Critical Review in Designing Plant-Based Anticancer Nanoparticles against Hepatocellular Carcinoma
Abstract
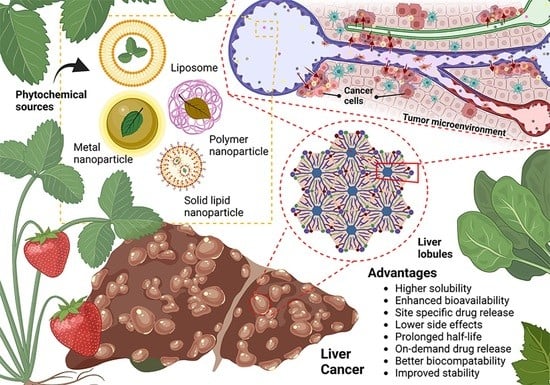
:1. Introduction
2. Tumor Biology: HCC and Current Limitations of Drug Delivery Design


3. Molecular Mechanisms of Plant Bioactives
3.1. Apoptosis Induction
3.2. Oncogene Inhibition and Tumor-Suppression Gene Expression
3.3. Cell Cycle Arrest
3.4. Antioxidant Effects
3.5. Anti-Angiogenesis
3.6. Interference in Cell Signaling Pathways
4. Current Nanoparticle-Based Delivery Systems for Plant Bioactives in HCC Therapy
4.1. Liposomes and Their Derivatives
4.2. Solid Lipid Nanoparticles
4.3. Polymer-Based Nanoparticles
4.4. Metallic-Based Nanoparticles
5. Current Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, M.; Chen, S. Comparison of Surgical Resection and Transcatheter Arterial Chemoembolization for Large Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Ann. Hepatol. 2023, 28, 100890. [Google Scholar] [CrossRef] [PubMed]
- De Minicis, S.; Marzioni, M.; Benedetti, A.; Svegliati-Baroni, G. New Insights in Hepatocellular Carcinoma: From Bench to Bedside. Ann. Transl. Med. 2013, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.; Hyman, D. Hepatocellular Cancer: A Guide for the Internist. Am. J. Med. 2007, 120, 194–202. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Siegel, A.B.; Zhu, A.X. Metabolic Syndrome and Hepatocellular Carcinoma. Cancer 2009, 115, 5651–5661. [Google Scholar] [CrossRef]
- Fan, J.-G.; Farrell, G.C. Prevention of Hepatocellular Carcinoma in Nonviral-Related Liver Diseases. J. Gastroenterol. Hepatol. 2009, 24, 712–719. [Google Scholar] [CrossRef]
- Ogunwobi, O.O.; Harricharran, T.; Huaman, J.; Galuza, A.; Odumuwagun, O.; Tan, Y.; Ma, G.X.; Nguyen, M.T. Mechanisms of Hepatocellular Carcinoma Progression. World J. Gastroenterol. 2019, 25, 2279–2293. [Google Scholar] [CrossRef]
- Kudo, M. Early Hepatocellular Carcinoma: Definition and Diagnosis. Liver Cancer 2013, 2, 69–72. [Google Scholar] [CrossRef]
- Sacco, R.; Tapete, G.; Simonetti, N.; Sellitri, R.; Natali, V.; Melissari, S.; Cabibbo, G.; Biscaglia, L.; Bresci, G.; Giacomelli, L. Transarterial Chemoembolization for the Treatment of Hepatocellular Carcinoma: A Review. J. Hepatocell. Carcinoma 2017, 4, 105–110. [Google Scholar] [CrossRef]
- Lang, L. FDA Approves Sorafenib for Patients with Inoperable Liver Cancer. Gastroenterology 2008, 134, 379. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.-C.; Galle, P.R.; Marquardt, J.U. The Role of Molecular Enrichment on Future Therapies in Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 237–247. [Google Scholar] [CrossRef]
- Dyhl-Polk, A.; Mikkelsen, M.K.; Ladekarl, M.; Nielsen, D.L. Clinical Trials of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma. J. Clin. Med. 2021, 10, 2662. [Google Scholar] [CrossRef]
- Luo, X.-Y.; Wu, K.-M.; He, X.-X. Advances in Drug Development for Hepatocellular Carcinoma: Clinical Trials and Potential Therapeutic Targets. J. Exp. Clin. Cancer Res. 2021, 40, 172. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Dadduzio, V.; Ricci, A.D.; Massari, F.; Di Federico, A.; Gadaleta-Caldarola, G.; Brandi, G. Lenvatinib plus Pembrolizumab: The next Frontier for the Treatment of Hepatocellular Carcinoma? Expert Opin. Investig. Drugs 2022, 31, 371–378. [Google Scholar] [CrossRef]
- Wege, H.; Li, J.; Ittrich, H. Treatment Lines in Hepatocellular Carcinoma. Visc. Med. 2019, 35, 266–272. [Google Scholar] [CrossRef]
- Tella, S.H.; Kommalapati, A.; Mahipal, A.; Jin, Z. First-Line Targeted Therapy for Hepatocellular Carcinoma: Role of Atezolizumab/Bevacizumab Combination. Biomedicines 2022, 10, 1304. [Google Scholar] [CrossRef]
- Llovet, J.M.; Di Bisceglie, A.M.; Bruix, J.; Kramer, B.S.; Lencioni, R.; Zhu, A.X.; Sherman, M.; Schwartz, M.; Lotze, M.; Talwalkar, J.; et al. Design and Endpoints of Clinical Trials in Hepatocellular Carcinoma. JNCI J. Natl. Cancer Inst. 2008, 100, 698–711. [Google Scholar] [CrossRef]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.-Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.-H.; Tak, W.Y.; et al. Adjuvant Sorafenib for Hepatocellular Carcinoma after Resection or Ablation (STORM): A Phase 3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Liu, Y.; Wang, J.; Liu, L. Adverse Effects of Immune-Checkpoint Inhibitors in Hepatocellular Carcinoma. OncoTargets Ther. 2020, 13, 11725–11740. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.-E.F.; Abdelfatah, S.; Hamed, A.R.; Mohamed, T.A.; Elshamy, A.A.; Saleh, I.A.; Reda, E.H.; Abdel-Azim, N.S.; Shams, K.A.; Sakr, M.; et al. Cytotoxicity of 40 Egyptian Plant Extracts Targeting Mechanisms of Drug-Resistant Cancer Cells. Phytomedicine 2019, 59, 152771. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Liu, M.; Xiang, X.; Li, Y.; Xi, Z.; Xu, H. OMICS Applications for Medicinal Plants in Gastrointestinal Cancers: Current Advancements and Future Perspectives. Front. Pharmacol. 2022, 13, 842203. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in Nanoparticle and Microparticle Delivery Systems for Increasing the Dispersibility, Stability, and Bioactivity of Phytochemicals. Biotechnol. Adv. 2020, 38, 107287. [Google Scholar] [CrossRef]
- Kumar, P.; Yadav, N.; Chaudhary, B.; Jain, V.; Balaramnavar, V.M.; Alharbi, K.S.; Alenezi, S.K.; Al-Malki, W.H.; Ghoneim, M.M.; Alshehri, S.; et al. Promises of Phytochemical Based Nano Drug Delivery Systems in the Management of Cancer. Chem. Biol. Interact. 2022, 351, 109745. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, M.-J.; Gao, B. Hepatocytes: A Key Cell Type for Innate Immunity. Cell. Mol. Immunol. 2016, 13, 301–315. [Google Scholar] [CrossRef]
- Cabrera, R.; Nelson, D.R. Review Article: The Management of Hepatocellular Carcinoma. Aliment. Pharmacol. Ther. 2010, 31, 461–476. [Google Scholar] [CrossRef]
- Hu, M.; Wang, Y.; Xu, L.; An, S.; Tang, Y.; Zhou, X.; Li, J.; Liu, R.; Huang, L. Relaxin Gene Delivery Mitigates Liver Metastasis and Synergizes with Check Point Therapy. Nat. Commun. 2019, 10, 2993. [Google Scholar] [CrossRef]
- Schulze, K.; Imbeaud, S.; Letouzé, E.; Alexandrov, L.B.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Meiller, C.; Shinde, J.; Soysouvanh, F.; et al. Exome Sequencing of Hepatocellular Carcinomas Identifies New Mutational Signatures and Potential Therapeutic Targets. Nat. Genet. 2015, 47, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, A.; Zhao, Y.; Ying, W.; Sun, H.; Yang, X.; Xing, B.; Sun, W.; Ren, L.; Hu, B.; et al. Proteomics Identifies New Therapeutic Targets of Early-Stage Hepatocellular Carcinoma. Nature 2019, 567, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jiang, L.; Liang, L.; Koral, K.; Zhang, Q.; Zhao, L.; Lu, S.; Tao, J. The Role of Fibroblast Growth Factor 19 in Hepatocellular Carcinoma. Am. J. Pathol. 2021, 191, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, C.; Zhai, Y.; Cai, Y.; Lee, R.J.; Xing, J.; Wang, H.; Zhu, H.H.; Teng, L.; Li, Y.; et al. High-Density Lipoprotein Modulates Tumor-Associated Macrophage for Chemoimmunotherapy of Hepatocellular Carcinoma. Nano Today 2021, 37, 101064. [Google Scholar] [CrossRef]
- Cai, H.; Yang, Y.; Peng, F.; Liu, Y.; Fu, X.; Ji, B. Gold Nanoparticles-Loaded Anti-MiR221 Enhances Antitumor Effect of Sorafenib in Hepatocellular Carcinoma Cells. Int. J. Med. Sci. 2019, 16, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kim, S.; Kim, K.M.; Choi, E.K.; Kim, J.; Seo, H.R. Activated Hepatic Stellate Cells Play Pivotal Roles in Hepatocellular Carcinoma Cell Chemoresistance and Migration in Multicellular Tumor Spheroids. Sci. Rep. 2016, 6, 36750. [Google Scholar] [CrossRef]
- Khan, A.R.; Yang, X.; Du, X.; Yang, H.; Liu, Y.; Khan, A.Q.; Zhai, G. Chondroitin Sulfate Derived Theranostic and Therapeutic Nanocarriers for Tumor-Targeted Drug Delivery. Carbohydr. Polym. 2020, 233, 115837. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, N.; Xu, W.; Ling, G.; Zhang, P. Potential Therapies and Diagnosis Based on Golgi-Targeted Nano Drug Delivery Systems. Pharmacol. Res. 2022, 175, 105861. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Zhang, A.; Miao, K.; Sun, H.; Deng, C.-X. Tumor Heterogeneity Reshapes the Tumor Microenvironment to Influence Drug Resistance. Int. J. Biol. Sci. 2022, 18, 3019–3033. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.J.; von Felden, J.; Garcia-Lezana, T.; Sarcognato, S.; Villanueva, A. Tumour Evolution in Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-C.; Hsu, C.-H.; Hsu, C.; Cheng, A.-L. Tumor Heterogeneity in Hepatocellular Carcinoma: Facing the Challenges. Liver Cancer 2016, 5, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour Heterogeneity and Resistance to Cancer Therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- D’Souza, A.A.; Devarajan, P.V. Asialoglycoprotein Receptor Mediated Hepatocyte Targeting—Strategies and Applications. J. Control. Release 2015, 203, 126–139. [Google Scholar] [CrossRef]
- Calzolari, A.; Oliviero, I.; Deaglio, S.; Mariani, G.; Biffoni, M.; Sposi, N.M.; Malavasi, F.; Peschle, C.; Testa, U. Transferrin Receptor 2 Is Frequently Expressed in Human Cancer Cell Lines. Blood Cells Mol. Dis. 2007, 39, 82–91. [Google Scholar] [CrossRef]
- Pang, H.-B.; Braun, G.B.; Ruoslahti, E. Neuropilin-1 and Heparan Sulfate Proteoglycans Cooperate in Cellular Uptake of Nanoparticles Functionalized by Cationic Cell-Penetrating Peptides. Sci. Adv. 2015, 1, e1500821. [Google Scholar] [CrossRef]
- Ahmed, M.; Narain, R. Carbohydrate-Based Materials for Targeted Delivery of Drugs and Genes to the Liver. Nanomedicine 2015, 10, 2263–2288. [Google Scholar] [CrossRef]
- Böttger, R.; Pauli, G.; Chao, P.-H.; AL Fayez, N.; Hohenwarter, L.; Li, S.-D. Lipid-Based Nanoparticle Technologies for Liver Targeting. Adv. Drug Deliv. Rev. 2020, 154–155, 79–101. [Google Scholar] [CrossRef]
- Wang, H.; Thorling, C.A.; Liang, X.; Bridle, K.R.; Grice, J.E.; Zhu, Y.; Crawford, D.H.G.; Xu, Z.P.; Liu, X.; Roberts, M.S. Diagnostic Imaging and Therapeutic Application of Nanoparticles Targeting the Liver. J. Mater. Chem. B 2015, 3, 939–958. [Google Scholar] [CrossRef]
- Marquez, J.; Fernandez-Piñeiro, I.; Araúzo-Bravo, M.J.; Poschmann, G.; Stühler, K.; Khatib, A.-M.; Sanchez, A.; Unda, F.; Ibarretxe, G.; Bernales, I.; et al. Targeting Liver Sinusoidal Endothelial Cells with MiR-20a-Loaded Nanoparticles Reduces Murine Colon Cancer Metastasis to the Liver. Int. J. Cancer 2018, 143, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Arechederra, M.; Bazai, S.K.; Abdouni, A.; Sequera, C.; Mead, T.J.; Richelme, S.; Daian, F.; Audebert, S.; Dono, R.; Lozano, A.; et al. ADAMTSL5 Is an Epigenetically Activated Gene Underlying Tumorigenesis and Drug Resistance in Hepatocellular Carcinoma. J. Hepatol. 2021, 74, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barrena, M.G.; Arechederra, M.; Colyn, L.; Berasain, C.; Avila, M.A. Epigenetics in Hepatocellular Carcinoma Development and Therapy: The Tip of the Iceberg. JHEP Rep. 2020, 2, 100167. [Google Scholar] [CrossRef]
- Tacke, F. Targeting Hepatic Macrophages to Treat Liver Diseases. J. Hepatol. 2017, 66, 1300–1312. [Google Scholar] [CrossRef]
- Kaps, L.; Schuppan, D. Targeting Cancer Associated Fibroblasts in Liver Fibrosis and Liver Cancer Using Nanocarriers. Cells 2020, 9, 2027. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Gavini, E.; Rassu, G.; Maestri, M.; Giunchedi, P. Chitosan Nanoparticles for Therapy and Theranostics of Hepatocellular Carcinoma (HCC) and Liver-Targeting. Nanomaterials 2020, 10, 870. [Google Scholar] [CrossRef]
- Zein, R.; Sharrouf, W.; Selting, K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J. Oncol. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.-D. Factors Controlling the Pharmacokinetics, Biodistribution and Intratumoral Penetration of Nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Günday Türeli, N. Key for Crossing the BBB with Nanoparticles: The Rational Design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef]
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A Critical Review of the Use of Surfactant-Coated Nanoparticles in Nanomedicine and Food Nanotechnology. Int. J. Nanomed. 2021, 16, 3937–3999. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Aldalaen, S.M.; Faisal, W.; Tawfeek, H.M. Somatostatin Receptors as a New Active Targeting Sites for Nanoparticles. Saudi Pharm. J. 2018, 26, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Kira, S.; Nakanishi, T.; Suemori, S.; Kitamoto, M.; Watanabe, Y.; Kajiyama, G. Expression of Transforming Growth Factor Alpha and Epidermal Growth Factor Receptor in Human Hepatocellular Carcinoma. Liver 2008, 17, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Scoble, J.A.; Li, N.; Lovrecz, G.; Waddington, L.J.; Tran, N.; Muir, B.W.; Coia, G.; Kirby, N.; Drummond, C.J.; et al. Epidermal Growth Factor Receptor-Targeted Lipid Nanoparticles Retain Self-Assembled Nanostructures and Provide High Specificity. Nanoscale 2015, 7, 2905–2913. [Google Scholar] [CrossRef]
- Da Silva Santos, E.; Nogueira, K.A.B.; Fernandes, L.C.C.; Martins, J.R.P.; Reis, A.V.F.; Neto, J.D.B.V.; da Silva Júnior, I.J.; Pessoa, C.; Petrilli, R.; Eloy, J.O. EGFR Targeting for Cancer Therapy: Pharmacology and Immunoconjugates with Drugs and Nanoparticles. Int. J. Pharm. 2021, 592, 120082. [Google Scholar] [CrossRef]
- Singh, V.K.; Chau, E.; Mishra, A.; DeAnda, A.; Hegde, V.L.; Sastry, J.K.; Haviland, D.; Jagannath, C.; Godin, B.; Khan, A. CD44 Receptor Targeted Nanoparticles Augment Immunity against Tuberculosis in Mice. J. Control. Release 2022, 349, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Su, W.; Liu, Z.; Zhou, M.; Chen, S.; Chen, Y.; Lu, D.; Liu, Y.; Fan, Y.; Zheng, Y.; et al. CD44 Antibody-Targeted Liposomal Nanoparticles for Molecular Imaging and Therapy of Hepatocellular Carcinoma. Biomaterials 2012, 33, 5107–5114. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Y.; Zhang, D.; Wu, M.; Wu, L.; Huang, A.; Yang, H.; Liu, X.; Liu, J. Glypican-3 Antibody Functionalized Prussian Blue Nanoparticles for Targeted MR Imaging and Photothermal Therapy of Hepatocellular Carcinoma. J. Mater. Chem. B 2014, 2, 3686–3696. [Google Scholar] [CrossRef]
- Gong, T.; Wang, X.; Ma, Q.; Li, J.; Li, M.; Huang, Y.; Liang, W.; Su, D.; Guo, R. Triformyl Cholic Acid and Folic Acid Functionalized Magnetic Graphene Oxide Nanocomposites: Multiple-Targeted Dual-Modal Synergistic Chemotherapy/Photothermal Therapy for Liver Cancer. J. Inorg. Biochem. 2021, 223, 111558. [Google Scholar] [CrossRef]
- Wei, Y.; Gu, X.; Cheng, L.; Meng, F.; Storm, G.; Zhong, Z. Low-Toxicity Transferrin-Guided Polymersomal Doxorubicin for Potent Chemotherapy of Orthotopic Hepatocellular Carcinoma in Vivo. Acta Biomater. 2019, 92, 196–204. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wu, X.; Gao, Y.; Zhang, J.; Zhang, D.; Gu, S.; Zhu, G.; Liu, G. Aptamer-Functionalized Peptide H3CR5C as a Novel Nanovehicle for Codelivery of Fasudil and MiRNA-195 Targeting Hepatocellular Carcinoma. Int. J. Nanomed. 2016, 11, 3891–3905. [Google Scholar] [CrossRef]
- Lou, S.; Gao, S.; Wang, W.; Zhang, M.; Zhang, J.; Wang, C.; Li, C.; Kong, D.; Zhao, Q. Galactose-Functionalized Multi-Responsive Nanogels for Hepatoma-Targeted Drug Delivery. Nanoscale 2015, 7, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Biscaglia, F.; Quarta, S.; Villano, G.; Turato, C.; Biasiolo, A.; Litti, L.; Ruzzene, M.; Meneghetti, M.; Pontisso, P.; Gobbo, M. PreS1 Peptide-Functionalized Gold Nanostructures with SERRS Tags for Efficient Liver Cancer Cell Targeting. Mater. Sci. Eng. C 2019, 103, 109762. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Dawson, K.A. Protein-Nanoparticle Interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Yang, S.; Cai, C.; Wang, H.; Ma, X.; Shao, A.; Sheng, J.; Yu, C. Drug Delivery Strategy in Hepatocellular Carcinoma Therapy. Cell Commun. Signal. 2022, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Chen, H.; Liu, R.H. Potential Mechanisms of Action of Dietary Phytochemicals for Cancer Prevention by Targeting Cellular Signaling Transduction Pathways. J. Agric. Food Chem. 2018, 66, 3260–3276. [Google Scholar] [CrossRef]
- Mondal, A.; Guria, T.; Maity, T.; Bishayee, A. A Novel Tetraenoic Fatty Acid Isolated from Amaranthus Spinosus Inhibits Proliferation and Induces Apoptosis of Human Liver Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1604. [Google Scholar] [CrossRef]
- Malik, Z.; Parveen, R.; Parveen, B.; Zahiruddin, S.; Aasif Khan, M.; Khan, A.; Massey, S.; Ahmad, S.; Husain, S.A. Anticancer Potential of Andrographolide from Andrographis Paniculata (Burm.f.) Nees and Its Mechanisms of Action. J. Ethnopharmacol. 2021, 272, 113936. [Google Scholar] [CrossRef]
- Chowdhury, K.D.; Sarkar, A.; Chatterjee, S.; Patra, D.; Sengupta, D.; Banerjee, S.; Chakraborty, P.; Sadhukhan, G.C. Cathepsin B Mediated Scramblase Activation Triggers Cytotoxicity and Cell Cycle Arrest by Andrographolide to Overcome Cellular Resistance in Cisplatin Resistant Human Hepatocellular Carcinoma HepG2 Cells. Environ. Toxicol. Pharmacol. 2019, 68, 120–132. [Google Scholar] [CrossRef]
- Dasgupta, A.; Dey, D.; Ghosh, D.; Lai, T.K.; Bhuvanesh, N.; Dolui, S.; Velayutham, R.; Acharya, K. Astrakurkurone, a Sesquiterpenoid from Wild Edible Mushroom, Targets Liver Cancer Cells by Modulating Bcl-2 Family Proteins. IUBMB Life 2019, 71, 992–1002. [Google Scholar] [CrossRef]
- Chu, Y.-L.; Ho, C.-T.; Chung, J.-G.; Raghu, R.; Lo, Y.-C.; Sheen, L.-Y. Allicin Induces Anti-Human Liver Cancer Cells through the P53 Gene Modulating Apoptosis and Autophagy. J. Agric. Food Chem. 2013, 61, 9839–9848. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Ye, W.; Chen, Y.; Wu, S.; Jin, L.; He, J.; Tao, X.; Zhu, J.; Chen, X.; Deng, A.; et al. Ardipusilloside Inhibits Survival, Invasion and Metastasis of Human Hepatocellular Carcinoma Cells. Phytomedicine 2012, 19, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Rumman, M.; Yan, H.; Cheon, M.J.; Choi, J.G.; Jin, X.; Park, S.; Oh, M.S.; Hong, S.-S. An Ethyl Acetate Fraction of Artemisia Capillaris (ACE-63) Induced Apoptosis and Anti-Angiogenesis via Inhibition of PI3K/AKT Signaling in Hepatocellular Carcinoma. Phyther. Res. 2018, 32, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, M.; Zhang, Z.; Liu, N.; Han, X.; Liu, Q.; Deng, W.; Liao, C. Induction of Apoptosis by Berberine in Hepatocellular Carcinoma HepG2 Cells via Downregulation of NF-ΚB. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Tong, N.; Zhang, J.; Chen, Y.; Li, Z.; Luo, Y.; Zuo, H.; Zhao, X. Berberine Sensitizes Mutliple Human Cancer Cells to the Anticancer Effects of Doxorubicin in Vitro. Oncol. Lett. 2012, 3, 1263–1267. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Wang, X.; Fang, X.; He, K.; Guo, X.; Zhan, Z.; Sun, C.; Jin, Y.-H. Co-Treatment with Ginsenoside Rh2 and Betulinic Acid Synergistically Induces Apoptosis in Human Cancer Cells in Association with Enhanced Capsase-8 Activation, Bax Translocation, and Cytochrome c Release. Mol. Carcinog. 2011, 50, 760–769. [Google Scholar] [CrossRef]
- Liu, W.; Li, S.; Qu, Z.; Luo, Y.; Chen, R.; Wei, S.; Yang, X.; Wang, Q. Betulinic Acid Induces Autophagy-Mediated Apoptosis through Suppression of the PI3K/AKT/MTOR Signaling Pathway and Inhibits Hepatocellular Carcinoma. Am. J. Transl. Res. 2019, 11, 6952. [Google Scholar]
- Noureini, S.K.; Wink, M. Dose-Dependent Cytotoxic Effects of Boldine in HepG-2 Cells—Telomerase Inhibition and Apoptosis Induction. Molecules 2015, 20, 3730–3743. [Google Scholar] [CrossRef]
- Subramaniam, N.; Kannan, P.; Ashokkumar, K.; Thiruvengadam, D. Hepatoprotective Effect of Boldine against Diethylnitrosamine-induced Hepatocarcinogenesis in Wistar Rats. J. Biochem. Mol. Toxicol. 2019, 33, e22404. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, C.; Wang, X.; Lang, Y.; Wu, Y.; Wu, X.; Zhu, X.; Wang, K.; Yang, H. Caffeine Enhances the Anti-Tumor Effect of 5-Fluorouracil via Increasing the Production of Reactive Oxygen Species in Hepatocellular Carcinoma. Med. Oncol. 2019, 36, 97. [Google Scholar] [CrossRef]
- Bort, A.; Spínola, E.; Rodríguez-Henche, N.; Díaz-Laviada, I. Capsaicin Exerts Synergistic Antitumor Effect with Sorafenib in Hepatocellular Carcinoma Cells through AMPK Activation. Oncotarget 2017, 8, 87684–87698. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kang, Y.S.; Lee, J.-S.; Nicolova, S.; Kim, J.-A. Involvement of NADPH Oxidase-Mediated Generation of Reactive Oxygen Species in the Apototic Cell Death by Capsaicin in HepG2 Human Hepatoma Cells. Free Radic. Res. 2004, 38, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ni, Y.; Zhao, C.; Qiao, Z.; Yu, H.; Wang, L.; Sun, J.; Du, C.; Zhang, J.; Dong, L.; et al. Capsaicin Enhances the Antitumor Activity of Sorafenib in Hepatocellular Carcinoma Cells and Mouse Xenograft Tumors through Increased ERK Signaling. Acta Pharmacol. Sin. 2018, 39, 438–448. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Cai, G.; Li, X.; Wang, D. Carnosic Acid Induces Apoptosis of Hepatocellular Carcinoma Cells via ROS-Mediated Mitochondrial Pathway. Chem. Biol. Interact. 2017, 277, 91–100. [Google Scholar] [CrossRef]
- Yao, C.; Liu, B.-B.; Qian, X.-D.; Li, L.-Q.; Cao, H.-B.; Guo, Q.-S.; Zhou, G.-F. Crocin Induces Autophagic Apoptosis in Hepatocellular Carcinoma by Inhibiting Akt/MTOR Activity. OncoTargets Ther. 2018, 11, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Abouzied, M.M.M.; Eltahir, H.M.; Abdel Aziz, M.A.; Ahmed, N.S.; Abd El-Ghany, A.A.; Abd El-Aziz, E.A.; Abd El-Aziz, H.O. Curcumin Ameliorate DENA-Induced HCC via Modulating TGF-β, AKT, and Caspase-3 Expression in Experimental Rat Model. Tumor Biol. 2015, 36, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, J.U.; Gomez-Quiroz, L.; Arreguin Camacho, L.O.; Pinna, F.; Lee, Y.-H.; Kitade, M.; Domínguez, M.P.; Castven, D.; Breuhahn, K.; Conner, E.A.; et al. Curcumin Effectively Inhibits Oncogenic NF-ΚB Signaling and Restrains Stemness Features in Liver Cancer. J. Hepatol. 2015, 63, 661–669. [Google Scholar] [CrossRef]
- García-Vilas, J.A.; Quesada, A.R.; Medina, M.A. Damnacanthal, a Noni Anthraquinone, Inhibits c-Met and Is a Potent Antitumor Compound against Hep G2 Human Hepatocellular Carcinoma Cells. Sci. Rep. 2015, 5, 8021. [Google Scholar] [CrossRef]
- Delle Monache, S.; Sanità, P.; Trapasso, E.; Ursino, M.R.; Dugo, P.; Russo, M.; Ferlazzo, N.; Calapai, G.; Angelucci, A.; Navarra, M. Mechanisms Underlying the Anti-Tumoral Effects of Citrus Bergamia Juice. PLoS ONE 2013, 8, e61484. [Google Scholar] [CrossRef]
- Zhang, Y.; Owusu, L.; Duan, W.; Jiang, T.; Zang, S.; Ahmed, A.; Xin, Y. Anti-Metastatic and Differential Effects on Protein Expression of Epigallocatechin-3-Gallate in HCCLM6 Hepatocellular Carcinoma Cells. Int. J. Mol. Med. 2013, 32, 959–964. [Google Scholar] [CrossRef]
- Chen, J.; Chen, L.; Lu, T.; Xie, Y.; Li, C.; Jia, Z.; Cao, J. ERα36 Is an Effective Target of Epigallocatechin-3-Gallate in Hepatocellular Carcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 3222–3234. [Google Scholar] [PubMed]
- Li, S.; Wu, L.; Feng, J.; Li, J.; Liu, T.; Zhang, R.; Xu, S.; Cheng, K.; Zhou, Y.; Zhou, S.; et al. In Vitro and in Vivo Study of Epigallocatechin-3-Gallate-Induced Apoptosis in Aerobic Glycolytic Hepatocellular Carcinoma Cells Involving Inhibition of Phosphofructokinase Activity. Sci. Rep. 2016, 6, 28479. [Google Scholar] [CrossRef]
- Hsu, C.-M.; Hsu, Y.-A.; Tsai, Y.; Shieh, F.-K.; Huang, S.-H.; Wan, L.; Tsai, F.-J. Emodin Inhibits the Growth of Hepatoma Cells: Finding the Common Anti-Cancer Pathway Using Huh7, Hep3B, and HepG2 Cells. Biochem. Biophys. Res. Commun. 2010, 392, 473–478. [Google Scholar] [CrossRef]
- Sundarraj, K.; Raghunath, A.; Panneerselvam, L.; Perumal, E. Fisetin, a Phytopolyphenol, Targets Apoptotic and Necroptotic Cell Death in HepG2 Cells. BioFactors 2020, 46, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Youns, M.; Abdel Halim Hegazy, W. The Natural Flavonoid Fisetin Inhibits Cellular Proliferation of Hepatic, Colorectal, and Pancreatic Cancer Cells through Modulation of Multiple Signaling Pathways. PLoS ONE 2017, 12, e0169335. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y. Genistein Inhibits Invasive Potential of Human Hepatocellular Carcinoma by Altering Cell Cycle, Apoptosis, and Angiogenesis. World J. Gastroenterol. 2005, 11, 6512. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-C.; Hsu, C.-L.; Lin, H.-T.; Yen, G.-C. Anticancer Effects of Flavonoid Derivatives Isolated from Millettia Reticulata Benth in SK-Hep-1 Human Hepatocellular Carcinoma Cells. J. Agric. Food Chem. 2010, 58, 814–820. [Google Scholar] [CrossRef]
- Shi, Q.; Shi, X.; Zuo, G.; Xiong, W.; Li, H.; Guo, P.; Wang, F.; Chen, Y.; Li, J.; Chen, D.-L. Anticancer Effect of 20(S)-Ginsenoside Rh2 on HepG2 Liver Carcinoma Cells: Activating GSK-3β and Degrading β-Catenin. Oncol. Rep. 2016, 36, 2059–2070. [Google Scholar] [CrossRef]
- Jiang, F.; Mu, J.; Wang, X.; Ye, X.; Si, L.; Ning, S.; Li, Z.; Li, Y. The Repressive Effect of MiR-148a on TGF Beta-SMADs Signal Pathway Is Involved in the Glabridin-Induced Inhibition of the Cancer Stem Cells-Like Properties in Hepatocellular Carcinoma Cells. PLoS ONE 2014, 9, e96698. [Google Scholar] [CrossRef]
- Kapkoti, D.S.; Singh, S.; Alam, S.; Khan, F.; Luqman, S.; Bhakuni, R.S. In Vitro Antiproliferative Activity of Glabridin Derivatives and Their In Silico Target Identification. Nat. Prod. Res. 2020, 34, 1735–1742. [Google Scholar] [CrossRef]
- Mylonis, I.; Lakka, A.; Tsakalof, A.; Simos, G. The Dietary Flavonoid Kaempferol Effectively Inhibits HIF-1 Activity and Hepatoma Cancer Cell Viability under Hypoxic Conditions. Biochem. Biophys. Res. Commun. 2010, 398, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, I.; Guven, E.B.; Ersahin, T.; Ozturk, M.; Calis, I.; Cetin-Atalay, R. Liver Cancer Cells Are Sensitive to Lanatoside C Induced Cell Death Independent of Their PTEN Status. Phytomedicine 2016, 23, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Seydi, E.; Salimi, A.; Rasekh, H.R.; Mohsenifar, Z.; Pourahmad, J. Selective Cytotoxicity of Luteolin and Kaempferol on Cancerous Hepatocytes Obtained from Rat Model of Hepatocellular Carcinoma: Involvement of ROS-Mediated Mitochondrial Targeting. Nutr. Cancer 2018, 70, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Yuan, P.; Wang, D.; Jin, H.; Chen, H. Effects of Naringin on the Expression of MiR-19b and Cell Apoptosis in Human Hepatocellular Carcinoma. Oncol. Lett. 2017, 14, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-S.; Kim, H.-M.; Yadunandam, A.K.; Kim, N.-H.; Jung, H.-A.; Choi, J.-S.; Kim, C.-Y.; Kim, G.-D. Neferine Isolated from Nelumbo Nucifera Enhances Anti-Cancer Activities in Hep3B Cells: Molecular Mechanisms of Cell Cycle Arrest, ER Stress Induced Apoptosis and Anti-Angiogenic Response. Phytomedicine 2013, 20, 1013–1022. [Google Scholar] [CrossRef]
- Law, B.Y.K.; Michelangeli, F.; Qu, Y.Q.; Xu, S.-W.; Han, Y.; Mok, S.W.F.; Dias, I.R.D.S.R.; Javed, M.-H.; Chan, W.-K.; Xue, W.-W.; et al. Neferine Induces Autophagy-Dependent Cell Death in Apoptosis-Resistant Cancers via Ryanodine Receptor and Ca2+-Dependent Mechanism. Sci. Rep. 2019, 9, 20034. [Google Scholar] [CrossRef]
- Shyu, M.-H.; Kao, T.-C.; Yen, G.-C. Oleanolic Acid and Ursolic Acid Induce Apoptosis in HuH7 Human Hepatocellular Carcinoma Cells through a Mitochondrial-Dependent Pathway and Downregulation of XIAP. J. Agric. Food Chem. 2010, 58, 6110–6118. [Google Scholar] [CrossRef]
- Dai, Q.; Yin, Q.; Wei, L.; Zhou, Y.; Qiao, C.; Guo, Y.; Wang, X.; Ma, S.; Lu, N. Oroxylin A Regulates Glucose Metabolism in Response to Hypoxic Stress with the Involvement of Hypoxia-Inducible Factor-1 in Human Hepatoma HepG2 Cells. Mol. Carcinog. 2016, 55, 1275–1289. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Li, K.; Li, A.; Yang, W.; Yang, R.; Wang, P.; Zhao, Z.; Cui, F.; Qin, Y.; et al. Protopanaxadiol Inhibits Epithelial–Mesenchymal Transition of Hepatocellular Carcinoma by Targeting STAT3 Pathway. Cell Death Dis. 2019, 10, 630. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, Z.; Yan, H.; Yuan, Y.; Levenson, A.S.; Li, K. Pterostilbene Inhibits MTA1/HDAC1 Complex Leading to PTEN Acetylation in Hepatocellular Carcinoma. Biomed. Pharmacother. 2018, 101, 852–859. [Google Scholar] [CrossRef]
- Anand David, A.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pan, L.; Gao, C.; Xu, H.; Li, Y.; Zhang, L.; Ma, L.; Meng, L.; Sun, X.; Qin, H. Quercetin Inhibits the Proliferation of Glycolysis-Addicted HCC Cells by Reducing Hexokinase 2 and Akt-MTOR Pathway. Molecules 2019, 24, 1993. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Mao, J.-M.; Zhang, S.-Y.; Zhou, Z.-Q.; Tan, Y.; Zhang, Y. Quercetin Induces HepG2 Cell Apoptosis by Inhibiting Fatty Acid Biosynthesis. Oncol. Lett. 2014, 8, 765–769. [Google Scholar] [CrossRef]
- Malhotra, A.; Bath, S.; Elbarbry, F. An Organ System Approach to Explore the Antioxidative, Anti-Inflammatory, and Cytoprotective Actions of Resveratrol. Oxid. Med. Cell. Longev. 2015, 2015, 803971. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Abdel–Mottaleb, Y.; Eissa Ahmed, A.A.; El-Maraghy, N.N. Novel Combination of Thymoquinone and Resveratrol Enhances Anticancer Effect on Hepatocellular Carcinoma Cell Line. Future J. Pharm. Sci. 2018, 4, 41–46. [Google Scholar] [CrossRef]
- Karakurt, S. Modulatory Effects of Rutin on the Expression of Cytochrome P450s and Antioxidant Enzymes in Human Hepatoma Cells. Acta Pharm. 2016, 66, 491–502. [Google Scholar] [CrossRef]
- Peng, W.; Hu, C.; Shu, Z.; Han, T.; Qin, L.; Zheng, C. Antitumor Activity of Tatariside F Isolated from Roots of Fagopyrum Tataricum (L.) Gaertn against H22 Hepatocellular Carcinoma via up-Regulation of P53. Phytomedicine 2015, 22, 730–736. [Google Scholar] [CrossRef]
- Jehan, S.; Zhong, C.; Li, G.; Bakhtiar, S.Z.; Li, D.; Sui, G. Thymoquinone Selectively Induces Hepatocellular Carcinoma Cell Apoptosis in Synergism with Clinical Therapeutics and Dependence of P53 Status. Front. Pharmacol. 2020, 11, 555283. [Google Scholar] [CrossRef]
- Kim, G.-H.; Kan, S.-Y.; Kang, H.; Lee, S.; Ko, H.M.; Kim, J.H.; Lim, J.-H. Ursolic Acid Suppresses Cholesterol Biosynthesis and Exerts Anti-Cancer Effects in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 4767. [Google Scholar] [CrossRef]
- Liao, W.; Fan, L.; Zheng, Z.; Liu, H.; Deng, H.; Li, M.; Liu, F.; Yang, A. Ziyuglycoside II Exerts Antiproliferative and Antimetastasis Effects on Hepatocellular Carcinoma Cells. Anticancer Drugs 2020, 31, 819–827. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Panda, K.C.; Das, S.; Jena, M.; Bhutia, S.K. Apoptosis and Autophagy Modulating Dietary Phytochemicals in Cancer Therapeutics: Current Evidences and Future Perspectives. Phyther. Res. 2021, 35, 4194–4214. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin Induces Apoptosis via Caspase Activation, Regulation of Bcl-2, and Inhibition of PI-3-Kinase/Akt and ERK Pathways in a Human Hepatoma Cell Line (HepG2). J. Nutr. 2006, 136, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Nakajima, T.; Moriguchi, M.; Jo, M.; Sekoguchi, S.; Ishii, M.; Takashima, H.; Katagishi, T.; Kimura, H.; Minami, M.; et al. A Green Tea Polyphenol, Epigalocatechin-3-Gallate, Induces Apoptosis of Human Hepatocellular Carcinoma, Possibly through Inhibition of Bcl-2 Family Proteins. J. Hepatol. 2006, 44, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Islam, A.U.; Prakash, H.; Singh, S. Phytochemicals Targeting NF-ΚB Signaling: Potential Anti-Cancer Interventions. J. Pharm. Anal. 2022, 12, 394–405. [Google Scholar] [CrossRef]
- Wu, J.-M.; Sheng, H.; Saxena, R.; Skill, N.J.; Bhat-Nakshatri, P.; Yu, M.; Nakshatri, H.; Maluccio, M.A. NF-ΚB Inhibition in Human Hepatocellular Carcinoma and Its Potential as Adjunct to Sorafenib Based Therapy. Cancer Lett. 2009, 278, 145–155. [Google Scholar] [CrossRef]
- Kavitha, K.; Priyadarsini, R.V.; Anitha, P.; Ramalingam, K.; Sakthivel, R.; Purushothaman, G.; Singh, A.K.; Karunagaran, D.; Nagini, S. Nimbolide, a Neem Limonoid Abrogates Canonical NF-ΚB and Wnt Signaling to Induce Caspase-Dependent Apoptosis in Human Hepatocarcinoma (HepG2) Cells. Eur. J. Pharmacol. 2012, 681, 6–14. [Google Scholar] [CrossRef]
- Shu, G.; Yue, L.; Zhao, W.; Xu, C.; Yang, J.; Wang, S.; Yang, X. Isoliensinine, a Bioactive Alkaloid Derived from Embryos of Nelumbo Nucifera, Induces Hepatocellular Carcinoma Cell Apoptosis through Suppression of NF-ΚB Signaling. J. Agric. Food Chem. 2015, 63, 8793–8803. [Google Scholar] [CrossRef]
- Chuang, T.-Y.; Wu, H.-L.; Min, J.; Diamond, M.; Azziz, R.; Chen, Y.-H. Berberine Regulates the Protein Expression of Multiple Tumorigenesis-Related Genes in Hepatocellular Carcinoma Cell Lines. Cancer Cell Int. 2017, 17, 59. [Google Scholar] [CrossRef]
- Lee, W.J.; Shim, J.-Y.; Zhu, B.T. Mechanisms for the Inhibition of DNA Methyltransferases by Tea Catechins and Bioflavonoids. Mol. Pharmacol. 2005, 68, 1018–1030. [Google Scholar] [CrossRef]
- Liu, A.; Wu, Q.; Peng, D.; Ares, I.; Anadón, A.; Lopez-Torres, B.; Martínez-Larrañaga, M.; Wang, X.; Martínez, M. A Novel Strategy for the Diagnosis, Prognosis, Treatment, and Chemoresistance of Hepatocellular Carcinoma: DNA Methylation. Med. Res. Rev. 2020, 40, 1973–2018. [Google Scholar] [CrossRef]
- Bhatia, N.; Zhao, J.; Wolf, D.M.; Agarwal, R. Inhibition of Human Carcinoma Cell Growth and DNA Synthesis by Silibinin, an Active Constituent of Milk Thistle: Comparison with Silymarin. Cancer Lett. 1999, 147, 77–84. [Google Scholar] [CrossRef]
- Hsieh, T.; Halicka, D.; Lu, X.; Kunicki, J.; Guo, J.; Darzynkiewicz, Z.; Wu, J.M. Effects of Resveratrol on the G0–G1 Transition and Cell Cycle Progression of Mitogenically Stimulated Human Lymphocytes. Biochem. Biophys. Res. Commun. 2002, 297, 1311–1317. [Google Scholar] [CrossRef]
- Tyagi, A.; Agarwal, R.; Agarwal, C. Grape Seed Extract Inhibits EGF-Induced and Constitutively Active Mitogenic Signaling but Activates JNK in Human Prostate Carcinoma DU145 Cells: Possible Role in Antiproliferation and Apoptosis. Oncogene 2003, 22, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.; Skeet, K.; Mehmetoglu-Gurbuz, T.; Goldfarb, M.; Karri, S.; Rocha, J.; Shahinian, M.; Yazadi, A.; Poudel, S.; Subramani, R. Phytochemicals as an Alternative or Integrative Option, in Conjunction with Conventional Treatments for Hepatocellular Carcinoma. Cancers 2021, 13, 5753. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic Potential of Flavonoids in Cancer: ROS-Mediated Mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Fundamental Concepts of the Angiogenic Process. Curr. Mol. Med. 2003, 3, 643–651. [Google Scholar] [CrossRef]
- Varghese, E.; Liskova, A.; Kubatka, P.; Samuel, S.M.; Büsselberg, D. Anti-Angiogenic Effects of Phytochemicals on MiRNA Regulating Breast Cancer Progression. Biomolecules 2020, 10, 191. [Google Scholar] [CrossRef]
- Mendell, J.T.; Olson, E.N. MicroRNAs in Stress Signaling and Human Disease. Cell 2012, 148, 1172–1187. [Google Scholar] [CrossRef]
- Lah, J.J. Effects and Mechanisms of Silibinin on Human Hepatoma Cell Lines. World J. Gastroenterol. 2007, 13, 5299. [Google Scholar] [CrossRef]
- Pan, Z.; Zhuang, J.; Ji, C.; Cai, Z.; Liao, W.; Huang, Z. Curcumin Inhibits Hepatocellular Carcinoma Growth by Targeting VEGF Expression. Oncol. Lett. 2018, 15, 4821–4826. [Google Scholar] [CrossRef]
- Sur, S.; Pal, D.; Roy, R.; Barua, A.; Roy, A.; Saha, P.; Panda, C.K. Tea Polyphenols EGCG and TF Restrict Tongue and Liver Carcinogenesis Simultaneously Induced by N-Nitrosodiethylamine in Mice. Toxicol. Appl. Pharmacol. 2016, 300, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cai, G.; Song, D.; Gao, R.; Teng, P.; Zhou, L.; Ji, Q.; Sui, H.; Cai, J.; Li, Q.; et al. Development of EGFR-Targeted Evodiamine Nanoparticles for the Treatment of Colorectal Cancer. Biomater. Sci. 2019, 7, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Qu, H.; Meng, X.; Jiang, Y.; Liu, D.; Ye, S.; Chen, H.; Jin, Y.; Fu, S.; Geng, J. S-Allylmercaptocysteine Promotes MAPK Inhibitor-Induced Apoptosis by Activating the TGF-β Signaling Pathway in Cancer Cells. Oncol. Rep. 2014, 32, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Furtado, N.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Tan, O.J.; Loo, H.L.; Thiagarajah, G.; Palanisamy, U.D.; Sundralingam, U. Improving Oral Bioavailability of Medicinal Herbal Compounds through Lipid-Based Formulations—A Scoping Review. Phytomedicine 2021, 90, 153651. [Google Scholar] [CrossRef]
- González-Ruiz, V.; Cores, Á.; Martín-Cámara, O.; Orellana, K.; Cervera-Carrascón, V.; Michalska, P.; Olives, A.I.; León, R.; Martín, M.A.; Menéndez, J.C. Enhanced Stability and Bioactivity of Natural Anticancer Topoisomerase I Inhibitors through Cyclodextrin Complexation. Pharmaceutics 2021, 13, 1609. [Google Scholar] [CrossRef]
- Mirhadi, E.; Rezaee, M.; Malaekeh-Nikouei, B. Nano Strategies for Berberine Delivery, a Natural Alkaloid of Berberis. Biomed. Pharmacother. 2018, 104, 465–473. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, J.; Xu, X. Emerging and Innovative Theranostic Approaches for Mesoporous Silica Nanoparticles in Hepatocellular Carcinoma: Current Status and Advances. Front. Bioeng. Biotechnol. 2020, 8, 184. [Google Scholar] [CrossRef]
- Kashkooli, F.M.; Soltani, M.; Souri, M. Controlled Anti-Cancer Drug Release through Advanced Nano-Drug Delivery Systems: Static and Dynamic Targeting Strategies. J. Control. Release 2020, 327, 316–349. [Google Scholar] [CrossRef]
- Bilal, M.; Qindeel, M.; Raza, A.; Mehmood, S.; Rahdar, A. Stimuli-Responsive Nanoliposomes as Prospective Nanocarriers for Targeted Drug Delivery. J. Drug Deliv. Sci. Technol. 2021, 66, 102916. [Google Scholar] [CrossRef]
- Yao, J.; Feng, J.; Chen, J. External-Stimuli Responsive Systems for Cancer Theranostic. Asian J. Pharm. Sci. 2016, 11, 585–595. [Google Scholar] [CrossRef]
- Fang, Z.; Pan, S.; Gao, P.; Sheng, H.; Li, L.; Shi, L.; Zhang, Y.; Cai, X. Stimuli-Responsive Charge-Reversal Nano Drug Delivery System: The Promising Targeted Carriers for Tumor Therapy. Int. J. Pharm. 2020, 575, 118841. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, R.; Lu, X.; Dai, Y.; Chen, T.; Xing, Y.; Xue, L.; Duan, Z.; Zhou, W.; Li, J. Fabrication and Characterization of L-Ascorbyl Palmitate and Phospholipid-Based Hybrid Liposomes and Their Impacts on the Stability of Loaded Hydrophobic Polyphenols. Food Chem. 2023, 398, 133953. [Google Scholar] [CrossRef]
- Domínguez-Arca, V.; Sabín, J.; García-Río, L.; Bastos, M.; Taboada, P.; Barbosa, S.; Prieto, G. On the Structure and Stability of Novel Cationic DPPC Liposomes Doped with Gemini Surfactants. J. Mol. Liq. 2022, 366, 120230. [Google Scholar] [CrossRef]
- Jagwani, S.; Jalalpure, S.; Dhamecha, D.; Jadhav, K.; Bohara, R. Pharmacokinetic and Pharmacodynamic Evaluation of Resveratrol Loaded Cationic Liposomes for Targeting Hepatocellular Carcinoma. ACS Biomater. Sci. Eng. 2020, 6, 4969–4984. [Google Scholar] [CrossRef]
- Yue, Y.; Yang, Y.; Shi, L.; Wang, Z. Basic Research Suppression of Human Hepatocellular Cancer Cell Proliferation by Brucea Javanica Oil-Loaded Liposomes via Induction of Apoptosis. Arch. Med. Sci. 2015, 4, 856–862. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Lu, Q.; Liu, X.; Wen, J.; Qi, X.; Liu, J.; Lian, B.; Zhang, B.; Sun, H.; et al. GA&HA-Modified Liposomes for Co-Delivery of Aprepitant and Curcumin to Inhibit Drug-Resistance and Metastasis of Hepatocellular Carcinoma. Int. J. Nanomed. 2022, 17, 2559–2575. [Google Scholar] [CrossRef]
- Singh, M.; Devi, S.; Rana, V.S.; Mishra, B.B.; Kumar, J.; Ahluwalia, V. Delivery of Phytochemicals by Liposome Cargos: Recent Progress, Challenges and Opportunities. J. Microencapsul. 2019, 36, 215–235. [Google Scholar] [CrossRef]
- Shu, Q.; Wu, J.; Chen, Q. Synthesis, Characterization of Liposomes Modified with Biosurfactant MEL-A Loading Betulinic Acid and Its Anticancer Effect in HepG2 Cell. Molecules 2019, 24, 3939. [Google Scholar] [CrossRef]
- Batool, S.; Asad, M.J.; Arshad, M.; Ahmed, W.; Sohail, M.F.; Abbasi, S.W.; Ahmad, S.; Saleem, R.S.Z.; Ahmed, M.S. In Silico Validation, Fabrication and Evaluation of Nano-Liposomes of Bistorta Amplexicaulis Extract for Improved Anticancer Activity Against Hepatoma Cell Line (HepG2). Curr. Drug Deliv. 2021, 18, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, X.; Hu, J.; Qiu, Z.; Yuan, M.; Zheng, G. Celastrol-Loaded Galactosylated Liposomes Effectively Inhibit AKT/c-Met-Triggered Rapid Hepatocarcinogenesis in Mice. Mol. Pharm. 2020, 17, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, R.; Zhang, Z.; Zhong, C.; Wang, J.; Wang, M. Curcumin-Loaded Liposomes with the Hepatic and Lysosomal Dual-Targeted Effects for Therapy of Hepatocellular Carcinoma. Int. J. Pharm. 2021, 602, 120628. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, P.; Wu, S.; Yang, T.; Chen, Y.; Zhang, X.; He, C.; Zheng, C.; Li, K.; Ma, X.; et al. Cisplatin and Curcumin Co-Loaded Nano-Liposomes for the Treatment of Hepatocellular Carcinoma. Int. J. Pharm. 2018, 545, 261–273. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Z.; Chen, Y.; Liu, J.; Wang, Y.; Liu, H. Targeted Delivery of Garcinia Glycosides by Reconstituted High-Density Lipoprotein Nano-Complexes. J. Microencapsul. 2018, 35, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pei, H.; Luo, H.; Fu, A.; Yang, H.; Hu, J.; Zhao, C.; Chai, L.; Chen, X.; Shao, X.; et al. Non-Toxic Dose of Liposomal Honokiol Suppresses Metastasis of Hepatocellular Carcinoma through Destabilizing EGFR and Inhibiting the Downstream Pathways. Oncotarget 2017, 8, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, S.; Zhu, J.; Shen, L.; Zhang, Q.Y.; Zhu, H. Folic Acid Modified TPGS as a Novel Nano-Micelle for Delivery of Nitidine Chloride to Improve Apoptosis Induction in Huh7 Human Hepatocellular Carcinoma. BMC Pharmacol. Toxicol. 2021, 22, 1. [Google Scholar] [CrossRef]
- Zhong, Z.; Liu, Z.; Zhang, X.; Huang, J.; Yu, X.; Li, J.; Xiong, D.; Sun, X.; Luo, Y. Effect of a Controlled-Release Drug Delivery System Made of Oleanolic Acid Formulated into Multivesicular Liposomes on Hepatocellular Carcinoma in Vitro and in Vivo. Int. J. Nanomed. 2016, 11, 3111–3129. [Google Scholar] [CrossRef]
- Ochi, M.M.; Amoabediny, G.; Rezayat, S.M.; Akbarzadeh, A.; Ebrahimi, B. In Vitro Co-Delivery Evaluation of Novel Pegylated Nano-Liposomal Herbal Drugs of Silibinin and Glycyrrhizic Acid (Nano-Phytosome) to Hepatocellular Carcinoma Cells. Cell J. 2016, 18, 135–148. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, J.; Deng, L.; Hu, H.; Hu, J.; Zheng, G. Galactose-Modified PH-Sensitive Niosomes for Controlled Release and Hepatocellular Carcinoma Target Delivery of Tanshinone IIA. AAPS PharmSciTech 2021, 22, 96. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Jiang, M.; Lu, L.; Ding, Y.; Ma, N.; Zhao, Y.; Xuchen, S.; Zhang, N. Novel Timosaponin AIII-Based Multifunctional Liposomal Delivery System for Synergistic Therapy Against Hepatocellular Carcinoma Cancer. Int. J. Nanomed. 2021, 16, 5531–5550. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Kong, F.; Liu, S.; Liu, X.; Pei, D.; Miao, X. Membrane Protein-Chimeric Liposome-Mediated Delivery of Triptolide for Targeted Hepatocellular Carcinoma Therapy. Drug Deliv. 2021, 28, 2033–2043. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Z.; Mo, Z.; Zou, B.; Yang, Y.; Sun, R.; Ma, W.; Yu, M.; Zhang, S.; Yu, Z. Synergetic Delivery of Triptolide and Ce6 with Light-Activatable Liposomes for Efficient Hepatocellular Carcinoma Therapy. Acta Pharm. Sin. B 2021, 11, 2004–2015. [Google Scholar] [CrossRef]
- Li, Z.; Yang, G.; Han, L.; Wang, R.; Gong, C.; Yuan, Y. Sorafenib and Triptolide Loaded Cancer Cell-Platelet Hybrid Membrane-Camouflaged Liquid Crystalline Lipid Nanoparticles for the Treatment of Hepatocellular Carcinoma. J. Nanobiotechnol. 2021, 19, 360. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, Q.; Zhou, C.; Lin, Y. Liposomes Co-Loaded with Ursolic Acid and Ginsenoside Rg3 in the Treatment of Hepatocellular Carcinoma. Acta Biochim. Pol. 2021, 68, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Nisha, R.; Kumar, P.; Gautam, A.K.; Bera, H.; Bhattacharya, B.; Parashar, P.; Saraf, S.A.; Saha, S. Assessments of in Vitro and in Vivo Antineoplastic Potentials of β-Sitosterol-Loaded PEGylated Niosomes against Hepatocellular Carcinoma. J. Liposome Res. 2021, 31, 304–315. [Google Scholar] [CrossRef]
- Guo, S.-J.; Ma, C.-G.; Hu, Y.-Y.; Bai, G.; Song, Z.-J.; Cao, X.-Q. Solid Lipid Nanoparticles for Phytosterols Delivery: The Acyl Chain Number of the Glyceride Matrix Affects the Arrangement, Stability, and Release. Food Chem. 2022, 394, 133412. [Google Scholar] [CrossRef]
- Sun, S.; Shang, E.; Ju, A.; Li, Y.; Wu, Q.; Li, Q.; Yang, Y.; Guo, Y.; Yang, D.; Lv, S. Tumor-Targeted Hyaluronic Acid-MPEG Modified Nanostructured Lipid Carriers for Cantharidin Delivery: An in Vivo and in Vitro Study. Fitoterapia 2021, 155, 105033. [Google Scholar] [CrossRef]
- Kunjiappan, S.; Sankaranarayanan, M.; Karan Kumar, B.; Pavadai, P.; Babkiewicz, E.; Maszczyk, P.; Glodkowska-Mrowka, E.; Arunachalam, S.; Ram Kumar Pandian, S.; Ravishankar, V.; et al. Capsaicin-Loaded Solid Lipid Nanoparticles: Design, Biodistribution, in Silico Modeling and in Vitro Cytotoxicity Evaluation. Nanotechnology 2021, 32, 095101. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Q.; Li, Y.; Tang, H.; Liu, W.; Yang, X. Doxorubicin and Curcumin Co-Delivery by Lipid Nanoparticles for Enhanced Treatment of Diethylnitrosamine-Induced Hepatocellular Carcinoma in Mice. Eur. J. Pharm. Biopharm. 2015, 93, 27–36. [Google Scholar] [CrossRef]
- Rahman, M.; Al-Ghamdi, S.A.; Alharbi, K.S.; Beg, S.; Sharma, K.; Anwar, F.; Al-Abbasi, F.A.; Kumar, V. Ganoderic Acid Loaded Nano-Lipidic Carriers Improvise Treatment of Hepatocellular Carcinoma. Drug Deliv. 2019, 26, 782–793. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, Y.; Zhang, J.; Feng, Y.; Shen, L. Formulation, Preparation and Evaluation of Nanostructured Lipid Carrier Containing Naringin and Coix Seed Oil for Anti-Tumor Application Based on “Unification of Medicines and Excipients”. Drug Des. Dev. Ther. 2020, 14, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Jafarian, A.; Salehi, G.; Zolfaghari, B. Comparing Different Sterol Containing Solid Lipid Nanoparticles for Targeted Delivery of Quercetin in Hepatocellular Carcinoma. J. Liposome Res. 2014, 24, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Almalki, W.H.; Afzal, O.; Altamimi, A.S.A.; Kazmi, I.; Al-Abbasi, F.A.; Choudhry, H.; Alenezi, S.; Barkat, M.A.; Beg, S.; et al. Cationic Solid Lipid Nanoparticles of Resveratrol for Hepatocellular Carcinoma Treatment: Systematic Optimization, in Vitro Characterization and Preclinical Investigation. Int. J. Nanomed. 2020, 15, 9283–9299. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in Phytochemical Delivery Systems for Improved Anticancer Activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Mondal, L.; Mukherjee, B.; Dutta, L.; Ehsan, I.; Debnath, M.C.; Gaonkar, R.H.; Pal, M.M.; Majumdar, S. Apigenin Loaded Nanoparticle Delayed Development of Hepatocellular Carcinoma in Rats. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1905–1917. [Google Scholar] [CrossRef]
- Tian, H.; Huang, Y.; He, J.; Zhang, M.; Ni, P. CD147 Monoclonal Antibody Targeted Reduction-Responsive Camptothecin Polyphosphoester Nanomedicine for Drug Delivery in Hepatocellular Carcinoma Cells. ACS Appl. Bio Mater. 2021, 4, 4422–4431. [Google Scholar] [CrossRef]
- Sarika, P.R.; James, N.R.; Kumar, P.R.A.; Raj, D.K.; Kumary, T.V. Gum Arabic-Curcumin Conjugate Micelles with Enhanced Loading for Curcumin Delivery to Hepatocarcinoma Cells. Carbohydr. Polym. 2015, 134, 167–174. [Google Scholar] [CrossRef]
- Mondal, J.; Khuda-Bukhsh, A.R. Cisplatin and Farnesol Co-Encapsulated PLGA Nano-Particles Demonstrate Enhanced Anti-Cancer Potential against Hepatocellular Carcinoma Cells in Vitro. Mol. Biol. Rep. 2020, 47, 3615–3628. [Google Scholar] [CrossRef]
- Abd-Rabou, A.A.; Ahmed, H.H. CS-PEG Decorated PLGA Nano-Prototype for Delivery of Bioactive Compounds: A Novel Approach for Induction of Apoptosis in HepG2 Cell Line. Adv. Med. Sci. 2017, 62, 357–367. [Google Scholar] [CrossRef]
- Kumar, V.; Bhatt, P.; Rahman, M.; Kaithwas, G.; Choudhry, H.; Al-Abbasi, F.; Anwar, F.; Verma, A. Fabrication, Optimization, and Characterization of Umbelliferone β-D-Galactopyranoside-Loaded PLGA Nanoparticles in Treatment of Hepatocellular Carcinoma: In Vitro and in Vivo Studies. Int. J. Nanomed. 2017, 12, 6747–6758. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yi, Y.; Liu, L.; Lin, Y.; Li, J.; Ruan, J.; Zhong, Z. Polymeric Micelles Loading with Ursolic Acid Enhancing Anti-Tumor Effect on Hepatocellular Carcinoma. J. Cancer 2019, 10, 5820–5831. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Nallappan, D.; Madhavi, K.; Rahman, S.; Jun Wei, L.; Gan, S.H. Phytochemicals and Biogenic Metallic Nanoparticles as Anticancer Agents. Oxid. Med. Cell. Longev. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, G.; Subramaniyan, J.; Chengalvarayan Subramani, P.; Muralidharan, B.; Thiruvengadam, D. Hesperetin Conjugated PEGylated Gold Nanoparticles Exploring the Potential Role in Anti-Inflammation and Anti-Proliferation during Diethylnitrosamine-Induced Hepatocarcinogenesis in Rats. Asian J. Pharm. Sci. 2017, 12, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, J.; Zeng, J.; Li, Z.; Zuo, H.; Huang, C.; Zhao, X. Nano-Gold Loaded with Resveratrol Enhance the Anti-Hepatoma Effect of Resveratrol In Vitro and In Vivo. J. Biomed. Nanotechnol. 2019, 15, 288–300. [Google Scholar] [CrossRef]
- Gao, W.; Fan, X.; Bi, Y.; Zhou, Z.; Yuan, Y. Preparation of NIR-Responsive Gold Nanocages as Efficient Carrier for Controlling Release of EGCG in Anticancer Application. Front. Chem. 2022, 10, 926002. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef]
- Ruenraroengsak, P.; Novak, P.; Berhanu, D.; Thorley, A.J.; Valsami-Jones, E.; Gorelik, J.; Korchev, Y.E.; Tetley, T.D. Respiratory Epithelial Cytotoxicity and Membrane Damage (Holes) Caused by Amine-Modified Nanoparticles. Nanotoxicology 2012, 6, 94–108. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Zhang, J.; Song, J.; Wu, D.; Wang, J.; Dong, W. Hesperetin Induces the Apoptosis of Hepatocellular Carcinoma Cells via Mitochondrial Pathway Mediated by the Increased Intracellular Reactive Oxygen Species, ATP and Calcium. Med. Oncol. 2015, 32, 101. [Google Scholar] [CrossRef] [PubMed]
- Dobrzynska, M.; Napierala, M.; Florek, E. Flavonoid Nanoparticles: A Promising Approach for Cancer Therapy. Biomolecules 2020, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Gong, T.; Zhao, T.; Fu, Y.; Zhang, Z.; Gong, T. A Comparison Study between Lycobetaine-Loaded Nanoemulsion and Liposome Using NRGD as Therapeutic Adjuvant for Lung Cancer Therapy. Eur. J. Pharm. Sci. 2018, 111, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Kaur, V.; Kumar, M.; Kumar, A.; Kaur, K.; Dhillon, V.S.; Kaur, S. Pharmacotherapeutic Potential of Phytochemicals: Implications in Cancer Chemoprevention and Future Perspectives. Biomed. Pharmacother. 2018, 97, 564–586. [Google Scholar] [CrossRef] [PubMed]
- Korga, A.; Ostrowska, M.; Jozefczyk, A.; Iwan, M.; Wojcik, R.; Zgorka, G.; Herbet, M.; Vilarrubla, G.G.; Dudka, J. Apigenin and Hesperidin Augment the Toxic Effect of Doxorubicin against HepG2 Cells. BMC Pharmacol. Toxicol. 2019, 20, 22. [Google Scholar] [CrossRef]
- Li, J.; Duan, B.; Guo, Y.; Zhou, R.; Sun, J.; Bie, B.; Yang, S.; Huang, C.; Yang, J.; Li, Z. Baicalein Sensitizes Hepatocellular Carcinoma Cells to 5-FU and Epirubicin by Activating Apoptosis and Ameliorating P-Glycoprotein Activity. Biomed. Pharmacother. 2018, 98, 806–812. [Google Scholar] [CrossRef]
- Chang, Y.-F.; Chi, C.-W.; Wang, J.-J. Reactive Oxygen Species Production Is Involved in Quercetin-Induced Apoptosis in Human Hepatoma Cells. Nutr. Cancer 2006, 55, 201–209. [Google Scholar] [CrossRef]
- Ganta, S.; Amiji, M. Coadministration of Paclitaxel and Curcumin in Nanoemulsion Formulations to Overcome Multidrug Resistance in Tumor Cells. Mol. Pharm. 2009, 6, 928–939. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, R.; Zhang, X.; Zhang, B.; Yao, Q. Curcumin May Reverse 5-Fluorouracil Resistance on Colonic Cancer Cells by Regulating TET1-NKD-Wnt Signal Pathway to Inhibit the EMT Progress. Biomed. Pharmacother. 2020, 129, 110381. [Google Scholar] [CrossRef]
- Duan, J.; Mansour, H.M.; Zhang, Y.; Deng, X.; Chen, Y.; Wang, J.; Pan, Y.; Zhao, J. Reversion of Multidrug Resistance by Co-Encapsulation of Doxorubicin and Curcumin in Chitosan/Poly(Butyl Cyanoacrylate) Nanoparticles. Int. J. Pharm. 2012, 426, 193–201. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Gao, X.; Zhang, D.; Feng, X.; Yang, P.; Li, H.; Mao, S. Co-Delivery of Daunorubicin and Homoharringtonine in Folic Acid Modified-Liposomes for Enhancing Therapeutic Effect on Acute Myeloid Leukemia. J. Pharm. Sci. 2022, 112, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Fu, M.; Yang, X.; Jia, G.; Shi, X.; Ji, J.; Liu, X.; Zhai, G. Paclitaxel and Quercetin Co-Loaded Functional Mesoporous Silica Nanoparticles Overcoming Multidrug Resistance in Breast Cancer. Colloids Surf. B Biointerfaces 2020, 196, 111284. [Google Scholar] [CrossRef]
- Singh, D.; Khan, M.A.; Akhtar, K.; Arjmand, F.; Siddique, H.R. Apigenin Alleviates Cancer Drug Sorafenib Induced Multiple Toxic Effects in Swiss Albino Mice via Anti-Oxidative Stress. Toxicol. Appl. Pharmacol. 2022, 447, 116072. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, G.; Hu, J.; Zhu, Y.; Lan, H.; Shen, X.; Lv, Y.; Huang, L. Rutin Attenuates Sorafenib-Induced Chemoresistance and Autophagy in Hepatocellular Carcinoma by Regulating BANCR/MiRNA-590-5P/OLR1 Axis. Int. J. Biol. Sci. 2021, 17, 3595–3607. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-T.; Lin, C.-L.; Lin, T.-Y.; Cheng, C.-W.; Yang, S.-F.; Lin, C.-L.; Wu, C.-C.; Hsieh, Y.-H.; Tsai, J.-P. Synergistic Effect of Fisetin Combined with Sorafenib in Human Cervical Cancer HeLa Cells through Activation of Death Receptor-5 Mediated Caspase-8/Caspase-3 and the Mitochondria-Dependent Apoptotic Pathway. Tumor Biol. 2016, 37, 6987–6996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hagan, C.T.; Min, Y.; Foley, H.; Tian, X.; Yang, F.; Mi, Y.; Au, K.M.; Medik, Y.; Roche, K.; et al. Nanoparticle Co-Delivery of Wortmannin and Cisplatin Synergistically Enhances Chemoradiotherapy and Reverses Platinum Resistance in Ovarian Cancer Models. Biomaterials 2018, 169, 1–10. [Google Scholar] [CrossRef]
- Singh, M.; Bhatnagar, P.; Mishra, S.; Kumar, P.; Shukla, Y.; Gupta, K.C. PLGA-Encapsulated Tea Polyphenols Enhance the Chemotherapeutic Efficacy of Cisplatin against Human Cancer Cells and Mice Bearing Ehrlich Ascites Carcinoma. Int. J. Nanomed. 2015, 10, 6789. [Google Scholar] [CrossRef]
- Sperrin, M.; Thygesen, H.; Su, T.-L.; Harbron, C.; Whitehead, A. Experimental Designs for Detecting Synergy and Antagonism between Two Drugs in a Pre-Clinical Study. Pharm. Stat. 2015, 14, 216–225. [Google Scholar] [CrossRef]
- Conti, L.; Lanzardo, S.; Ruiu, R.; Cadenazzi, M.; Cavallo, F.; Aime, S.; Crich, S.G. L-Ferritin Targets Breast Cancer Stem Cells and Delivers Therapeutic and Imaging Agents. Oncotarget 2016, 7, 66713–66727. [Google Scholar] [CrossRef]
- Malekzadeh, A.M.; Ramazani, A.; Rezaei, S.J.T.; Niknejad, H. Design and Construction of Multifunctional Hyperbranched Polymers Coated Magnetite Nanoparticles for Both Targeting Magnetic Resonance Imaging and Cancer Therapy. J. Colloid Interface Sci. 2017, 490, 64–73. [Google Scholar] [CrossRef]
- Usman, M.; Hussein, M.; Kura, A.; Fakurazi, S.; Masarudin, M.; Ahmad Saad, F. Graphene Oxide as a Nanocarrier for a Theranostics Delivery System of Protocatechuic Acid and Gadolinium/Gold Nanoparticles. Molecules 2018, 23, 500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; García-Gabilondo, M.; Rosell, A.; Roig, A. MRI/Photoluminescence Dual-Modal Imaging Magnetic PLGA Nanocapsules for Theranostics. Pharmaceutics 2019, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Shim, G.; Lee, S.; Lee, S.; Choe, Y.S.; Oh, Y.-K. Safety and Tumor Tissue Accumulation of Pegylated Graphene Oxide Nanosheets for Co-Delivery of Anticancer Drug and Photosensitizer. Biomaterials 2013, 34, 3402–3410. [Google Scholar] [CrossRef]
- Wu, L.-P.; Wang, D.; Li, Z. Grand Challenges in Nanomedicine. Mater. Sci. Eng. C 2020, 106, 110302. [Google Scholar] [CrossRef]
- Zou, M.-Z.; Liu, W.-L.; Li, C.-X.; Zheng, D.-W.; Zeng, J.-Y.; Gao, F.; Ye, J.-J.; Zhang, X.-Z. A Multifunctional Biomimetic Nanoplatform for Relieving Hypoxia to Enhance Chemotherapy and Inhibit the PD-1/PD-L1 Axis. Small 2018, 14, 1801120. [Google Scholar] [CrossRef]
- Donoso-Quezada, J.; Guajardo-Flores, D.; González-Valdez, J. Enhanced Exosome-Mediated Delivery of Black Bean Phytochemicals (Phaseolus Vulgaris L.) for Cancer Treatment Applications. Biomed. Pharmacother. 2020, 131, 110771. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, M.; Yan, L.; Zhang, H.; Shi, C.; Liu, J.; Zhao, S.; Liu, H.; Wang, B. Exosomes Derived from Baicalin-Pretreated Mesenchymal Stem Cells Alleviate Hepatocyte Ferroptosis after Acute Liver Injury via the Keap1-NRF2 Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 8287227. [Google Scholar] [CrossRef]
- Du, J.; Wan, Z.; Wang, C.; Lu, F.; Wei, M.; Wang, D.; Hao, Q. Designer Exosomes for Targeted and Efficient Ferroptosis Induction in Cancer via Chemo-Photodynamic Therapy. Theranostics 2021, 11, 8185–8196. [Google Scholar] [CrossRef]
- Brar, G.; Greten, T.F.; Graubard, B.I.; McNeel, T.S.; Petrick, J.L.; McGlynn, K.A.; Altekruse, S.F. Hepatocellular Carcinoma Survival by Etiology: A SEER-Medicare Database Analysis. Hepatol. Commun. 2020, 4, 1541–1551. [Google Scholar] [CrossRef]



| Plant Bioactives (Biological Source) | Animal Model/Cell Lines | Mechanism of Action | IC50 | References |
|---|---|---|---|---|
| (14E, 18E, 22E, 26E)-methylnonacosa- 14, 18, 22, 26 tetraenoate (Amaranthus spinosus) | HepG2 | Inhibition of proliferation by upregulation of Bax; downregulation of Bcl-2 and cyclin B, resulting in G2/M arrest | 25.52 µM | [77] |
| Andrographolide (Andrographis paniculata) | Cisplatin-resistant HepG2 (HepG2CR) | Sub-G1 phase arrest; apoptosis; antiangiogenesis | 40 µM | [78,79] |
| Astrakurkurone (Astraeus hygrometricus) | HepG2, Hep3B | Inhibition of proliferation through cycle arrest at sub-G0/G1 phase; upregulation of pro-apoptotic markers Bax and cleaved caspase 9, with downregulation of antiapoptotic marker Bcl-2 | 150 µM in HepG2, 40 µM in Hep3B | [80] |
| Allicin (Allium sativum) | HepG2, Hep3B | Autophagic and apoptotic cell death through ROS generation | 35 µM in HepG2, 35 µM in Hep3B | [81] |
| Ardipusilloside I (Ardisia pusilla) | HepG2, SMMC-7721 | Inhibition of growth, invasion, and metastasis through suppression of MEK/ERK and Akt signaling pathways; inhibition of metastasis through upregulation of E-cadherin | - | [82] |
| Artemisinin (Artemisia capillaris) | SMMC-7721 | Inhibition of proliferation through blocking of PI3K/Akt and mTOR signaling channels; induction of apoptosis through downregulating antiapoptotic proteins XIAP and survivin and upregulating proapoptotic proteins cleaved caspase-3 and PARP; impeding metastasis through increasing cell–cell adhesion; inhibiting invasive and migratory ability | - | [83] |
| Berberine (Berberis vulgaris) | HepG2 | Decreased proliferation and induced apoptosis through suppression via p65 of NF-kB pathway | 3587.9 µM | [84,85] |
| Betulinic acid (Betula pubescens) | HepG2, SMMC-7721. | Causing apoptosis through mitochondrial pathway | 24.8 µM in HepG2, 28.9 µM in SMMC-7721. | [86,87] |
| Boldine (Peumus boldus) | HepG2, Wistar rats | Induced apoptosis; overexpression of Bax and cleaved caspase 3 | 170 µM in HepG2 | [88,89] |
| Caffeine (Coffea arabica) | SMMC-7721, Hep3B | Working in combination with 5-fluorouracil to reduce proliferation and induce apoptosis through intracellular ROS production | 2.2 mM in SMMC-7721, 2.02 mM in Hep3B | [90] |
| Capsaicin (Capsicum annuum) | HepG2 | Improved antitumor effect of sorafenib and induced apoptosis through intracellular ROS production | 150 µM | [91,92,93] |
| Carnosic acid (Rosmarinus officinalis) | HepG2, SMMC-7721 | Inhibited cell proliferation; induced apoptosis through increased production of ROS | 43.7 µM in HepG2, 74.8 µM in SMMC-7721 | [94] |
| Crocin (Crocus sativus) | HepG2, HCCLM3 cells | Induced autophagic apoptosis in an Akt/mTOR-dependent mechanism; inhibition of IL-6/STAT3 pathways | - | [95] |
| Curcumin (Curcuma longa) | HepG2, rat model | Modulated TGF-β, AkT, and caspase-3 expression; protective effects against toxins through expression of nuclear factor E2-related factor 2 and glutathione | 23.15 µM | [96,97] |
| Damnacanthal (Morinda citrifolia) | HepG2 | Decreased the phosphorylation levels of Akt; targets matrix metalloproteinase-2 secretion; induces apoptosis | 5.1 µM | [98] |
| Eriocitrin (Citrus limon) | HepG2 | Decreased proliferation through cell cycle arrest at G2 phase; induced apoptosis through increased expression of pro-apoptotic proteins Bcl-2, caspase 3, caspase 8, caspase 9, PARP, TNF receptor, NF-κB, and IkB; downregulated antiapoptotic genes. | - | [99] |
| Epigallocatechin-3- gallate (Camellia sinensis) | HepG2, Hep3B, Huh-7 | Reduced proliferation through inhibiting ERalpha36 and PI3K/Akt and MAPK/ERK pathways; caused apoptosis by caspase 3 activation and induction of the ER-36-EGFR-Her-2 feedback loop | 74.04 µM in HepG2, 50.8 µM in Hep3B, 83.8 µM in Huh-7 | [100,101,102] |
| Emodin (Rheum palmatum) | Huh7, Hep3B, HepG2 | Cell cycle arrested at G2/M phase | 101.5 µM in Huh7, 66.9 µM in Hep3B, 74.36 µM in HepG2 | [103] |
| Fisetin (Rhus cotinus) | HepG2 | Prevented proliferation through cell cycle arrest; stimulated apoptosis and necroptosis through increased expression of Bax, caspase-3, TNF-alpha, and PARP and through increased expression of RIPK1, RIPK3, pRIPK1, pRIPK3, and MLKL; reduced expression of pNF-κB, NF-κB, and pIKKB | 3.2 µM | [104,105] |
| Genistein (Millettia reticulata) | SK-Hep-1, Huh-7, Hep3B | Increased protein expression of Fas, FasL, and p5; impeded tumor growth through cell cycle arrest at G0/G1 and G2/M phases | 16.23 µM in SK-Hep-1, 18.67 µM in Huh7 | [106,107] |
| Ginsenoside Rh2 (Panax ginseng) | HepG2 | Causing apoptosis through mitochondrial pathway. | 100 µM | [86,108] |
| Glabridin (Glycyrrhiza glabra) | HepG2, Huh-7, MHCC97H, Sk-Hep-1 | Reducing stemness by inhibition of TGF-beta/SMAD2 signaling channel; reduced invasive ability through downregulation of MMP-9 and MMP-1; preventing tumor formation in xenograft model | 7.22 µM in HepG2 | [109,110] |
| Kaempferol (Camellia sinensis) | Huh-7 | Inhibits p44/42 MAPK and hypoxia-inducible factor 1 activity | 4.75 µM | [111] |
| Lanatoside C (Digitalis lanata) | Huh-7 | Inhibition of proliferation through cell cycle arrest; induction of apoptosis through JNK pathway activation and ROS generation | - | [112] |
| Luteolin (Verbascum lychnitis) | HCC cells from rats | Causing cancer cell death through increased production of ROS and release of cytochrome-c; prevented growth through increased expression of miR-6809-5p, blocking activation of growth cell signaling regulator FLOT1 | 12 µM | [113] |
| Naringin (Vitis vinifera) | HepG2 | Upregulates the expression of miR-19b mRNA and induces cell apoptosis | 20 µM | [114] |
| Neferine (Nelumbo nucifera) | Hep3B | Causes apoptosis through downregulation of cell cycle markers and induction of ER stress | 14.8 µM | [115,116] |
| Oleanolic acid (Ophiopogon japonicus) | Huh-7 | Induction of apoptosis through increased mitochondrial permeability, causing activation of certain proapoptotic markers; inhibition of expression of XIAP in cancer cells | 100 µM | [117] |
| Oroxylin A (Oroxylum indicum) | HepG2 | Reduced metabolic ability of cancer cells under hypoxic conditions by inhibiting the generation of lactate and glucose; suppresses expression of metabolic regulator HIF-1a; caused differentiation of cancer cells through activation of HNF-4a, thereby reducing metastatic ability | - | [118] |
| Protopanaxadiol (Panax ginseng) | HepG2, PLC/PRF/5 | Inhibition of EMT through higher expression of E-cadherin and reduced expression of vimentin; inhibition of EMT also through restriction of STAT3 activation and through inhibition of Twist1 expression | ~70 µM in all cell types | [119] |
| Pterostilbene (Pterocarpus marsupium) | HepG2 | Prevented migration, invasion, and proliferation through downregulation of MMP-9 and through suppression of TPA-induced PI3K-Akt-NF-κB signaling; inhibits metastasis | 39.06 µM | [120] |
| Quercetin (Allium cepa) | HepG2 | Caused apoptosis through upregulation of p53 and Bax; impeded glycolysis through reduction in glycolysis enzyme HK-2 and by reducing expression of phosphorylated mTOR and Akt | 24 µM | [121,122,123] |
| Resveratrol (Vitis vinifera) | SMMC-7721, HepG2 | Limited cell growth through inhibition of metabolic phenotypes that facilitate anaerobic growth | 100 µM in SMMC-7721, 64.5 µM in HepG2 | [124,125] |
| Rutin (Fagopyrum esculentum) | HepG2 | Inhibition of cell proliferation; inhibited protein expression of cytochrome P450-dependent CYP3A4 | 52.7 µM | [126] |
| Tatariside F (Fagopyrum tataricum) | H22 | Caused apoptosis through upregulation of p53 and Bax and down-regulation of Blc-2; inhibits tumor growth in vivo | 1.31 µM | [127] |
| Thymoquinone (Nigella sativa) | HepG2, SMMC-7721 | Activation of caspases and generation of ROS | 84.2 µM in HepG2, 91.6 µM in SMMC-7721. | [128] |
| Ursolic acid (Vaccinium macrocarpon) | HepG2, Huh-7 | Inhibition of proliferation through disruption of DNA structures, leading to cell cycle arrest; increased expression of p21/WAF1, inducing cell cycle arrest and apoptosis; inhibition of expression of XIAP in cancer cells | - | [129] |
| Ziyuglycoside II (Sanguisorba officinalis) | HepG2, SMMC-7721 | Inhibited cell cycle proliferation and caused apoptosis through cell cycle arrest; suppression of migration and invasion through downregulation of MMP2 and MMP9, while also inhibiting the EGFR/NF-kB pathway | 13.1 µM in HepG2, 15.6 µM in SMMC-7721. | [130] |
| Plant Bioactives | Observations and Outcomes | Cellular/Intracellular Target | References |
|---|---|---|---|
| Liposomes [169] | |||
| Aprepitant and curcumin | Reduced ECM deposition and tumor angiogenesis | Drug accumulation in tumor tissues by EPR effect and GA and/or CD44 receptor-medicated endocytosis | [168] |
| Betulinic acid | Enhanced cell apoptosis and mitochondrial membrane disruption in HepG2 cells | Mitochondrial membrane of HepG2 cells | [170] |
| Bistorta amplexicaulis extract | Plant extract containing nanoliposomes demonstrated higher cytotoxicity toward HepG2 cells | HepG2 cells in vitro | [171] |
| Brucea javanica extract | Increased apoptosis of HepG2 cells | DNA synthesis inhibition and blockage of G0/G1 development to S phase | [167] |
| Celastrol | Suppressed AKT activation, induced apoptosis, and retarded cell proliferation | Uptake in HepG2 cells in vitro through receptor-mediated endocytosis | [172] |
| Curcumin | Galactose-morpholine modification resulted in better lysosomal targeting efficacy | ASGPR receptors on liver cells in mice | [173] |
| Curcumin and cisplatin | Exhibited synergistic effects in mouse hepatoma H22 and human HCC HepG2 xenograft models | Nanoliposomes delivered both curcumin and cisplatin to tumor tissues | [174] |
| Garcinia | Drug loaded nanolipoprotein complex showed higher cell death rate compared to free drug | Scavenger receptor class B type 1 receptors | [175] |
| Honokiol | Inhibited tumor metastasis by destabilizing EGFR and reducing the downstream pathways | Cellular uptake study was not performed | [176] |
| Nitidine chloride | Exhibited sustained release and higher cytotoxicity toward Huh-7 cells | Huh-7 cells in vitro | [177] |
| Oleanolic acid | Suppressed growth of murine H22 hepatoma and prolonged the survival of tumor-bearing mice | Cellular uptake study was not performed | [178] |
| Resveratrol | Improved localization of drug in cancer tissue by 3.2 and 2.2 fold increases, respectively, in AUC and Cmax | HepG2 cells in vitro; cancer tissues in rat liver | [166] |
| Silibinin and glycyrrhizic acid | Synergistic effect of silibinin with glycyrrhizic acid on HepG2 cell line | Cellular uptake study was not performed | [179] |
| Tanshinone IIA | Promoted apoptosis in HepG2 and Huh-7 cells | Galactose modified niosomes targeted ASGPR receptors on hepatocytes | [180] |
| Timosaponin AIII and doxorubicin | TAIII improved uptake of doxorubicin HCC cells and exhibited synergistic effect | HepG2 cells in vitro, and tumor bearing mice model | [181] |
| Triptolide | Induced cell proliferation arrest and apoptosis via the mitochondrial pathway | Huh-7 cells in vitro, and tumor sites in mice model | [182] |
| Triptolide and Ce6 | Under NIR laser irradiation, liposome released triptolide and, along with Ce6, caused apoptosis of HCC cells | HepG2 cells in vitro, and patient-derived tumor xenograft | [183] |
| Triptolide and sorafenib | Long circulating liposomes promoted cancer cell apoptosis and inhibited tumor growth through synergistic effects | Huh-7 cells in-vitro, and tumor sites in mice model | [184] |
| Ursolic acid and ginsenoside | Intervened cell proliferation, apoptosis, and cell cycle of HepG2 cells | Cellular uptake study was not performed | [185] |
| β-sitosterol | Improved cellular uptake and cytotoxicity in HepG2 cells; increased drug-plasma concentrations by 8 fold | HepG2 cells in vitro | [186] |
| Solid lipid nanoparticles [187] | |||
| Cantharidin | Inhibited tumor growth and prolonged survival in tumor-bearing mice | Hyaluronic acid surface functionalization improved nanoparticle uptake in tumor tissues of rats | [188] |
| Capsaicin | Stable in circulation for a period of three days | Biodistribution studies revealed nanoparticles accumulated in the liver | [189] |
| Doxorubicin and curcumin | Synergistic activity was observed, including reversal of multidrug resistance | Cellular uptake and biodistribution study was not performed | [190] |
| Ganoderic acid | Exhibited significant antitumor effect in vivo by balancing hepatic injury markers, biochemicals, and antioxidants markers | Rapid internalization of nanoparticles in HepG2 cells | [191] |
| Naringin and coix seed oil | Exhibited synergistic effect by enhancing antitumor activity in xenograft model | Cellular uptake study was not performed | [192] |
| Quercetin | Creating better penetration into HepG2 cells | - | [193] |
| Resveratrol | Caused reduction in tumor volume and accumulation of drug in tumor tissues | Accumulation of drug in livers of rats | [194] |
| Polymer-based nanoparticles [195] | |||
| Apigenin | Sustained release of drug at target site with improved AUC and delayed liver clearance | Increased accumulation of nanoparticles in HepG2, Huh-7, and liver tissue in rats | [196] |
| Camptothecin | Provided higher uptake rate and accumulation in HepG2 cells | CD147 monoclonal antibody | [197] |
| Curcumin | Stability and aqueous solubility of curcumin were increased by several fold | Targeting HepG2 cells was achieved due to presence of galactose groups | [198] |
| Farnesol and cisplatin | Exhibited faster drug mobility, sustained particle release, site-specific action, and higher percentage of apoptotic death compared with single drug treatment | ROS generated DNA damage in HepG2 cells | [199] |
| Quercetin, ellagic acid, and gallic acid | Nanoformulation offered controlled release of bioactives with improved bioavailability | Induced apoptosis-mediated cell death in HepG2 cells | [200] |
| Umbelliferone β-D-galactopyranoside | Effectively mitigated diethyl nitrosamine-induced HCC as confirmed through both histopathological and biochemical assays. | High hepatic accumulation of drug in rat model | [201] |
| Ursolic acid | Inhibited the growth of H22 xenograft and prolonged the survival time of tumor-bearing mice | Specific targeting or cellular uptake study was not performed | [202] |
| Metallic-based nanoparticles [203] | |||
| Hesperetin | Suppression of tumor necrosis factor alpha, transcription factor NF-κB, glycoconjugates, and proliferating cell nuclear antigen | Though specific targeting was not performed, the nanoparticles arrested DNA replication at late G1- and early S-phase | [204] |
| Resveratrol | Suppressed of tumor growth, promoted apoptosis, and decreased the expression of vascular endothelial growth factor. | Accumulation of nanoparticles in liver tissue was reported, along with apoptosis of cancer cells through PI3/Akt pathway | [205] |
| Epigallo- catechin gallate (EGCG) | Nanocages irradiated by NIR significantly upregulated caspase-3 by nearly two-fold and downregulated B-cell lymphoma 2 and caused cell apoptosis | Induced cancer cell apoptosis through changes in mitochondrial activities | [206] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basu, A.; Namporn, T.; Ruenraroengsak, P. Critical Review in Designing Plant-Based Anticancer Nanoparticles against Hepatocellular Carcinoma. Pharmaceutics 2023, 15, 1611. https://doi.org/10.3390/pharmaceutics15061611
Basu A, Namporn T, Ruenraroengsak P. Critical Review in Designing Plant-Based Anticancer Nanoparticles against Hepatocellular Carcinoma. Pharmaceutics. 2023; 15(6):1611. https://doi.org/10.3390/pharmaceutics15061611
Chicago/Turabian StyleBasu, Aalok, Thanaphon Namporn, and Pakatip Ruenraroengsak. 2023. "Critical Review in Designing Plant-Based Anticancer Nanoparticles against Hepatocellular Carcinoma" Pharmaceutics 15, no. 6: 1611. https://doi.org/10.3390/pharmaceutics15061611