucoadhesion, precorneal retention, aqueous humor pharmacokinetics, and transcorneal permeability. Colloids Surf. B Biointerfaces 2016, 141, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Puras, G.; Mashal, M.; Zárate, J.; Agirre, M.; Ojeda, E.; Grijalvo, S.; Eritja, R.; Diaz-Tahoces, A.; Martínez Navarrete, G.; Avilés-Trigueros, M.; et al. A novel cationic niosome formulation for gene delivery to the retina. J. Control. Release 2014, 174, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Bengani, L.C.; Jung, H.J.; Leclerc, J.; Gupta, C.; Chauhan, A. Emulsions and microemulsions for ocular drug delivery. J. Drug Deliv. Sci. Technol. 2011, 21, 111–121. [Google Scholar] [CrossRef]
- Tiwari, R.; Pandey, V.; Asati, S.; Soni, V.; Jain, D. Therapeutic challenges in ocular delivery of lipid based emulsion. Egypt. J. Basic Appl. Sci. 2018, 5, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.; Chakrapani, M.; Kaza, R. Preparation and Evaluation of Vancomycin Microemulsion for Ocular Drug Delivery. Drug Deliv. Lett. 2012, 2, 26–34. [Google Scholar]
- Ameta, R.K.; Soni, K.; Bhattarai, A. Recent Advances in Improving the Bioavailability of Hydrophobic/Lipophilic Drugs and Their Delivery via Self-Emulsifying Formulations. Colloids Interfaces 2023, 7, 16. [Google Scholar] [CrossRef]
- Li, Y.; Guan, Q.; Xu, J.; Zhang, H.; Liu, S.; Ding, Z.; Wang, Q.; Wang, Z.; Liu, M.; Zhao, Y. Comparative study of cyclosporine A liposomes and emulsions for ophthalmic drug delivery: Process optimization through response surface methodology (RSM) and biocompatibility evaluation. Colloids Surf. B Biointerfaces 2023, 225, 113267. [Google Scholar] [CrossRef]
- Ying, L.; Tahara, K.; Takeuchi, H. Drug delivery to the ocular posterior segment using lipid emulsion via eye drop administration: Effect of emulsion formulations and surface modification. Int. J. Pharm. 2013, 453, 329–335. [Google Scholar] [CrossRef]
- Liu, C.; Lan, Q.; He, W.; Nie, C.; Zhang, C.; Xu, T.; Jiang, T.; Wang, S. Octa-arginine modified lipid emulsions as a potential ocular delivery system for disulfiram: A study of the corneal permeation, transcorneal mechanism and anti-cataract effect. Colloids Surf. B Biointerfaces 2017, 160, 305–314. [Google Scholar] [CrossRef]
- Ahad, A.; Raish, M.; Ahmad, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Eprosartan mesylate loaded bilosomes as potential nano-carriers against diabetic nephropathy in streptozotocin-induced diabetic rats. Eur. J. Pharm. Sci. 2018, 111, 409–417. [Google Scholar] [CrossRef]
- Mondal, D.; Mandal, R.P.; De, S. Addressing the Superior Drug Delivery Performance of Bilosomes─A Microscopy and Fluorescence Study. ACS Appl. Bio. Mater. 2022, 5, 3896–3911. [Google Scholar] [CrossRef]
- Csaba, N.; Garcia-Fuentes, M.; Alonso, M.J. The performance of nanocarriers for transmucosal drug delivery. Expert Opin. Drug Deliv. 2006, 3, 463–478. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-mahallawi, A.M. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: In vitro characterization, ex vivo permeation and in vivo safety assessment. Int. J. Pharm. 2016, 513, 688–696. [Google Scholar] [CrossRef]
- Alsaidan, O.A.; Zafar, A.; Yasir, M.; Alzarea, S.I.; Alqinyah, M.; Khalid, M. Development of Ciprofloxacin-Loaded Bilosomes In-Situ Gel for Ocular Delivery: Optimization, In-Vitro Characterization, Ex-Vivo Permeation, and Antimicrobial Study. Gels 2022, 8, 687. [Google Scholar] [CrossRef] [PubMed]
- Nemr, A.A.; El-Mahrouk, G.M.; Badie, H.A. Hyaluronic acid-enriched bilosomes: An approach to enhance ocular delivery of agomelatine via D-optimal design: Formulation, in vitro characterization, and in vivo pharmacodynamic evaluation in rabbits. Drug Deliv. 2022, 29, 2343–2356. [Google Scholar] [CrossRef] [PubMed]
- Quintanar-Guerrero, D.; Allémann, E.; Fessi, H.; Doelker, E. Preparation Techniques and Mechanisms of Formation of Biodegradable Nanoparticles from Preformed Polymers. Drug Dev. Ind. Pharm. 1998, 24, 1113–1128. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.L.; Legeay, S.; Bejaud, J.; Montich, G.G.; Ullio Gamboa, G.V.; Benoit, J.-P.; Palma, S.D. Novel hybrid lipid nanocapsules loaded with a therapeutic monoclonal antibody–Bevacizumab–and Triamcinolone acetonide for combined therapy in neovascular ocular pathologies. Mater. Sci. Eng. C 2021, 119, 111398. [Google Scholar] [CrossRef]
- Eldesouky, L.M.; El-Moslemany, R.M.; Ramadan, A.A.; Morsi, M.H.; Khalafallah, N.M. Cyclosporine Lipid Nanocapsules as Thermoresponsive Gel for Dry Eye Management: Promising Corneal Mucoadhesion, Biodistribution and Preclinical Efficacy in Rabbits. Pharmaceutics 2021, 13, 360. [Google Scholar] [CrossRef]
- Kakkar, S.; Kaur, I.P. Spanlastics—A novel nanovesicular carrier system for ocular delivery. Int. J. Pharm. 2011, 413, 202–210. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Yang, J.; Zhang, H.; Gan, L. Cationized hyaluronic acid coated spanlastics for cyclosporine A ocular delivery: Prolonged ocular retention, enhanced corneal permeation and improved tear production. Int. J. Pharm. 2019, 565, 133–142. [Google Scholar] [CrossRef]
- Abdelbari, M.A.; El-Mancy, S.S.; Elshafeey, A.H.; Abdelbary, A.A. Implementing Spanlastics for Improving the Ocular Delivery of Clotrimazole: In vitro Characterization, Ex vivo Permeability, Microbiological Assessment and In vivo Safety Study. Int. J. Nanomed. 2021, 16, 6249–6261. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Abd-allah, H. Spanlastic nanovesicles for enhanced ocular delivery of vanillic acid: Design, in vitro characterization, and in vivo anti-inflammatory evaluation. Int. J. Pharm. 2022, 625, 122068. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Sharma, P.; Garg, V.; Garg, G. Ocular inserts-Advancement in therapy of eye diseases. J. Adv. Pharm. Technol. Res. 2010, 1, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelkader, H.; Fathalla, Z.; Seyfoddin, A.; Farahani, M.; Thrimawithana, T.; Allahham, A.; Alani, A.W.G.; Al-Kinani, A.A.; Alany, R.G. Polymeric long-acting drug delivery systems (LADDS) for treatment of chronic diseases: Inserts, patches, wafers, and implants. Adv. Drug Deliv. Rev. 2021, 177, 113957. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, C.; Yadav, K.S. Formulation and evaluation of polymer-coated bimatoprost-chitosan matrix ocular inserts for sustained lowering of IOP in rabbits. J. Drug Deliv. Sci. Technol. 2022, 77, 103885. [Google Scholar] [CrossRef]
- Shanmugam, S.; Valarmathi, S.; Kumar, S.S.; Shanmugasundaram, P.; Senthilkumar, M. Formulation development and evaluation of opthalmic ocusert containing aciclovir. Res. J. Pharm. Technol. 2016, 9, 1858–1862. [Google Scholar] [CrossRef]
- Tatke, A.; Dudhipala, N.; Janga, K.Y.; Soneta, B.; Avula, B.; Majumdar, S. Melt-Cast Films Significantly Enhance Triamcinolone Acetonide Delivery to the Deeper Ocular Tissues. Pharmaceutics 2019, 11, 158. [Google Scholar] [CrossRef] [Green Version]
- Rakhmetova, A. Development of a New Polymer Ocular Insert to Treat Fungal Infections Threatening the Cornea; Nazarbayev University: Astana, Kazakhstan, 2020. [Google Scholar]
- Bertens, C.J.F.; Martino, C.; van Osch, M.C.; Lataster, A.; Dias, A.J.A.A.; van den Biggelaar, F.J.H.M.; Tuinier, R.; Nuijts, R.M.M.A.; Gijs, M. Design of the ocular coil, a new device for non-invasive drug delivery. Eur. J. Pharm. Biopharm. 2020, 150, 120–130. [Google Scholar] [CrossRef]
- Taghe, S.; Mirzaeei, S.; Alany, R.G.; Nokhodchi, A. Polymeric Inserts Containing Eudragit® L100 Nanoparticle for Improved Ocular Delivery of Azithromycin. Biomedicines 2020, 8, 466. [Google Scholar] [CrossRef]
- Hennig, R.; Goepferich, A. Nanoparticles for the treatment of ocular neovascularizations. Eur. J. Pharm. Biopharm. 2015, 95, 294–306. [Google Scholar] [CrossRef]
- Lim, C.-B.; Abuzar, S.M.; Karn, P.R.; Cho, W.; Park, H.J.; Cho, C.-W.; Hwang, S.-J. Preparation, Characterization, and In Vivo Pharmacokinetic Study of the Supercritical Fluid-Processed Liposomal Amphotericin B. Pharmaceutics 2019, 11, 589. [Google Scholar] [CrossRef] [Green Version]
- Robinson, R.F.; Nahata, M.C. A comparative review of conventional and lipid formulations of amphotericin B. J. Clin. Pharm. Ther. 1999, 24, 249–257. [Google Scholar] [CrossRef]
- Barratt, G.; Bretagne, S. Optimizing efficacy of Amphotericin B through nanomodification. Int. J. Nanomed. 2007, 2, 301–313. [Google Scholar]
- Grandi, G.; Cavallo, R.; Zanotto, E.; Cipriani, R.; Panico, C.; Protti, R.; Scapagnini, G.; Davinelli, S.; Costagliola, C. In vitro antimicrobial activity of ozonated oil in liposome eyedrop against multidrug-resistant bacteria. Open Med. 2022, 17, 1057–1063. [Google Scholar] [CrossRef]
- Garrigue, J.S.; Amrane, M.; Faure, M.O.; Holopainen, J.M.; Tong, L. Relevance of Lipid-Based Products in the Management of Dry Eye Disease. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2017, 33, 647–661. [Google Scholar] [CrossRef]
- Nagai, N.; Otake, H. Novel drug delivery systems for the management of dry eye. Adv. Drug Deliv. Rev. 2022, 191, 114582. [Google Scholar] [CrossRef] [PubMed]
- Aragona, P.; Spinella, R.; Rania, L.; Postorino, E.; Roszkowska, A.; Versura, P.; Profazio, V.; Rolando, M. Assessment of the efficacy of Cationorm® in patients with moderate dry eye compared with Optive® and Emustil® eye drops. Acta Ophthalmol. 2011, 89. [Google Scholar] [CrossRef]
- Di Pascuale, M.A.; Goto, E.; Tseng, S.C.G. Sequential changes of lipid tear film after the instillation of a single drop of a new emulsion eye drop in dry eye patients. Ophthalmology 2004, 111, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Khopade, A.J. Pharmacokinetics and Pharmacology of Latanoprost 0.005% without Benzalkonium Chloride Vs Latanoprost 0.005% with Benzalkonium Chloride in New Zealand White Rabbits and Beagles. Curr. Eye Res. 2021, 46, 1031–1037. [Google Scholar] [CrossRef]
- Mazet, R.; Yaméogo, J.B.G.; Wouessidjewe, D.; Choisnard, L.; Gèze, A. Recent Advances in the Design of Topical Ophthalmic Delivery Systems in the Treatment of Ocular Surface Inflammation and Their Biopharmaceutical Evaluation. Pharmaceutics 2020, 12, 570. [Google Scholar] [CrossRef]
- Luchs, J. Azithromycin in DuraSite® for the treatment of blepharitis. Clin. Ophthalmol. 2010, 4, 681–688. [Google Scholar] [CrossRef] [Green Version]
- Tobin, K.A. Macugen treatment for wet age-related macular degeneration. Insight 2006, 31, 11–14. [Google Scholar]
- Couch, S.M.; Bakri, S.J. Intravitreal triamcinolone for intraocular inflammation and associated macular edema. Clin. Ophthalmol. 2009, 3, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Li, S.; Xu, M.; Zhong, Y.; Fan, W.; Xu, J.; Zhou, T.; Ji, J.; Ye, J.; Yao, K. Polymer- and lipid-based nanocarriers for ocular drug delivery: Current status and future perspectives. Adv. Drug Deliv. Rev. 2023, 196, 114770. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.; Liu, C.K.-R.; Wakabayashi, T.; Mahmoudzadeh, R.; Salabati, M.; Spirn, M. Ocular Complications After Dexamethasone Implant Versus Intravitreal Triamcinolone in Patients with Post Vitrectomy Macular Edema. Investig. Ophthalmol. Vis. Sci. 2022, 63, 700-F0225. [Google Scholar]
- Hosseini, K.; Hutcheson, J.; Bowman, L. Aqueous humor concentration of Bromfenac 0.09% (Bromday) compared with Bromfenac in DuraSite 0.075% (Bromsite) in cataract patients undergoing phacoemulsification after 3 days dosing. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5061. [Google Scholar]
- McCarey, B.E.; Cockrum, P.C. Topical Besivance, Vigamox and BSS Affects on Corneal Epithelial Wound Closure. Investig. Ophthalmol. Vis. Sci. 2010, 51, 397. [Google Scholar]
- Costello, M.A.; Liu, J.; Wang, Y.; Qin, B.; Xu, X.; Li, Q.; Lynd, N.A.; Zhang, F. Reverse engineering the Ozurdex dexamethasone intravitreal implant. Int. J. Pharm. 2023, 634, 122625. [Google Scholar] [CrossRef]
- García-Estrada, P.; García-Bon, M.A.; López-Naranjo, E.J.; Basaldúa-Pérez, D.N.; Santos, A.; Navarro-Partida, J. Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials. Pharmaceutics 2021, 13, 701. [Google Scholar] [CrossRef]
- Zeroni, J.; Slager, J.; Hegenrother, R.; Kloke, T.; Varner, S. Sustained Delivery of Bioactive Protein with the I-Vation Intravitreal Implant. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5805. [Google Scholar]
- Gaballa, S.A.; Kompella, U.B.; Elgarhy, O.; Alqahtani, A.M.; Pierscionek, B.; Alany, R.G.; Abdelkader, H. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv. Transl. Res. 2021, 11, 866–893. [Google Scholar] [CrossRef]
- Yasukawa, T.; Ogura, Y. Medical Devices for the Treatment of Eye Diseases. In Drug Delivery; Schäfer-Korting, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 469–489. [Google Scholar] [CrossRef]
- Kuppermann, B.D.; Blumenkranz, M.S.; Haller, J.A.; Williams, G.A.; Weinberg, D.V.; Chou, C.; Whitcup, S.M.; Group, D.D.P.I.S. Randomized Controlled Study of an Intravitreous Dexamethasone Drug Delivery System in Patients with Persistent Macular Edema. Arch. Ophthalmol. 2007, 125, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Seah, S.K.L.; Husain, R.; Gazzard, G.; Lim, M.C.C.; Hoh, S.-T.; Oen, F.T.S.; Aung, T. Use of Surodex in Phacotrabeculectomy Surgery. Am. J. Ophthalmol. 2005, 139, 927–928. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, K.B.; Jadach, B.; Muszalska-Kolos, I. Progress in drug formulation design and delivery of medicinal substances used in ophthalmology. Int. J. Pharm. 2021, 607, 121012. [Google Scholar] [CrossRef]
- Allyn, M.M.; Luo, R.H.; Hellwarth, E.B.; Swindle-Reilly, K.E. Considerations for Polymers Used in Ocular Drug Delivery. Front. Med. 2022, 8, 2963. [Google Scholar] [CrossRef]
- Nyamweya, N.N. Applications of polymer blends in drug delivery. Future J. Pharm. Sci. 2021, 7, 18. [Google Scholar] [CrossRef]
- Cao, Y.; Samy, K.E.; Bernards, D.A.; Desai, T.A. Recent advances in intraocular sustained-release drug delivery devices. Drug Discov. Today 2019, 24, 1694–1700. [Google Scholar] [CrossRef]
- Fayzullin, A.; Bakulina, A.; Mikaelyan, K.; Shekhter, A.; Guller, A. Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape. Bioengineering 2021, 8, 205. [Google Scholar] [CrossRef]
- Saettone, M.F.; Salminen, L. Ocular inserts for topical delivery. Adv. Drug Deliv. Rev. 1995, 16, 95–106. [Google Scholar] [CrossRef]
- Banker, G.S.; Siepmann, J.; Rhodes, C. Design and evaluation of ophthalmic pharmaceutical products. In Modern pharmaceutics; CRC Press: Boca Raton, FL, USA, 2002; pp. 647–738. [Google Scholar]
- Brandt, J.D.; DuBiner, H.B.; Benza, R.; Sall, K.N.; Walker, G.A.; Semba, C.P.; Budenz, D.; Day, D.; Flowers, B.; Lee, S.; et al. Long-term Safety and Efficacy of a Sustained-Release Bimatoprost Ocular Ring. Ophthalmology 2017, 124, 1565–1566. [Google Scholar] [CrossRef] [Green Version]
- Belamkar, A.; Harris, A.; Zukerman, R.; Siesky, B.; Oddone, F.; Verticchio Vercellin, A.; Ciulla, T.A. Sustained release glaucoma therapies: Novel modalities for overcoming key treatment barriers associated with topical medications. Ann. Med. 2022, 54, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Cagini, C.; Caricato, A.; Tosi, G.; Pascale, A.; Cesari, C.; Fiore, T. Evaluation of the efficacy and safety of the ophthalmic insert Mydriasert in patients undergoing retinal angiography. Eur. J. Ophthalmol. 2014, 24, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Kansara, V.S.; Hancock, S.E.; Muya, L.W.; Ciulla, T.A. Suprachoroidal delivery enables targeting, localization and durability of small molecule suspensions. J. Control. Release 2022, 349, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, S.H.; Lee, J.Y.; Ryu, W.H. Commercialized Microneedles. In Microneedling in Clinical Practice; CRC Press: Boca Raton, FL, USA, 2020; pp. 91–108. [Google Scholar] [CrossRef]
- Novack, G.D. US Regulatory Approval of a Drug-Eluting Contact Lens. Eye Contact Lens Sci. Clin. Pract. 2023, 49, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Brady, J.M.; Cutright, D.E. Degradation rates of oral resorbable implants (polylactates and polyglycolates): Rate modification with changes in PLA/PGA copolymer ratios. J. Biomed. Mater. Res. 1977, 11, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Haghjou, N.; Soheilian, M.; Abdekhodaie, M.J. Sustained release intraocular drug delivery devices for treatment of uveitis. J. Ophthalmic Vis. Res. 2011, 6, 317–329. [Google Scholar] [PubMed]
- Desai, A.R.; Maulvi, F.A.; Desai, D.M.; Shukla, M.R.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Sandeman, S.; Shah, D.O. Multiple drug delivery from the drug-implants-laden silicone contact lens: Addressing the issue of burst drug release. Mater. Sci. Eng. C 2020, 112, 110885. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Desai, D.T.; Shetty, K.H.; Shah, D.O.; Willcox, M.D.P. Advances and challenges in the nanoparticles-laden contact lenses for ocular drug delivery. Int. J. Pharm. 2021, 608, 121090. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef]
- Kudryavtseva, V.; Otero, M.; Zhang, J.; Bukatin, A.; Gould, D.; Sukhorukov, G.B. Drug-Eluting Sandwich Hydrogel Lenses Based on Microchamber Film Drug Encapsulation. ACS Nanosci. Au 2023. [Google Scholar] [CrossRef]
- Bin Sahadan, M.Y.; Tong, W.Y.; Tan, W.N.; Leong, C.R.; Bin Misri, M.N.; Chan, M.; Cheng, S.Y.; Shaharuddin, S. Phomopsidione nanoparticles coated contact lenses reduce microbial keratitis causing pathogens. Exp. Eye Res. 2019, 178, 10–14. [Google Scholar] [CrossRef]
- Horne, R.R.; Rich, J.T.; Bradley, M.W.; Pitt, W.G. Latanoprost uptake and release from commercial contact lenses. J. Biomater. Sci. Polym. Ed. 2020, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Torres-Luna, C.; Hu, N.; Tammareddy, T.; Domszy, R.; Yang, J.; Wang, N.S.; Yang, A. Extended delivery of non-steroidal anti-inflammatory drugs through contact lenses loaded with Vitamin E and cationic surfactants. Contact Lens Anterior Eye 2019, 42, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, I.I.; Karavasili, C.; Thomas, A.; Komnenou, A.; Tzimtzimis, M.; Tzetzis, D.; Andreadis, D.; Bouropoulos, N.; Fatouros, D.G. In Situ Gelling Electrospun Ocular Films Sustain the Intraocular Pressure-Lowering Effect of Timolol Maleate: In Vitro, Ex Vivo, and Pharmacodynamic Assessment. Mol. Pharm. 2022, 19, 274–286. [Google Scholar] [CrossRef] [PubMed]
- I Tartara, L.; D Palma, S.; Allemandi, D.; I Ahumada, M.; M Llabot, J. New Mucoadhesive Polymeric Film for Ophthalmic Administration of Acetazolamide. Recent Pat. Drug Deliv. Formul. 2014, 8, 224–232. [Google Scholar] [CrossRef]
- Sanap, S.N.; Bisen, A.C.; Kedar, A.; Yadav, K.S.; Krishna, A.; Akhir, A.; Chopra, S.; Mugale, M.N.; Bhatta, R.S. Chitosan/HPMC-based mucoadhesive film co-loaded with fluconazole and ofloxacin for management of polymicrobial keratitis. Int. J. Biol. Macromol. 2022, 222, 2785–2795. [Google Scholar] [CrossRef]
- Tandale, Y.N.; Wagh, V.D.J.I.J.P.R. Formulation and evaluation of dorzolamide hydrochloride polymeric film. Int. J. PharmTech Res. 2011, 3, 1817–1824. [Google Scholar]
- Gupta, P.; Yadav, K.S. Applications of microneedles in delivering drugs for various ocular diseases. Life Sci. 2019, 237, 116907. [Google Scholar] [CrossRef]
- Tariq, N.; Ashraf, M.W.; Tayyaba, S. A Review on Solid Microneedles for Biomedical Applications. J. Pharm. Innov. 2022, 17, 1464–1483. [Google Scholar] [CrossRef]
- Patel, S.R.; Edelhauser, H.F.; Prausnitz, M.R. Targeted Drug Delivery to the Eye Enabled by Microneedles. In Drug Product Development for the Back of the Eye; Kompella, U.B., Edelhauser, H.F., Eds.; Springer: Boston, MA, USA, 2011; pp. 331–360. [Google Scholar] [CrossRef]
- Jiang, J.; Gill, H.S.; Ghate, D.; McCarey, B.E.; Patel, S.R.; Edelhauser, H.F.; Prausnitz, M.R. Coated Microneedles for Drug Delivery to the Eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4038–4043. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.C.; Grossniklaus, H.E.; Edelhauser, H.F.; Prausnitz, M.R. Intrastromal Delivery of Bevacizumab Using Microneedles to Treat Corneal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7376–7386. [Google Scholar] [CrossRef]
- Cárcamo-Martínez, Á.; Mallon, B.; Domínguez-Robles, J.; Vora, L.K.; Anjani, Q.K.; Donnelly, R.F. Hollow microneedles: A perspective in biomedical applications. Int. J. Pharm. 2021, 599, 120455. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilger, B.C.; Abarca, E.M.; Salmon, J.H.; Patel, S. Treatment of Acute Posterior Uveitis in a Porcine Model by Injection of Triamcinolone Acetonide Into the Suprachoroidal Space Using Microneedles. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2483–2492. [Google Scholar] [CrossRef] [Green Version]
- Thakur, R.R.S.; Tekko, I.A.; Al-Shammari, F.; Ali, A.A.; McCarthy, H.; Donnelly, R.F. Rapidly dissolving polymeric microneedles for minimally invasive intraocular drug delivery. Drug Deliv. Transl. Res. 2016, 6, 800–815. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Namjoshi, S.; Benson, H.A.E.; Mohammed, Y.; Kumeria, T. Dissolvable polymer microneedles for drug delivery and diagnostics. J. Control. Release 2022, 347, 561–589. [Google Scholar] [CrossRef]
- Albadr, A.A.; Tekko, I.A.; Vora, L.K.; Ali, A.A.; Laverty, G.; Donnelly, R.F.; Thakur, R.R.S. Rapidly dissolving microneedle patch of amphotericin B for intracorneal fungal infections. Drug Deliv. Transl. Res. 2022, 12, 931–943. [Google Scholar] [CrossRef]
- Datta, D.; Roy, G.; Garg, P.; Venuganti, V.V.K. Ocular delivery of cyclosporine A using dissolvable microneedle contact lens. J. Drug Deliv. Sci. Technol. 2022, 70, 103211. [Google Scholar] [CrossRef]
- Isreb, A.; Baj, K.; Wojsz, M.; Isreb, M.; Peak, M.; Alhnan, M.A. 3D printed oral theophylline doses with innovative ‘radiator-like’ design: Impact of polyethylene oxide (PEO) molecular weight. Int. J. Pharm. 2019, 564, 98–105. [Google Scholar] [CrossRef]
- Pereira, B.C.; Isreb, A.; Forbes, R.T.; Dores, F.; Habashy, R.; Petit, J.-B.; Alhnan, M.A.; Oga, E.F. ‘Temporary Plasticiser’: A novel solution to fabricate 3D printed patient-centred cardiovascular ‘Polypill’ architectures. Eur. J. Pharm. Biopharm. 2019, 135, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.N.; Yong, W.P.; Kochhar, J.S.; Khanolkar, J.; Yao, X.; Sun, Y.; Ao, C.K.; Soh, S. On-demand fully customizable drug tablets via 3D printing technology for personalized medicine. J. Control. Release 2020, 322, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, I.I.; Gioumouxouzis, C.I.; Eleftheriadis, G.K.; Fatouros, D.G. The Advent of a New Era in Digital Healthcare: A Role for 3D Printing Technologies in Drug Manufacturing? Pharmaceutics 2022, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, R.D.; Mann, S.E.; Ifantides, C. 3D Printing in Eye Care. Ophthalmol. Ther. 2021, 10, 733–752. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.R.; Kim, S.H.; Ko, J.; Baek, S.W.; Park, Y.K.; Kim, Y.J.; Yoon, J.S. A Pilot Clinical Study of Ocular Prosthesis Fabricated by Three-dimensional Printing and Sublimation Technique. Korean J. Ophthalmol. 2021, 35, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ulag, S.; Ilhan, E.; Sahin, A.; Karademir Yilmaz, B.; Kalaskar, D.M.; Ekren, N.; Kilic, O.; Nuzhet Oktar, F.; Gunduz, O. 3D printed artificial cornea for corneal stromal transplantation. Eur. Polym. J. 2020, 133, 109744. [Google Scholar] [CrossRef]
- Holland, G.; Pandit, A.; Sánchez-Abella, L.; Haiek, A.; Loinaz, I.; Dupin, D.; Gonzalez, M.; Larra, E.; Bidaguren, A.; Lagali, N.; et al. Artificial Cornea: Past, Current, and Future Directions. Front. Med. 2021, 8, 770780. [Google Scholar] [CrossRef]
- Garg, U.; Jain, N.; Kaul, S.; Rai, V.K.; Pandey, M.; Nagaich, U.; Dua, K. The emerging role of 3D-printing in ocular drug delivery: Challenges, current status, and future prospects. J. Drug Deliv. Sci. Technol. 2022, 76, 103798. [Google Scholar] [CrossRef]
- Shi, P.; Tan, E.Y.S.; Yeong, W.Y.; Laude, A. Hybrid three-dimensional (3D) bioprinting of retina equivalent for ocular research. Int. J. Bioprint. 2017, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.R.; Worthington, K.S.; Green, B.J.; Mullin, N.K.; Jiao, C.; Kaalberg, E.E.; Wiley, L.A.; Han, I.C.; Russell, S.R.; Sohn, E.H.; et al. Two-photon polymerized poly(caprolactone) retinal cell delivery scaffolds and their systemic and retinal biocompatibility. Acta Biomater. 2019, 94, 204–218. [Google Scholar] [CrossRef]
- Tagami, T.; Goto, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. Lyophilized ophthalmologic patches as novel corneal drug formulations using a semi-solid extrusion 3D printer. Int. J. Pharm. 2022, 617, 121448. [Google Scholar] [CrossRef]
- Mohamdeen, Y.M.G.; Tabriz, A.G.; Tighsazzadeh, M.; Nandi, U.; Khalaj, R.; Andreadis, I.; Boateng, J.S.; Douroumis, D. Development of 3D printed drug-eluting contact lenses. J. Pharm. Pharmacol. 2021, 74, 1467–1476. [Google Scholar] [CrossRef]
- Yang, S.-A.; Mitchell, W.; Hall, N.; Elze, T.; Lorch, A.C.; Miller, J.W.; Zebardast, N. Trends and Usage Patterns of Minimally Invasive Glaucoma Surgery in the United States: IRIS® Registry Analysis 2013–2018. Ophthalmol. Glaucoma 2021, 4, 558–568. [Google Scholar] [CrossRef]
- Culmone, C.; Lussenburg, K.; Alkemade, J.; Smit, G.; Sakes, A.; Breedveld, P. A Fully 3D-Printed Steerable Instrument for Minimally Invasive Surgery. Materials 2021, 14, 7910. [Google Scholar] [CrossRef]
- Thakur, R.R.S.; McMillan, H.L.; Jones, D.S. Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J. Control. Release 2014, 176, 8–23. [Google Scholar] [CrossRef]
- Kempe, S.; Mäder, K. In situ forming implants—An attractive formulation principle for parenteral depot formulations. J. Control. Release 2012, 161, 668–679. [Google Scholar] [CrossRef]
- Sheshala, R.; Hong, G.C.; Yee, W.P.; Meka, V.S.; Thakur, R.R.S. In situ forming phase-inversion implants for sustained ocular delivery of triamcinolone acetonide. Drug Deliv. Transl. Res. 2019, 9, 534–542. [Google Scholar] [CrossRef] [Green Version]
- Bisht, R.; Jaiswal, J.K.; Rupenthal, I.D. Nanoparticle-loaded biodegradable light-responsive in situ forming injectable implants for effective peptide delivery to the posterior segment of the eye. Med. Hypotheses 2017, 103, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, H.A.M.; Copeland, N.A.; Hardy, J.G. Light-responsive biomaterials for ocular drug delivery. Drug Deliv. Transl. Res. 2022, 1–24. [Google Scholar] [CrossRef]
- Hsu, X.-L.; Wu, L.-C.; Hsieh, J.-Y.; Huang, Y.-Y. Nanoparticle-Hydrogel Composite Drug Delivery System for Potential Ocular Applications. Polymers 2021, 13, 642. [Google Scholar] [CrossRef]
- Danckwerts, M.; Fassihi, A. Implantable Controlled Release Drug Delivery Systems: A Review. Drug Dev. Ind. Pharm. 1991, 17, 1465–1502. [Google Scholar] [CrossRef]
- Baino, F.; Kargozar, S. Regulation of the Ocular Cell/Tissue Response by Implantable Biomaterials and Drug Delivery Systems. Bioengineering 2020, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Pearson, P.A.; Blandford, D.L.; Brown, J.D.; Goins, K.A.; Hollins, J.L.; Schmeisser, E.T.; Glavinos, P.; Baldwin, L.B.; Ashton, P. Intravitreal Sustained-Release Ganciclovir. Arch. Ophthalmol. 1992, 110, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Prata, A.I.; Coimbra, P.; Pina, M.E. Preparation of dexamethasone ophthalmic implants: A comparative study of in vitro release profiles. Pharm. Dev. Technol. 2018, 23, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Solano, A.G.R.; de Fátima Pereira, A.; de Faria, L.G.A.; Fialho, S.L.; de Oliveira Patricio, P.S.; da Silva-Cunha, A.; Fulgêncio, G.O.; da Silva, G.R.; Pianetti, G.A. Etoposide-Loaded Poly(Lactic-co-Glycolic Acid) Intravitreal Implants: In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2018, 19, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Natu, M.V.; Gaspar, M.N.; Fontes Ribeiro, C.A.; Cabrita, A.M.; de Sousa, H.C.; Gil, M.H. In vitro and in vivo evaluation of an intraocular implant for glaucoma treatment. Int. J. Pharm. 2011, 415, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Stanković, M.; Frijlink, H.W.; Hinrichs, W.L.J. Polymeric formulations for drug release prepared by hot melt extrusion: Application and characterization. Drug Discov. Today 2015, 20, 812–823. [Google Scholar] [CrossRef]
- Breitenbach, J. Melt extrusion: From process to drug delivery technology. Eur. J. Pharm. Biopharm. 2002, 54, 107–117. [Google Scholar] [CrossRef]
- Bhattarai, R.S.; Das, A.; Alzhrani, R.M.; Kang, D.; Bhaduri, S.B.; Boddu, S.H.S. Comparison of electrospun and solvent cast polylactic acid (PLA)/poly(vinyl alcohol) (PVA) inserts as potential ocular drug delivery vehicles. Mater. Sci. Eng. C 2017, 77, 895–903. [Google Scholar] [CrossRef]
- Thakkar, S.; Misra, M. Electrospun polymeric nanofibers: New horizons in drug delivery. Eur. J. Pharm. Sci. 2017, 107, 148–167. [Google Scholar] [CrossRef]
- Sommer, A.C.; Blumenthal, E.Z. Implementations of 3D printing in ophthalmology. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1815–1822. [Google Scholar] [CrossRef]
- Reddy Dumpa, N.; Bandari, S.A.; Repka, M. Novel Gastroretentive Floating Pulsatile Drug Delivery System Produced via Hot-Melt Extrusion and Fused Deposition Modeling 3D Printing. Pharmaceutics 2020, 12, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korelidou, A.; Domínguez-Robles, J.; Magill, E.R.; Eleftheriadou, M.; Cornelius, V.A.; Donnelly, R.F.; Margariti, A.; Larrañeta, E. 3D-printed reservoir-type implants containing poly(lactic acid)/poly(caprolactone) porous membranes for sustained drug delivery. Biomater. Adv. 2022, 139, 213024. [Google Scholar] [CrossRef] [PubMed]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef]
- Annuryanti, F.; Domínguez-Robles, J.; Anjani, Q.K.; Adrianto, M.F.; Larrañeta, E.; Thakur, R.R.S. Fabrication and Characterisation of 3D-Printed Triamcinolone Acetonide-Loaded Polycaprolactone-Based Ocular Implants. Pharmaceutics 2023, 15, 243. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.; Youssef, A.A.A.; Nyavanandi, D.; Tripathi, S.; Bandari, S.; Majumdar, S.; Repka, M.A. Design and optimization of ciprofloxacin hydrochloride biodegradable 3D printed ocular inserts: Full factorial design and in-vitro and ex-vivo evaluations: Part II. Int. J. Pharm. 2023, 631, 122533. [Google Scholar] [CrossRef]
- Atkinson, H.V.; Davies, S. Fundamental aspects of hot isostatic pressing: An overview. Metall. Mater. Trans. A 2000, 31, 2981–3000. [Google Scholar] [CrossRef]
- Adelmann, B.; Hellmann, R. Mechanical Properties of LPBF-Built Titanium Lattice Structures—A Comparative Study of As-Built and Hot Isostatic Pressed Structures for Medical Implants. Metals 2022, 12, 2072. [Google Scholar]
- Hassanin, H.; Al-Kinani, A.A.; ElShaer, A.; Polycarpou, E.; El-Sayed, M.A.; Essa, K.J.J.O.M.C.B. Stainless steel with tailored porosity using canister-free hot isostatic pressing for improved osseointegration implants. J. Mater. Chem. B 2017, 5, 9384–9394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullen, L.; Stamp, R.C.; Fox, P.; Jones, E.; Ngo, C.; Sutcliffe, C.J. Selective laser melting: A unit cell approach for the manufacture of porous, titanium, bone in-growth constructs, suitable for orthopedic applications. II. Randomized structures. J. Biomed. Mater. Res. 2010, 92B, 178–188. [Google Scholar] [CrossRef]
- Abdelkader, H.; Wertheim, D.; Pierscionek, B.; Alany, R.G. Curcumin In Situ Gelling Polymeric Insert with Enhanced Ocular Performance. Pharmaceutics 2020, 12, 1158. [Google Scholar] [CrossRef]
- Bode, C.; Kranz, H.; Siepmann, F.; Siepmann, J. In-situ forming PLGA implants for intraocular dexamethasone delivery. Int. J. Pharm. 2018, 548, 337–348. [Google Scholar] [CrossRef]
- Allababidi, S.; Shah, J.C. Kinetics and mechanism of release from glyceryl monostearate-based implants: Evaluation of release in a gel simulating in vivo implantation. J. Pharm. Sci. 1998, 87, 738–744. [Google Scholar] [CrossRef]
- Balasubramaniam, J.; Srinatha, A.; Pandit, J.K. Studies on Indomethacin Intraocular Implants Using Different in vitro Release Methods. Indian J. Pharm. Sci. 2008, 70, 216–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chennamaneni, S.R.; Mamalis, C.; Archer, B.; Oakey, Z.; Ambati, B.K. Development of a novel bioerodible dexamethasone implant for uveitis and postoperative cataract inflammation. J. Control. Release 2013, 167, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awwad, S.; Lockwood, A.; Brocchini, S.; Khaw, P.T. The PK-Eye: A Novel In Vitro Ocular Flow Model for Use in Preclinical Drug Development. J. Pharm. Sci. 2015, 104, 3330–3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, S.; Auel, T.; Kempin, W.; Bogdahn, M.; Weitschies, W.; Seidlitz, A. Influence of the test method on in vitro drug release from intravitreal model implants containing dexamethasone or fluorescein sodium in poly (d,l-lactide-co-glycolide) or polycaprolactone. Eur. J. Pharm. Biopharm. 2018, 127, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, R.S.; Garcia, A.; Robeson, R.; Gilger, B.C.; Culp, D.; Struble, C.; Hamm, L.; Navratil, T.; Yerxa, B. Nonclinical Development of ENV905 (Difluprednate) Ophthalmic Implant for the Treatment of Inflammation and Pain Associated with Ocular Surgery. J. Ocul. Pharmacol. Ther. 2017, 34, 161–169. [Google Scholar] [CrossRef]
- Sirinek, P.E.; Lin, M.M. Intracameral sustained release bimatoprost implants (Durysta). Semin. Ophthalmol. 2022, 37, 385–390. [Google Scholar] [CrossRef]
- Salama, H.A.; Ghorab, M.; Mahmoud, A.A.; Abdel Hady, M. PLGA Nanoparticles as Subconjunctival Injection for Management of Glaucoma. AAPS PharmSciTech 2017, 18, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- FDA. Regulatory Controls. Available online: https://www.fda.gov/medical-devices/overview-device-regulation/regulatory-controls (accessed on 28 April 2023).
- Premarket Notification 510(k)|FDA. Available online: https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-notification-510k (accessed on 28 April 2023).
- Premarket Approval (PMA)|FDA. Available online: https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-approval-pma (accessed on 29 April 2023).
- Design Control Guidance for Medical Device Manufacturers|FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/design-control-guidance-medical-device-manufacturers (accessed on 29 April 2023).
- Use of International Standard ISO 10993-1, “Biological Evaluation of Medical Devices-Part 1: Evaluation and Testing within a Risk Management Process”|FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-international-standard-iso-10993-1-biological-evaluation-medical-devices-part-1-evaluation-and (accessed on 29 April 2023).
- Guidance for the Preparation of a Premarket Notification Application for a Surgical Mesh-Guidance for Industry and/or for FDA Reviewers/Staff and/or Compliance|Guidance Portal. Available online: https://www.hhs.gov/guidance/document/guidance-preparation-premarket-notification-application-surgical-mesh-guidance-industry (accessed on 29 April 2023).








| Platform/Device | Commercial Brand | Therapeutic Agent (Approval Year, Country) | Excipient Composition | Clinical Implication | Route of Administration | Refs. |
|---|---|---|---|---|---|---|
| Liposomes | Visudyne® | Verteporfin (2000, USA) | Dimyristoylphosphatidylcholine and egg yolk phosphatidylglycerol | Choroidal neovascularization in AMD | IV | [393] |
| Amphotec® | Amphotericin B (1996, USA) | Cholesteryl sulfate | Fungal endophthalmitis | IV | [394] | |
| Abelcet® | Amphotericin B (1995, USA) | Dimyristoylphosphatidylcholine and dimyristoylphosphatidylglycerol | Fungal endophthalmitis | IV | [395] | |
| AmBisome® | Amphotericin B (1997, USA) | Hydrogenated soy phosphatidylcholine, cholesterol, and distearoylphosphatidylglycerol | Fungal endophthalmitis | IV | [396] | |
| Ozodrop® | Sunflower ozonized oil (NA, Italy) | LipozonEye, hypromellose and polyhexamethylene biguanide | Post-cataract surgery inflammation | Topical | [397] | |
| Lacrisek® | Vitamin A palmitate, vitamin E (NA, Italy) | Hydrogenated phospholipids | Dry eye syndrome | Topical | [398] | |
| Tears Again® | Vitamin A palmitate, vitamin E (NA, USA) | Soy lecithin, phenoxyethanol | Dry eye syndrome | Topical | [398] | |
| Emulsions | Restasis® | Cyclosporine A (2002, USA) | Polysorbate 80, castor oil | Dry eye syndrome | Topical | [399] |
| Emustil® | Cyclosporine A (NA, Italy) | Soybean oil, egg yolk phospholipids | Dry eye syndrome | Topical | [400] | |
| Refresh Endura® | Cyclosporine A (2020, USA) | Polysorbate 80, castor oil | Dry eye syndrome | Topical | [401] | |
| Xelpros® | Latanoprost (2018, USA) | Castor oil, propylene glycol | Glaucoma | Topical | [402] | |
| Durezol® | Difluprednate (2008, USA) | Castor oil, polysorbate 80 | Diabetic macular edema | Topical | [85] | |
| Verkazia® | Cyclosporine A (2017, UK) | Medium-chain triglyceride, Tyloxapol, and poloxamer 188 | Vernal keratoconjunctivitis | Topical | [403] | |
| Micelles | Cequa® | Cyclosporin A (2018, USA) | Polyoxyl hydrogenated castor oil, polyalkoxylated alcohol | Dry eye syndrome | Topical | [54] |
| AzaSite® | Azithromycin (2007, USA) | Polycarbophil | Blepharitis | Topical | [404] | |
| Micro and nanoparticles | Macugen® | Pegaptanib (2004, USA) | PEG-40kDa | AMD | IVT | [405] |
| Trivaris® | Triamcinolone acetonide (2005, USA) | Sodium hyaluronate | Uveitis | IVT | [406] | |
| Inveltys® | Loteprednol etabonate (2018, USA) | Pluronic F127 | Post-operative inflammations | Topical | [407] | |
| Eysuvis® | Loteprednol etabonate (2020, USA) | Pluronic F127 | Dry eye syndrome | Topical | [407] | |
| Triesence® | Triamcinolone acetonide (2005, USA) | Carboxymethyl cellulose | Dry eye syndrome | IVT | [408] | |
| Tobradex ST® | Tobramycin and dexamethasone (2003, USA) | Xanthan gum | Bacterial conjunctivitis | Topical | [403] | |
| BromSite® | Bromfenac (2016, USA) | Polycarbophil | Post-operative inflammation and pain reliver | Topical | [409] | |
| Besivance® | Besifloxacin (2009, USA) | Polycarbophil | Bacterial conjunctivitis | Topical | [410] | |
| Implants | Ozurdex® | Dexamethasone (2009, USA) | Acid-terminated PLGA (30%) + ester-terminated PLGA (10%) | Macular edema | IVT | [411] |
| Retisert® | Fluocinolone acetonide (2005, USA) | Ethylene-vinyl acetate coated with polyvinyl alcohol | Uveitis | IVT | [412] | |
| Vitrasert® | Ganciclovir (1996, USA) | Ethylene-vinyl acetate coated with polyvinyl alcohol | Cytomegalovirus retinitis | IVT | [412] | |
| I-vation® | Triamcinolone acetonide (2007, USA) | Poly (methyl methacrylate) and ethylene vinyl acetate | Diabetic macular edema | IVT | [413] | |
| Iluvien® | Fluocinolone acetonide (2014, USA) | Polyimide tube coated with Polyvinyl alcohol | Diabetic macular edema | IVT | [414] | |
| Medidur® | Fluocinolone acetonide (2014, USA) | Polyvinyl alcohol | Diabetic macular edema | IVT | [415] | |
| Posurdex® | Dexamethasone (2009, USA) | PLGA | Macular edema | IVT | [416] | |
| Surodex® | Dexamethasone (2008, USA) | PLGA | Post-operative inflammation | Subscleral placement | [417] | |
| Renexus® | Encapsulated cell technology (NA) | Polyethylene terephthalate | AMD | IVT | [418] | |
| Yutiq® | Fluocinolone acetonide (2018, USA) | Polyimide/polyvinyl alcohol | Diabetic macular edema | IVT | [419] | |
| Durysta® | Bimatoprost (2020, USA) | Poly(D,L-lactide), PLGA, and poly (D,L-lactide) with an acid end group | Glaucoma | ICI | [420] | |
| Dexycu® | Dexamethasone (2018, USA) | Acetyl triethyl citrate | Post-operative inflammation | Posterior chamber injection | [421] | |
| Susvimo® | Ranibizumab (2020, Germany) | Polysulphone, silicone | AMD | IVT | [422] | |
| Inserts | Ocusert® * | Pilocarpine (1972, USA) | Polyethylene co-vinyl acetate | Glaucoma | CI | [423] |
| Lacrisert® | Hydroxypropyl methyl cellulose (1992, USA) | Hydroxypropyl methyl cellulose | Moderate-to-severe dry eye syndrome | CI | [424] | |
| BIM ring® | Bimatoprost (NA) | Support made of polypropylene and covered in a silicone matrix. | Glaucoma | CI | [425,426] | |
| Dextenza® | Dexamethasone (2018, USA) | Polyethylene glycol | Post-operative inflammation | CI | [414] | |
| Mydriasert® | Tropicamid, phenylephrine hydrochloride, and hydroxypropyl methyl cellulose (2015, UK) | Ammonium methacrylate copolymer | Diagnosis (pupil dilator) | Intracanalicular insertion | [427] | |
| Microneedles | Xipere™ | Triamcinolone acetonide (2019, USA) | Carboxymethylcellulose sodium, and polysorbate 80 | Macular edema associated with uveitis | SCS | [428,429] |
| Drug eluting contact lens | Acuvue® | Ketotifen fumarate (2017, USA) | Etafilcon A | Ocular allergic itch | Topical | [430] |
| Polymer | Route | Pharmaceutical Forms | CAS Number |
|---|---|---|---|
| Carbomer a | Eye surface | Emulsion | |
| Carbomer b | Eye surface | Emulsion | |
| Carbomer b | Eye surface | Gel | |
| Carbomer b | Eye surface | Suspension | |
| Carbomer b | Eye surface | Suspension/drops | |
| Carbomer b | Eye surface | Suspension | |
| Carbomer b | Eye surface | Suspension/drops | |
| Carbomer c | Eye surface | Gel | |
| Ethylene-vinyl acetate copolymers (EVA) | Eye surface | Insert, extended release | 24937788 |
| Ethylene-vinyl acetate copolymers (EVA) | Eye surface | Solution | 24937788 |
| PEG/PPG-4/30 copolymer | Eye surface | Solution | |
| PLGA | Intravitreal | Implant | 26780507 |
| PLGA | Intravitreal | Injection | 26780507 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, M.; Al Fatease, A.; Alany, R.G.; Abdelkader, H. Recent Advances of Ocular Drug Delivery Systems: Prominence of Ocular Implants for Chronic Eye Diseases. Pharmaceutics 2023, 15, 1746. https://doi.org/10.3390/pharmaceutics15061746
Mostafa M, Al Fatease A, Alany RG, Abdelkader H. Recent Advances of Ocular Drug Delivery Systems: Prominence of Ocular Implants for Chronic Eye Diseases. Pharmaceutics. 2023; 15(6):1746. https://doi.org/10.3390/pharmaceutics15061746
Chicago/Turabian StyleMostafa, Mahmoud, Adel Al Fatease, Raid G. Alany, and Hamdy Abdelkader. 2023. "Recent Advances of Ocular Drug Delivery Systems: Prominence of Ocular Implants for Chronic Eye Diseases" Pharmaceutics 15, no. 6: 1746. https://doi.org/10.3390/pharmaceutics15061746