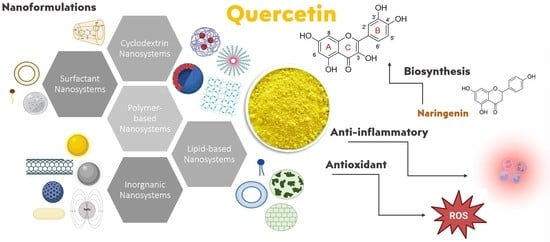
Recent Advances in Nanoformulations for Quercetin Delivery
Abstract
:1. Introduction
2. Materials and Methods
3. Origin, Physicochemical Characteristics, Biosynthesis, and Main Pharmacological Activities of QUE
3.1. Origin, Physicochemical Characteristics, Pharmacokinetics, and Metabolism
3.2. Biosynthesis
3.3. Main Pharmacological Properties: Anti-Oxidant and Anti-Inflammatory Activities
4. QUE Nanosystems
4.1. Polymer-Based Nanosystems
4.1.1. Polymeric Micelles
4.1.2. Polymeric Nanoparticles
4.1.3. Hydrogels
4.1.4. Polymersomes
4.2. Lipid-Based Nanoparticles
4.2.1. Liposomes and Liposome-Based Nanoparticles
4.2.2. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLC)
4.3. Surfactant-Based Nanoparticles
4.3.1. Niosomes
4.3.2. Nanoemulsions
4.4. Cyclodextrin-Based Nanoparticles
4.5. Inorganic Nanoparticles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, R.; Huang, H. Anti-Allergic Effects of Quercetin and Quercetin Liposomes in RBL-2H3 Cells. Endocr. Metab. Immune Disord. Drug Targets 2022, 23, 692–701. [Google Scholar]
- Dian, L.; Yu, E.; Chen, X.; Wen, X.; Zhang, Z.; Qin, L.; Wang, Q.; Li, G.; Wu, C. Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res. Lett. 2014, 9, 684. [Google Scholar] [CrossRef] [Green Version]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharm. 2019, 44, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamantis, D.; Ramesova, S.; Chatzigiannis, C.; Degano, I.; Gerogianni, P.; Karadima, C.; Perikleous, S.; Rekkas, D.; Gerothanassis, I.; Galaris, D.; et al. Exploring the oxidation and iron binding profile of a cyclodextrin encapsulated quercetin complex unveiled a controlled complex dissociation through a chemical stimulus. BBA-Gen. Subj. 2018, 1862, 1913–1924. [Google Scholar] [CrossRef]
- Hashemian, M.; Ghasemi-Kasman, M.; Ghasemi, S.; Akbari, A.; Moalem-Banhangi, M.; Zare, L.; Ahmadian, S.R. Fabrication and evaluation of novel quercetin-conjugated Fe3O4-β-cyclodextrin nanoparticles for potential use in epilepsy disorder. Int. J. Nanomed. 2019, 14, 6481–6495. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimpour, S.; Esmaeili, A.; Dehghanian, F.; Beheshti, S. Effects of quercetin-conjugated with superparamagnetic iron oxide nanoparticles on learning and memory improvement through targeting microRNAs/NF-κB pathway. Sci. Rep. 2020, 10, 15070. [Google Scholar] [CrossRef]
- Laffleur, F.; Keckeis, V. Advances in drug delivery systems: Work in progress still needed? Int. J. Pharm. 2020, 590, 119912. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, K. Advanced drug delivery 2020 and beyond: Perspectives on the future. Adv. Drug Deliv. Rev. 2020, 158, 4–16. [Google Scholar] [CrossRef]
- Ganesan, P.; Choi, D.K. Current application of phytocompound-based nanocosmeceuticals for beauty and skin therapy. Int. J. Nanomed. 2016, 11, 1987–2007. [Google Scholar] [CrossRef] [Green Version]
- Laffleur, F.; Bauer, B. Progress in nasal drug delivery systems. Int. J. Pharm. 2021, 607, 120994. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Drechsler, M.; Puglia, C.; Cortesi, R. New Strategies for the Delivery of Some Natural Anti-oxidants with Therapeutic Properties. Mini Rev. Med. Chem. 2019, 19, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Quercetin derivatives: Drug design, development, and biological activities, a review. Eur. J. Med. Chem. 2022, 229, 114068. [Google Scholar] [CrossRef]
- Batiha, G.E.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Materska, M. Quercetin and Its Derivatives: Chemical Structure and Bioactivity—A Review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef] [PubMed]
- Suganthy, N.; Devi, K.P.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Bioactive Effects of Quercetin in the Central Nervous System: Focusing on the Mechanisms of Actions. Biomed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Hai, Y.; Zhang, Y.; Liang, Y.; Ma, X.; Qi, X.; Xiao, J.; Xue, W.; Luo, Y.; Yue, T. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives. Food Front. 2020, 1, 420–434. [Google Scholar] [CrossRef]
- Chen, X.; Yin, O.Q.; Zuo, Z.; Chow, M.S. Pharmacokinetics and modeling of quercetin and metabolites. Pharm. Res. 2005, 22, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Van der Woude, H.; Bersma, M.G.; Vervoort, J.; Rietjens, I.M. Identification of 14 quercetin phase II mono- and mixed conjugates and their formation by rat and human phase II in vitro model systems. Chem. Res. Toxicol. 2004, 17, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.L.; Tsai, Y.J.; Huang, C.M.; Tsai, T.H. Lymphatic absorption of quercetin and rutin in rat and their pharmacokinetics in systemic plasma. J. Agric. Food Chem. 2010, 58, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, A.A.; Maghiar, T.A.; Alaya, A.; Olah, N.K.; Turcus, V.; Pelea, D.; Totolici, B.D.; Neamtu, C.; Maghiar, A.M.; Mathe, E. A Comprehensive View on the Quercetin Impact on Colorectal Cancer. Molecules 2022, 27, 1873. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant. Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef]
- Whinkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant. Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Dewick, P. Medicinal Natural Products: A Biosynthetic Approach, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Teskey, G.; Abrahem, R.; Cao, R.; Gyurjian, K.; Islamoglu, H.; Lucero, M.; Martinez, A.; Paredes, E.; Salaiz, O.; Robinson, B.; et al. Glutathione as a Marker for Human Disease. Adv. Clin. Chem. 2018, 87, 141–159. [Google Scholar] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.J.; Chee, K.M.; Lee, B.H. Anti- and prooxidant effects of chronic quercetin administration in rats. Eur. J. Pharmacol. 2003, 482, 281–285. [Google Scholar] [CrossRef]
- Awad, H.M.; Boersma, M.G.; Boeren, S.; van Bladeren, P.J.; Vervoort, J.; Rietjens, I.M. Structure-activity study on the quinone/quinone methide chemistry of flavonoids. Chem. Res. Toxicol. 2001, 14, 398–408. [Google Scholar] [CrossRef]
- Gao, W.; Pu, L.; Chen, M.; Wei, J.; Xin, Z.; Wang, Y.; Yao, Z.; Shi, T.; Guo, C. Glutathione homeostasis is significantly altered by quercetin via the Keap1/Nrf2 and MAPK signaling pathways in rats. J. Clin. Biochem. Nutr. 2018, 62, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.J.; Lee, B.H.; Lee, K.; Chee, K.M. Long-term combined administration of quercetin and daidzein inhibits quercetin-induced suppression of glutathione antioxidant defenses. Food Chem. Toxicol. 2005, 43, 793–798. [Google Scholar] [CrossRef]
- Hu, X.T.; Ding, C.; Zhou, N.; Xu, C. Quercetin protects gastric epithelial cell from oxidative damage in vitro and in vivo. Eur. J. Pharmacol. 2015, 754, 115–124. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Chen, G.-W.; Chen, Y.-C.; Shen, C.-K.; Lu, D.-Y.; Yang, L.-Y.; Chen, J.-H.; Yeh, W.-L. Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance. Nutrients 2022, 14, 67. [Google Scholar] [CrossRef]
- Hung, C.H.; Chan, S.H.; Chu, P.M.; Tsai, K.L. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol. Nutr. Food Res. 2015, 59, 1905–1917. [Google Scholar] [CrossRef]
- Odbayar, T.O.; Kimura, T.; Tsushida, T.; Ide, T. Isoenzyme-specific up-regulation of glutathione transferase and aldo-keto reductase mRNA expression by dietary quercetin in rat liver. Mol. Cell. Biochem. 2009, 325, 121–130. [Google Scholar] [CrossRef]
- Kim, H.P.; Mani, I.; Iversen, L.; Ziboh, V.A. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot. Essent. Fat. Acids 1998, 58, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.I.; Vicente-Sánchez, C.; Jerkic, M.; Santiago, J.M.; Sánchez-González, P.D.; Pérez-Barriocanal, F.; López-Novoa, J.M. Effect of quercetin on metallothionein, nitric oxide synthases and cyclooxygenase-2 expression on experimental chronic cadmium nephrotoxicity in rats. Toxicol. Appl. Pharmacol. 2006, 210, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Flórez, S.; Gutiérrez-Fernández, B.; Sánchez-Campos, S.; González-Gallego, J.; Tuñón, M.J. Quercetin attenuates nuclear factor-kappaB activation and nitric oxide production in interleukin-1beta-activated rat hepatocytes. J. Nutr. 2005, 135, 1359–1365. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.C.; Lee, W.R.; Lin, H.Y.; Huang, H.C.; Ko, C.H.; Yang, L.L.; Chen, Y.C. In vitro and in vivo inhibitory activities of rutin, wogonin, and quercetin on lipopolysaccharide-induced nitric oxide and prostaglandin E(2) production. Eur. J. Pharmacol. 2002, 446, 187–194. [Google Scholar] [CrossRef]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Manjeet, K.R.; Ghosh, B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-alpha production in murine macrophages. Int. J. Immunopharmacol. 1999, 21, 435–443. [Google Scholar]
- Bureau, G.; Longpré, F.; Martinoli, M.G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef]
- Yang, D.; Liu, X.; Liu, M.; Chi, H.; Liu, J.; Han, H. Protective effects of quercetin and taraxasterol against H2O2-induced human umbilical vein endothelial cell injury in vitro. Exp. Med. 2015, 10, 1253–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chroni, A.; Mavromoustakos, T.; Pispas, S. Biocompatible PEO-b-PCL Nanosized Micelles as Drug Carriers: Structure and Drug-Polymer Interactions. Nanomaterials 2020, 10, 1872. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Pispas, S.; Demetzos, C. Polymer Self-Assembled Nanostructures as Innovative Drug Nanocarrier Platforms. Curr. Pharm. Des. 2016, 22, 2788–2795. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.S.; Shaikh, S.J.; Ray, D.; Aswal, V.K.; Vaidya, F.; Pathak, C.; Sharma, R.K. Formulation, Solubilization, and In Vitro Characterization of Quercetin-Incorporated Mixed Micelles of PEO-PPO-PEO Block Copolymers. Appl. Biochem. Biotechnol. 2022, 194, 445–463. [Google Scholar] [CrossRef]
- Qi, X.; Gao, C.; Yin, C.; Fan, J.; Wu, X.; Di, G.; Wang, J.; Guo, C. Development of quercetin-loaded PVCL-PVA-PEG micelles and application in inhibiting tumor angiogenesis through the PI3K/Akt/VEGF pathway. Toxicol. Appl. Pharmacol. 2022, 437, 115889. [Google Scholar] [CrossRef]
- Paranthaman, S.; Uthaiah, C.A.; Osmani, R.A.M.; Hani, U.; Ghazwani, M.; Alamri, A.H.; Fatease, A.A.; Madhunapantula, S.V.; Gowda, D.V. Anti-Proliferative Potential of Quercetin Loaded Polymeric Mixed Micelles on Rat C6 and Human U87MG Glioma Cells. Pharmaceutics 2022, 14, 1643. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wu, Q.; Peng, F.; Liu, L.; Gong, C. Strategies of polymeric nanoparticles for enhanced internalization in cancer therapy. Colloids Surf. B Biointerfaces 2015, 135, 56–72. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Abujamous, L. pH-sensitive polymeric nanoparticles of quercetin as a potential colon cancer-targeted nanomedicine. J. Drug Deliv. Sci. Technol. 2019, 52, 670–676. [Google Scholar] [CrossRef]
- Bagad, M.; Khan, Z.A. Poly(n-butylcyanoacrylate) nanoparticles for oral delivery of quercetin: Preparation, characterization, and pharmacokinetics and biodistribution studies in Wistar rats. Int. J. Nanomed. 2015, 10, 3921–3935. [Google Scholar]
- Huang, K.T.; Wu, C.T.; Chang, Y.; Ho, F.M.; Chiang, C.K.; Liu, S.H. Therapeutic effect of quercetin polymeric nanoparticles on ischemia/reperfusion-induced acute kidney injury in mice. Biochem. Biophys. Res. Commun. 2022, 608, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, D.; Xue, G.; Yu, S.; Yuan, C.; Huang, M.; Jiang, L. Improved therapeutic efficacy of quercetin-loaded polymeric nanoparticles on triple-negative breast cancer by inhibiting uPA. RSC Adv. 2020, 10, 34517–34526. [Google Scholar] [CrossRef] [PubMed]
- Doosti, M.; Seyed Dorraji, M.S.; Mousavi, S.N.; Rasoulifard, M.H.; Hosseini, S.H. Enhancing quercetin bioavailability by super paramagnetic starch-based hydrogel grafted with fumaric acid: An in vitro and in vivo study. Colloids Surf. B Biointerfaces 2019, 183, 110487. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Pispas, S.; Demetzos, C. Recent advances in micellar-like polyelectrolyte/protein complexes. In Design and Development of New Nanocarriers; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 57–88. ISBN 978-0-12-813627-0. [Google Scholar]
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K. Synthesis and characterization of karaya gum-g- poly (acrylic acid) hydrogels and in vitro release of hydrophobic quercetin. Polymer 2018, 147, 108–120. [Google Scholar] [CrossRef]
- Esposito, L.; Barbosa, A.I.; Moniz, T.; Costa Lima, S.; Costa, P.; Celia, C.; Reis, S. Design and Characterization of Sodium Alginate and Poly(vinyl) Alcohol Hydrogels for Enhanced Skin Delivery of Quercetin. Pharmaceutics 2020, 12, 1149. [Google Scholar] [CrossRef]
- Mok, S.W.; Fu, S.C.; Cheuk, Y.C.; Chu, I.M.; Chan, K.M.; Qin, L.; Yung, S.H.; Kevin Ho, K.W. Intra-Articular Delivery of Quercetin Using Thermosensitive Hydrogel Attenuate Cartilage Degradation in an Osteoarthritis Rat Model. Cartilage 2020, 11, 490–499. [Google Scholar] [CrossRef]
- Safar Sajadi, S.M.; Khoee, S. The simultaneous role of porphyrins’ H- and J- aggregates and host-guest chemistry on the fabrication of reversible Dextran-PMMA polymersome. Sci. Rep. 2021, 11, 2832. [Google Scholar] [CrossRef]
- Gomes, C.P.; Dias, R.C.S.; Costa, M.R.P.F.N. Design of RAFT synthesized amphiphilic and stimuli-responsive block copolymers for encapsulation of polyphenols in polymersomes. CHEMPOR 2018, P-IM02, 579–580. [Google Scholar]
- Chavda, V.P.; Vihol, D.; Mehta, B.; Shah, D.; Patel, M.; Vora, L.K.; Pereira-Silva, M.; Paiva-Santos, A.C. Phytochemical-loaded liposomes for anticancer therapy: An updated review. Nanomedicine 2022, 17, 547–568. [Google Scholar] [CrossRef]
- Liu, H.; Xue, J.X.; Li, X.; Ao, R.; Lu, Y. Quercetin liposomes protect against radiation-induced pulmonary injury in a murine model. Oncol. Lett. 2013, 6, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Oh, K.T.; Youn, Y.S.; Lee, E.S. pH-Sensitive Twin Liposomes Containing Quercetin and Laccase for Tumor Therapy. Biomacromolecules 2022, 23, 3688–3697. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, K.; Zhang, Y.; Li, H.; Li, A.; Xu, Y.; Wei, B. Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci. Rep. 2020, 10, 2440. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Yang, D.; Xing, Z.; Zhao, C.; Wang, L.; Fan, Y.; Nie, H.; Liu, H. Quercetin loaded liposomes modified with galactosylated chitosan prevent LPS/D-GalN induced acute liver injury. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112527. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Wu, W.; Chen, X.; Xiao, H.; Zhang, Z.; Chen, F.; Zhang, Z.; Liu, J.; Shao, Z.; Pu, F. Quercetin encapsulated in folic acid-modified liposomes is therapeutic against osteosarcoma by non-covalent binding to the JH2 domain of JAK2 via the JAK2-STAT3-PDL1. Pharmacol. Res. 2022, 182, 106287. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Gabriele, M.; Fernàndez-Busquets, X.; Valenti, D.; Fadda, A.M.; Pucci, L.; Manconi, M. Antioxidant activity of quercetin in Eudragit-coated liposomes for intestinal delivery. Int. J. Pharm. 2019, 565, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Bonechi, C.; Donati, A.; Tamasi, G.; Leone, G.; Consumi, M.; Rossi, C.; Lamponi, S.; Magnani, A. Protective effect of quercetin and rutin encapsulated liposomes on induced oxidative stress. Biophys. Chem. 2018, 233, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Mureşan, M.; Olteanu, D.; Filip, G.A.; Clichici, S.; Baldea, I.; Jurca, T.; Pallag, A.; Marian, E.; Frum, A.; Gligor, F.G.; et al. Comparative Study of the Pharmacological Properties and Biological Effects of Polygonum aviculare L. herba Extract-Entrapped Liposomes versus Quercetin-Entrapped Liposomes on Doxorubicin-Induced Toxicity on HUVECs. Pharmaceutics 2021, 13, 1418. [Google Scholar] [CrossRef]
- Kim, Y.A.; Tarahovsky, Y.S.; Yagolnik, E.A.; Kuznetsova, S.M.; Muzafarov, E.N. Integration of Quercetin-Iron Complexes into Phosphatidylcholine or Phosphatidylethanolamine Liposomes. Appl. Biochem. Biotechnol. 2015, 176, 1904–1913. [Google Scholar] [CrossRef]
- Munot, N.; Kandekar, U.; Giram, P.S.; Khot, K.; Patil, A.; Cavalu, S. A Comparative Study of Quercetin-Loaded Nanocochleates and Liposomes: Formulation, Characterization, Assessment of Degradation and In Vitro Anticancer Potential. Pharmaceutics 2022, 14, 1601. [Google Scholar] [CrossRef]
- Román-Aguirre, M.; Leyva-Porras, C.; Cruz-Alcantar, P.; Aguilar-Elguézabal, A.; Saavedra-Leos, M.Z. Comparison of Polysaccharides as Coatings for Quercetin-Loaded Liposomes (QLL) and Their Effect as Antioxidants on Radical Scavenging Activity. Polymers 2020, 12, 2793. [Google Scholar] [CrossRef]
- Ferreira-Silva, M.; Faria-Silva, C.; Carvalheiro, M.C.; Simões, S.; Marinho, H.S.; Marcelino, P.; Campos, M.C.; Metselaar, J.M.; Fernandes, E.; Baptista, P.V.; et al. Quercetin Liposomal Nanoformulation for Ischemia and Reperfusion Injury Treatment. Pharmaceutics 2022, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.M.; Müller, R.H.; Bégu, S. Liposomes, lipid nanocapsules and smartCrystals®: A comparative study for an effective quercetin delivery to the skin. Int. J. Pharm. 2018, 542, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Manconi, M.; Allaw, M.; Perra, M.; Orrù, G.; Fais, S.; Scano, A.; Escribano-Ferrer, E.; Ghavam, M.; Rezvani, M.; et al. Mouthwash Formulation Co-Delivering Quercetin and Mint Oil in Liposomes Improved with Glycol and Ethanol and Tailored for Protecting and Tackling Oral Cavity. Antioxidants 2022, 11, 367. [Google Scholar] [CrossRef]
- Seong, J.S.; Yun, M.E.; Park, S.N. Surfactant-stable and pH-sensitive liposomes coated with N-succinyl-chitosan and chitooligosaccharide for delivery of quercetin. Carbohydr. Polym. 2018, 181, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.J.; Anantha, M.; Leung, A.W.Y.; Kulkarni, J.A.; Militao, G.G.C.; Wehbe, M.; Sutherland, B.; Cullis, P.R.; Bally, M.B. Characterization of a liposomal copper(II)-quercetin formulation suitable for parenteral use. Drug Deliv. Transl. Res. 2020, 10, 202–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, F.; Benedusi, M.; Sguizzato, M.; Cortesi, R.; Baldisserotto, A.; Buzzi, R.; Valacchi, G.; Esposito, E. Ethosomes and Transethosomes as Cutaneous Delivery Systems for Quercetin: A Preliminary Study on Melanoma Cells. Pharmaceutics 2022, 14, 1038. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, H.M.; Abd El Aziz, A.E.; Mohamed, S.A.; AbuelEzz, N.Z. The tiny big world of solid lipid nanoparticles and nanostructured lipid carriers: An updated review. J. Microencapsul. 2022, 39, 72–94. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Ahmadi, F.; Hashemi, S.M.H.; Babaei, A.; Yaddollahi, S.; Rostamkalaei, S.S.; Asare-Addo, K.; Nokhodchi, A. Solid lipid nanoparticles and nanostructured lipid carriers: A review of the methods of manufacture and routes of administration. Pharm. Dev. Technol. 2022, 27, 525–544. [Google Scholar] [CrossRef]
- Salah, E.; Abouelfetouh, M.M.; Pan, Y.; Chen, D.; Xie, S. Solid lipid nanoparticles for enhanced oral absorption: A review. Colloids Surf. B Biointerfaces. 2020, 196, 111305. [Google Scholar] [CrossRef]
- Talarico, L.; Consumi, M.; Leone, G.; Tamasi, G.; Magnani, A. Solid Lipid Nanoparticles Produced via a Coacervation Method as Promising Carriers for Controlled Release of Quercetin. Molecules 2021, 26, 2694. [Google Scholar] [CrossRef]
- Shawky, S.; Makled, S.; Awaad, A.; Boraie, N. Quercetin Loaded Cationic Solid Lipid Nanoparticles in a Mucoadhesive In Situ Gel-A Novel Intravesical Therapy Tackling Bladder Cancer. Pharmaceutics 2022, 14, 2527. [Google Scholar] [CrossRef] [PubMed]
- Weerapol, Y.; Manmuan, S.; Chaothanaphat, N.; Limmatvapirat, S.; Sirirak, J.; Tamdee, P.; Tubtimsri, S. New Approach for Preparing Solid Lipid Nanoparticles with Volatile Oil-Loaded Quercetin Using the Phase-Inversion Temperature Method. Pharmaceutics 2022, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.G.R.; Granja, A.; Loureiro, J.A.; Pereira, M.C.; Pinheiro, M.; Neves, A.R.; Reis, S. Quercetin lipid nanoparticles functionalized with transferrin for Alzheimer’s disease. Eur. J. Pharm. Sci. 2020, 148, 105314. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.G.R.; Granja, A.; Loureiro, J.A.; Pereira, M.C.; Pinheiro, M.; Neves, A.R.; Reis, S. RVG29-Functionalized Lipid Nanoparticles for Quercetin Brain Delivery and Alzheimer’s Disease. Pharm. Res. 2020, 37, 139. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Baskaran, R.; Jang, Y.S.; Oh, S.H.; Yoo, B.K. Quercetin-Loaded Solid Lipid Nanoparticle Dispersion with Improved Physicochemical Properties and Cellular Uptake. AAPS PharmSciTech 2017, 18, 875–883. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar] [CrossRef]
- Ganthala, P.D.; Alavala, S.; Chella, N.; Andugulapati, S.B.; Bathini, N.B.; Sistla, R. Co-encapsulated nanoparticles of Erlotinib and Quercetin for targeting lung cancer through nuclear EGFR and PI3K/AKT inhibition. Colloids Surf. B Biointerfaces 2022, 211, 112305. [Google Scholar] [CrossRef]
- De Barros, D.P.C.; Santos, R.; Reed, P.; Fonseca, L.P.; Oliva, A. Design of Quercetin-Loaded Natural Oil-Based Nanostructured Lipid Carriers for the Treatment of Bacterial Skin Infections. Molecules 2022, 27, 8818. [Google Scholar] [CrossRef]
- Chaudhari, V.S.; Gawali, B.; Saha, P.; Naidu, V.G.M.; Murty, U.S.; Banerjee, S. Quercetin and piperine enriched nanostructured lipid carriers (NLCs) to improve apoptosis in oral squamous cellular carcinoma (FaDu cells) with improved biodistribution profile. Eur. J. Pharmacol. 2021, 909, 174400. [Google Scholar] [CrossRef]
- Sun, M.; Nie, S.; Pan, X.; Zhang, R.; Fan, Z.; Wang, S. Quercetin-nanostructured lipid carriers: Characteristics and anti-breast cancer activities in vitro. Colloids Surf. B Biointerfaces 2014, 113, 15–24. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Kavetskyy, T.; Khosroushahi, A.Y.; Turksoy, V.A.; Khalilov, R. Effects of quercetin loaded nanostructured lipid carriers on the paraquat-induced toxicity in human lymphocytes. Pestic. Biochem. Physiol. 2020, 167, 104586. [Google Scholar] [CrossRef]
- Liu, L.; Tang, Y.; Gao, C.; Li, Y.; Chen, S.; Xiong, T.; Li, J.; Du, M.; Gong, Z.; Chen, H.; et al. Characterization and biodistribution in vivo of quercetin-loaded cationic nanostructured lipid carriers. Colloids Surf. B Biointerfaces 2014, 115, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, V.S.; Murty, U.S.; Banerjee, S. Nanostructured lipid carriers as a strategy for encapsulation of active plant constituents: Formulation and in vitro physicochemical characterizations. Chem. Phys. Lipids 2021, 235, 105037. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Cui, H.; Zhang, F.; Zhao, L.; Liu, Y.; Meng, Q. Combined and targeted drugs delivery system for colorectal cancer treatment: Conatumumab decorated, reactive oxygen species sensitive irinotecan prodrug and quercetin co-loaded nanostructured lipid carriers. Drug Deliv. 2022, 29, 342–350. [Google Scholar] [CrossRef]
- Costa-Fernandez, S.; Matos, J.K.R.; Scheunemann, G.S.; Salata, G.C.; Chorilli, M.; Watanabe, I.S.; de Araujo, G.L.B.; Santos, M.F.; Ishida, K.; Lopes, L.B. Nanostructured lipid carriers containing chitosan or sodium alginate for co-encapsulation of antioxidants and an antimicrobial agent for potential application in wound healing. Int. J. Biol. Macromol. 2021, 183, 668–680. [Google Scholar] [CrossRef]
- Izza, N.; Watanabe, N.; Okamoto, Y.; Wibisono, Y.; Umakoshi, H. Characterization of entrapment behavior of polyphenols in nanostructured lipid carriers and its effect on their antioxidative activity. J. Biosci. Bioeng. 2022, 134, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Iqubal, M.K.; Imtiyaz, K.; Saleem, S.; Mittal, S.; Rizvi, M.M.A.; Ali, J.; Baboota, S. Topical nanostructured lipid carrier gel of quercetin and resveratrol: Formulation, optimization, in vitro and ex vivo study for the treatment of skin cancer. Int. J. Pharm. 2020, 587, 119705. [Google Scholar] [CrossRef]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar]
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Adv. Pharm. Sci. 2018, 2018, 6847971. [Google Scholar] [CrossRef]
- Ag Seleci, D.; Seleci, M.; Walter, J.-G.; Stahl, F.; Scheper, T. Niosomes as Nanoparticular Drug Carriers: Fundamentals and Recent Applications. J. Nanomater. 2016, 2016, 7372306. [Google Scholar] [CrossRef] [Green Version]
- Khoee, S.; Yaghoobian, M. Niosomes: A novel approach in modern drug delivery systems. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 207–237. [Google Scholar]
- Kauslya, A.; Borawake, P.D.; Shinde, J.V.; Chavan, R.S. Niosomes: A Novel Carrier Drug Delivery System. J. Drug Deliv. Ther. 2021, 11, 162–170. [Google Scholar] [CrossRef]
- Sadeghi-Ghadi, Z.; Ebrahimnejad, P.; Talebpour Amiri, F.; Nokhodchi, A. Improved oral delivery of quercetin with hyaluronic acid containing niosomes as a promising formulation. J. Drug Target. 2021, 29, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Tavano, L.; Mauro, L.; Naimo, G.D.; Bruno, L.; Picci, N.; Andò, S.; Muzzalupo, R. Further Evolution of Multifunctional Niosomes Based on Pluronic Surfactant: Dual Active Targeting and Drug Combination Properties. Langmuir 2016, 32, 8926–8933. [Google Scholar] [CrossRef] [PubMed]
- Sayyad, N.; Maji, R.; Omolo, C.A.; Ganai, A.M.; Ibrahim, U.H.; Pathan, T.K.; Devnarain, N.; Karpoormath, R.; Dhawan, S.; Obakachi, V.A.; et al. Development of niosomes for encapsulating captopril-quercetin prodrug to combat hypertension. Int. J. Pharm. 2021, 609, 121191. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, E.; El-Derany, M.O.; Biondo, F.; Tiboni, M.; Casettari, L.; Soliman, M.E. Quercetin Loaded Monolaurate Sugar Esters-Based Niosomes: Sustained Release and Mutual Antioxidant-Hepatoprotective Interplay. Pharmaceutics 2020, 12, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, B.; Huang, Y.; Chen, Z.; Ye, J.; Xu, H.; Chen, W.; Long, X. Niosomal Nanocarriers for Enhanced Skin Delivery of Quercetin with Functions of Anti-Tyrosinase and Antioxidant. Molecules 2019, 24, 2322. [Google Scholar] [CrossRef] [Green Version]
- Javani, R.; Hashemi, F.S.; Ghanbarzadeh, B.; Hamishehkar, H. Quercetin-loaded niosomal nanoparticles prepared by the thin-layer hydration method: Formulation development, colloidal stability, and structural properties. LWT 2021, 141, 110865. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2014, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Tayeb, H.H.; Sainsbury, F. Nanoemulsions in drug delivery: Formulation to medical application. Nanomedicine 2018, 13, 2507–2525. [Google Scholar] [CrossRef]
- Wilson, R.J.; Li, Y.; Yang, G.; Zhao, C.-X. Nanoemulsions for drug delivery. Particuology 2021, 64, 85–97. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Nanoemulsions for health, food, and cosmetics: A review. Env. Chem. Lett. 2021, 19, 3381–3395. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, K.B.; Amin, M.L. Nanoemulsions: Increasing possibilities in drug delivery. Eur. J. Nanomed. 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Yen, C.C.; Chen, Y.C.; Wu, M.T.; Wang, C.C.; Wu, Y.T. Nanoemulsion as a strategy for improving the oral bioavailability and anti-inflammatory activity of andrographolide. Int. J. Nanomed. 2018, 13, 669–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.L.; Stanslas, J.; Basri, M.; Abedi Karjiban, R.A.; Kirby, B.P.; Sani, D.; Basri, H.B. Nanoemulsion-based Parenteral Drug Delivery System of Carbamazepine: Preparation, Characterization, Stability Evaluation and Blood-Brain Pharmacokinetics. Curr. Drug Deliv. 2015, 12, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, K.; Zimmer, A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J. Control. Release 2016, 223, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Oskooei, F.; Mehrzad, J.; Asoodeh, A.; Motavalizadehkakhky, A. Olive oil-based quercetin nanoemulsion (QuNE)’s interactions with human serum proteins (HSA and HTF) and its anticancer activity. J. Biomol. Struct. Dyn. 2023, 41, 778–791. [Google Scholar] [CrossRef]
- Samadi, A.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Eufrasio-da-Silva, T. Ameliorating quercetin constraints in cancer therapy with pH-responsive agarose-polyvinylpyrrolidone -hydroxyapatite nanocomposite encapsulated in double nanoemulsion. Int. J. Biol. Macromol. 2021, 182, 11–25. [Google Scholar] [CrossRef]
- Rahman, M.A.; Mittal, V.; Wahab, S.; Alsayari, A.; Bin Muhsinah, A.; Almaghaslah, D. Intravenous Nanocarrier for Improved Efficacy of Quercetin and Curcumin against Breast Cancer Cells: Development and Comparison of Single and Dual Drug-Loaded Formulations Using Hemolysis, Cytotoxicity and Cellular Uptake Studies. Membranes 2022, 12, 713. [Google Scholar] [CrossRef]
- Arbain, N.H.; Basri, M.; Salim, N.; Wui, W.T.; Abdul Rahman, M.B. Development and Characterization of Aerosol Nanoemulsion System Encapsulating Low Water Soluble Quercetin for Lung Cancer Treatment. Mater. Today Proc. 2018, 5, S137–S142. [Google Scholar] [CrossRef]
- Arbain, N.H.; Salim, N.; Masoumi, H.R.F.; Wong, T.W.; Basri, M.; Abdul Rahman, M.B. In vitro evaluation of the inhalable quercetin loaded nanoemulsion for pulmonary delivery. Drug Deliv. Transl. Res. 2019, 9, 497–507. [Google Scholar] [CrossRef]
- Lotfi, M.; Kazemi, S.; Shirafkan, F.; Hosseinzadeh, R.; Ebrahimpour, A.; Barary, M.; Sio, T.T.; Hosseini, S.M.; Moghadamnia, A.A. The protective effects of quercetin nano-emulsion on intestinal mucositis induced by 5-fluorouracil in mice. Biochem. Biophys. Res. Commun. 2021, 585, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Groo, A.-C.; Iacopetta, D.; Séguy, L.; Mariconda, A.; Puoci, F.; Saturnino, C.; Leroy, F.; Since, M.; Longo, P.; et al. A winning strategy to improve the anticancer properties of Cisplatin and quercetin based on the nanoemulsions formulation. J. Drug Deliv. Sci. Technol. 2021, 66, 102907. [Google Scholar] [CrossRef]
- Papakyriakopoulou, P.; Velidakis, N.; Khattab, E.; Valsami, G.; Korakianitis, I.; Kadoglou, N.P. Potential Pharmaceutical Applications of Quercetin in Cardiovascular Diseases. Pharmaceuticals 2022, 15, 1019. [Google Scholar] [CrossRef] [PubMed]
- Mahadev, M.; Nandini, H.S.; Ramu, R.; Gowda, D.V.; Almarhoon, Z.M.; Al-Ghorbani, M.; Mabkhot, Y.N. Fabrication and Evaluation of Quercetin Nanoemulsion: A Delivery System with Improved Bioavailability and Therapeutic Efficacy in Diabetes Mellitus. Pharmaceuticals 2022, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Son, H.Y.; Lee, M.S.; Chang, E.; Kim, S.Y.; Kang, B.; Ko, H.; Kim, I.H.; Zhong, Q.; Jo, Y.H.; Kim, C.T.; et al. Formulation and Characterization of Quercetin-loaded Oil in Water Nanoemulsion and Evaluation of Hypocholesterolemic Activity in Rats. Nutrients 2019, 11, 244. [Google Scholar] [CrossRef] [Green Version]
- Hussein, J.; El-Naggar, M.E. Synthesis of an environmentally quercetin nanoemulsion to ameliorate diabetic-induced cardiotoxicity. Biocatal. Agric. Biotechnol. 2021, 33, 101983. [Google Scholar] [CrossRef]
- Pangeni, R.; Kang, S.-W.; Oak, M.; Park, E.Y.; Park, J.W. Oral delivery of quercetin in oil-in-water nanoemulsion: In vitro characterization and in vivo anti-obesity efficacy in mice. J. Funct. Foods 2017, 38, 571–581. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Wadhwa, Κ.; Kadian, V.; Puri, V.; Yogeshvar Bhardwaj, B.; Sharma, A.; Pahwa, R.; Rao, R.; Gupta, M.; Singh, I. New insights into quercetin nanoformulations for topical delivery. Phytomedicine Plus 2022, 2, 100257. [Google Scholar] [CrossRef]
- Bennet, D.; Kim, S. A transdermal delivery system to enhance quercetin nanoparticle permeability. J. Biomater. Sci. Polym. Ed. 2013, 24, 185–209. [Google Scholar] [CrossRef]
- Hosny, K.M.; Al Nahyah, K.S.; Alhakamy, N.A. Self-Nanoemulsion Loaded with a Combination of Isotretinoin, an Anti-Acne Drug, and Quercetin: Preparation, Optimization, and In Vivo Assessment. Pharmaceutics 2020, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Amanzadeh, E.; Esmaeili, A.; Rahgozar, S.; Nourbakhshnia, M. Application of quercetin in neurological disorders: From nutrition to nanomedicine. Rev. Neurosci. 2019, 30, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential Implications of Quercetin in Autoimmune Diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zeng, Y.; Peng, W.; Xie, X.; Yang, Y.; Ji, B.; Li, F. Pharmacological Aspects of Natural Quercetin in Rheumatoid Arthritis. Drug Des. Dev. Ther. 2022, 16, 2043–2053. [Google Scholar] [CrossRef] [PubMed]
- Alaqeel, N.K.; AlSheikh, M.H.; Al-Hariri, M.T. Quercetin Nanoemulsion Ameliorates Neuronal Dysfunction in Experimental Alzheimer’s Disease Model. Antioxidants 2022, 11, 1986. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, J.P.; Mahajan, H.S.; Surana, S.S. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: In vivo and in vitro studies. Biomed. Pharmacother. 2019, 112, 108622. [Google Scholar] [CrossRef] [PubMed]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin Based Nanoparticles for Drug Delivery and Theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef] [Green Version]
- Aiassa, V.; Garnero, C.; Longhi, M.R.; Zoppi, A. Cyclodextrin Multicomponent Complexes: Pharmaceutical Applications. Pharmaceutics 2021, 13, 1099. [Google Scholar] [CrossRef]
- Shelley, H.; Babu, R.J. Role of Cyclodextrins in Nanoparticle-Based Drug Delivery Systems. J. Pharm. Sci. 2018, 107, 1741–1753. [Google Scholar] [CrossRef]
- Ghafelehbashi, R.; Yaraki, M.T.; Saremi, L.H.; Lajevardi, A.; Haratian, M.; Astinchap, B.; Rashidi, A.M.; Moradian, R. A pH-responsive citric-acid/α-cyclodextrin-functionalized Fe3O4 nanoparticles as a nanocarrier for quercetin: An experimental and DFT study. Mater. Sci. Eng. C 2019, 109, 110597. [Google Scholar] [CrossRef] [PubMed]
- Abdul Karim, A.; Chee, P.L.; Chan, M.F.; Loh, X.J. Micellized α-Cyclodextrin-Based Supramolecular Hydrogel Exhibiting pH-Responsive Sustained Release and Corresponding Oscillatory Shear Behavior Analysis. ACS Biomater. Sci. Eng. 2016, 2, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Thanh Nguyen, H.; Goycoolea, F.M. Chitosan/Cyclodextrin/TPP Nanoparticles Loaded with Quercetin as Novel Bacterial Quorum Sensing Inhibitors. Molecules 2017, 22, 1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Zou, Y.; Song, L.; Han, S.; Yang, H.; Chu, D.; Dai, Y.; Ma, J.; O’Driscoll, C.M.; Yu, Z.; et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm. Sin. B 2021, 12, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Peñalva, R.; Esparza, I.; Morales-Gracia, J.; González-Navarro, C.J.; Larrañeta, E.; Irache, J.M. Casein nanoparticles in combination with 2-hydroxypropyl-β-cyclodextrin improves the oral bioavailability of quercetin. Int. J. Pharm. 2019, 570, 118652. [Google Scholar] [CrossRef] [PubMed]
- Nasirzadeh, K.; Nazarian, S.; Hayat, S.M.G. Inorganic nanomaterials: A brief overview of the applications and developments in sensing and drug delivery. Appl. Biotechnol. Rep. 2016, 3, 395–402. [Google Scholar]
- Asad, S.; Jacobsen, A.C.; Teleki, A. Inorganic nanoparticles for oral drug delivery: Opportunities, barriers, and future perspectives. Curr. Opin. Chem. Eng. 2022, 38, 100869. [Google Scholar] [CrossRef]
- Dechsupa, N.; Kosintarajit, P.; Kamkan, K.; Khanjina, T.; Sirikul, C.; Innuan, P.; Suwan, A.; Anukul, N.; Kantapan, J. Iron(III)-Quercetin Complexes’ Safety for MRI Cell Tracking in Cell Therapy Applications: Cytotoxic and Genotoxic Assessment. Nanomaterials 2022, 12, 2776. [Google Scholar] [CrossRef]
- Renner, A.M.; Ilyas, S.; Schlößer, H.A.; Szymura, A.; Roitsch, S.; Wennhold, K.; Langmuir, S.M. Receptor-Mediated In Vivo Targeting of Breast Cancer Cells with 17α-Ethynylestradiol-Conjugated Silica-Coated Gold Nanoparticles. Langmuir 2020, 36, 14819–14828. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Aminianfar, A.; Asemi, Z.; Yousefi, B. Application of nanoparticles for efficient delivery of quercetin in cancer cells. Curr. Med. Chem. 2023. [Google Scholar] [CrossRef]
- Dini, S.; Zakeri, M.; Ebrahimpour, S.; Dehghanian, F.; Esmaeili, A. Quercetin-conjugated superparamagnetic iron oxide nanoparticles modulate glucose metabolism-related genes and miR-29 family in the hippocampus of diabetic rats. Sci. Rep. 2021, 11, 8618. [Google Scholar] [CrossRef]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Mukherjee, S.; Das, S.; Bhat, F.A.; Raja Singh, P.; Patra, C.R.; Arunakaran, J. Gold nanoparticles-conjugated quercetin induces apoptosis via inhibition of EGFR/PI3K/Akt-mediated pathway in breast cancer cell lines (MCF-7 and MDA-MB-231). Cell. Biochem. Funct. 2017, 35, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, P.; Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.M.; Sherif, N.M.; Hassan, N.S.; Althobaiti, F.; Hanafy, N.A.N.; Sahyon, H.A. Novel quercetin encapsulated chitosan functionalized copper oxide nanoparticles as anti-breast cancer agent via regulating p53 in rat model. Int. J. Biol. Macromol. 2021, 185, 134–152. [Google Scholar] [CrossRef]
- Ramalingam, V.; Muthukumar Sathya, P.; Srivalli, T.; Mohan, H. Synthesis of quercetin functionalized wurtzite type zinc oxide nanoparticles and their potential to regulate intrinsic apoptosis signaling pathway in human metastatic ovarian cancer. Life Sci. 2022, 309, 121022. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhou, L.; Zhang, Q.; Liu, X.; Wei, X.; Li, Y. Inorganic Nanomaterial for Biomedical Imaging of Brain Diseases. Molecules 2021, 26, 7340. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neuronanomedicine: An Up-to-Date Overview. Pharmaceutics 2019, 11, 101. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimpour, S.; Esmaeili, A.; Beheshti, S. Effect of quercetin-conjugated superparamagnetic iron oxide nanoparticles on diabetes-induced learning and memory impairment in rats. Int. J. Nanomed. 2018, 13, 6311–6324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanzadeh, E.; Esmaeili, A.; Abadi, R.E.N.; Kazemipour, N.; Pahlevanneshan, Z.; Beheshti, S. Quercetin conjugated with superparamagnetic iron oxide nanoparticles improves learning and memory better than free quercetin via interacting with proteins involved in LTP. Sci. Rep. 2019, 9, 6876. [Google Scholar] [CrossRef] [Green Version]
- Bardestani, A.; Ebrahimpour, S.; Esmaeili, A.; Esmaeili, A. Quercetin attenuates neurotoxicity induced by iron oxide nanoparticles. J. Nanobiotechnol. 2021, 19, 327. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, H.; Yin, T.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer’s disease. J. Colloid Interface Sci. 2019, 552, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Lenders, V.; Koutsoumpou, X.; Sargsian, A.; Manshian, B.B. Biomedical nanomaterials for immunological applications: Ongoing research and clinical trials. Nanoscale Adv. 2020, 2, 5046. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomed. Pharmacother. 2019, 109, 2561–2572. [Google Scholar] [CrossRef]
- Alemzadeh, E.; Karamian, M.; Abedi, F.; Hanafi-Bojd, M.Y. Topical treatment of cutaneous leishmaniasis lesions using quercetin/ Artemisia-capped silver nanoparticles ointment: Modulation of inflammatory response. Acta Trop. 2022, 228, 106325. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, M.; Madhyastha, H.; Singh, M.; Revaprasadu, N.; Srinivas, S.P.; Daima, H.K. Double functionalized haemocompatible silver nanoparticles control cell inflammatory homeostasis. PLoS ONE 2022, 17, e0276296. [Google Scholar] [CrossRef]
- Madhyastha, H.; Halder, S.; Queen Intan, N.; Madhyastha, R.; Mohanapriya, A.; Sudhakaran, R.; Sajitha, L.S.; Banerjee, K.; Bethasiwi, P.; Daima, H.; et al. Surface refined AuQuercetin nanoconjugate stimulates dermal cell migration: Possible implication in wound healing. RSC Adv. 2020, 10, 37683–37694. [Google Scholar]
- Badhwar, R.; Mangla, B.; Neupane, Y.R.; Khanna, K.; Popli, H. Quercetin loaded silver nanoparticles in hydrogel matrices for diabetic wound healing. Nanotechnology 2021, 32, 505102. [Google Scholar] [CrossRef]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Zarrabi, A.; Rahgozar, S. The antitoxic effects of quercetin and quercetin-conjugated iron oxide nanoparticles (QNPs) against H2O2-induced toxicity in PC12 cells. Int. J. Nanomed. 2019, 14, 6813–6830. [Google Scholar] [CrossRef] [Green Version]







| Polymer-Based Nanosystems | Lipid-Based Nanosystems | Surfactant-Based Nanosystems | Cyclodextrin-Based Nanoparticles | Inorganic Nanoparticles | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Polymeric Micelles | Polymeric Nanoparticles | Hydrogels | Polymersomes | Liposomes | SLN-NLC | Niosomes | Nanoemulsions | |||
| Advantages | ||||||||||
| Biocompatibility | ++ | ++ | ++ | ++ | +++ | +++ | ++ | +++ | +++ | + |
| Biodegradability | + | ++ | +++ | ++ | ++ | ++ | ++ | ++ | +++ | + |
| High Loading efficiency | ++ | ++ | +++ | +++ | ++ | +++ | ++ | +++ | ++ | + |
| Chemical versatility | +++ | +++ | + | +++ | + | + | + | + | + | + |
| Physicochemical stability | +++ | +++ | +++ | +++ | + | ++ | ++ | ++ | ++ | +++ |
| Controlled release properties | +++ | +++ | ++ | +++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Administration by different routes | ++ | ++ | + | ++ | +++ | ++ | ++ | ++ | ++ | ++ |
| Stimuli responsiveness | +++ | ++ | +++ | ++ | + | + | + | + | + | +++ |
| Improve of ADME(T) profile | +++ | +++ | + | ++ | ++ | ++ | ++ | ++ | ++ | + |
| Targeting | ++ | ++ | + | + | + | + | + | + | + | +++ |
| Imaging | + | + | + | + | + | + | + | + | + | +++ |
| Theragnostic | + | + | + | + | + | + | + | + | + | +++ |
| Precision medicine | + | + | + | + | +++ | + | + | + | + | ++ |
| Disadvantages | ||||||||||
| Nanotoxicity | ++ | ++ | + | ++ | ++ | ++ | ++ | + | + | +++ |
| High cost | + | + | + | ++ | ++ | ++ | ++ | + | + | +++ |
| Limitations in scale-up | + | + | +++ | ++ | ++ | ++ | ++ | + | ++ | +++ |
| Immunogenicity | ++ | ++ | + | ++ | + | + | + | + | + | +++ |
| Lack in regulatory framework | ++ | ++ | +++ | +++ | ++ | ++ | +++ | + | +++ | ++ |
| Polymer-Based Nanosystems | Lipid-Based Nanosystems | Surfactant-Based Nanosystems | Cyclodextrin-Based Nanoparticles | Inorganic Nanoparticles | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Properties of QUE | Polymeric Micelles | Polymeric Nanoparticles | Hydrogels | Polymersomes | Liposomes | SLN-NLC | Niosomes | Nanoemulsions | ||
| Improved solubility | x | x | x | x | x | x | x | x | x | |
| Improved EE | x | x | x | |||||||
| Co-loading with other APIs | x | x | x | x | ||||||
| Increased bioavailability | x | x | x | x | x | |||||
| Sustained release | x | x | ||||||||
| Controlled release | x | x | x | x | ||||||
| pH-responsive release | x | |||||||||
| Increased stability | x | x | ||||||||
| Targeting to tumors | x | x | x | x | x | |||||
| Targeting to brain | x | |||||||||
| Transdermal administration | x | x | ||||||||
| Facilitation of the uptake into cells | x | x | x | |||||||
| Improved anti-inflammatory properties | x | x | x | x | x | x | ||||
| Enhanced antioxidant activity | x | x | x | x | x | x | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomou, E.-M.; Papakyriakopoulou, P.; Saitani, E.-M.; Valsami, G.; Pippa, N.; Skaltsa, H. Recent Advances in Nanoformulations for Quercetin Delivery. Pharmaceutics 2023, 15, 1656. https://doi.org/10.3390/pharmaceutics15061656
Tomou E-M, Papakyriakopoulou P, Saitani E-M, Valsami G, Pippa N, Skaltsa H. Recent Advances in Nanoformulations for Quercetin Delivery. Pharmaceutics. 2023; 15(6):1656. https://doi.org/10.3390/pharmaceutics15061656
Chicago/Turabian StyleTomou, Ekaterina-Michaela, Paraskevi Papakyriakopoulou, Elmina-Marina Saitani, Georgia Valsami, Natassa Pippa, and Helen Skaltsa. 2023. "Recent Advances in Nanoformulations for Quercetin Delivery" Pharmaceutics 15, no. 6: 1656. https://doi.org/10.3390/pharmaceutics15061656