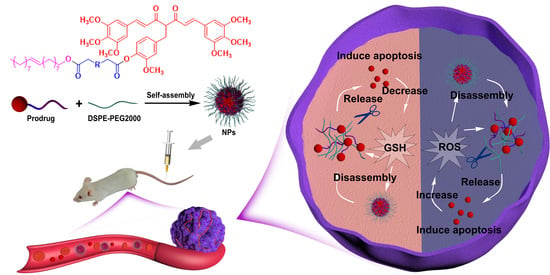
Redox-Responsive Lipidic Prodrug Nano-Delivery System Improves Antitumor Effect of Curcumin Derivative C210
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of C210 Prodrugs
2.3. Preparation and Characterization of C210 Prodrug Nanoparticles
2.4. Colloidal Stability
2.5. Drug release of C210 Prodrug Nanoparticles In Vitro
2.6. Cell Culture
2.7. Cellular Uptake of C210 Prodrug Nanoparticles
2.8. Cell Viability Assays
2.9. Animal Studies
2.10. Pharmacokinetic and Biodistribution of C210 Prodrug Nanoparticles
2.11. Antitumor Effect of C210 Prodrug Nanoparticles In Vivo
2.12. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of C210 Prodrugs
3.2. Preparation and Characterization of C210 Prodrug Nanoparticles
3.3. Redox-Responsive Release of C210 Prodrug Nanoparticles
3.4. Cellular Uptake of C210 Prodrug Nanoparticles
3.5. Cytotoxicity of C210 Prodrug Nanoparticles on Cancer Cells
3.6. Pharmacokinetics and Biodistribution of C210 Prodrug Nanoparticles
3.7. Antitumor Effects of C210 Prodrug Nanoparticles In Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Earnshaw, W.C. Induction of apoptosis by cancer chemotherapy. Exp. Cell Res. 2000, 256, 42–49. [Google Scholar] [CrossRef]
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef]
- Chen, Z.G. Small-molecule delivery by nanoparticles for anticancer therapy. Trends Mol. Med. 2010, 16, 594–602. [Google Scholar] [CrossRef]
- Kumar, A.; Harsha, C.; Parama, D.; Girisa, S.; Daimary, U.D.; Mao, X.; Kunnumakkara, A.B. Current clinical developments in curcumin-based therapeutics for cancer and chronic diseases. Phytother. Res. 2021, 35, 6768–6801. [Google Scholar] [CrossRef]
- Ning, P.; Lu, S.; Bai, X.; Wu, X.; Gao, C.; Wen, N.; Liu, M. High encapsulation and localized delivery of curcumin from an injectable hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 121–129. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.; Chen, Y.; Xu, J. C086, a novel analog of curcumin, induces growth inhibition and down-regulation of NFkappaB in colon cancer cells and xenograft tumors. Cancer Biol. Ther. 2011, 12, 797–807. [Google Scholar] [CrossRef]
- Wu, L.; Yu, J.; Chen, R.; Liu, Y.; Lou, L.; Wu, Y.; Huang, L.; Fan, Y.; Gao, P.; Huang, M.; et al. Dual inhibition of Bcr-Abl and Hsp90 by C086 potently inhibits the proliferation of imatinib-resistant CML cells. Clin. Cancer Res. 2015, 21, 833–843. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Zhang, L.; Cai, F.; Zhu, L.; Xu, J. C0818, a novel curcumin derivative, interacts with Hsp90 and inhibits Hsp90 ATPase activity. Acta Pharm. Sin. B 2017, 7, 91–96. [Google Scholar] [CrossRef]
- Fan, Y.J.; Zhou, Y.X.; Zhang, L.R.; Lin, Q.F.; Gao, P.Z.; Cai, F.; Zhu, L.P.; Liu, B.; Xu, J.H. C1206, a novel curcumin derivative, potently inhibits Hsp90 and human chronic myeloid leukemia cells in vitro. Acta Pharmacol Sin. 2018, 39, 649–658. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, M.; Wu, Q.D.; Wu, L.X.; Xu, J.H. Synthesis and Evaluation of 4-arylmethyl Curcumin Analgues as Potent Hsp90 Inhibitors. Lett. Drug Des. Discov. 2014, 11, 993–999. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, X.; Zhu, W.; Di, L. The Preparation of Curcumin Sustained-Release Solid Dispersion by Hot-Melt Extrusion-II. Optimization of Preparation Process and Evaluation In Vitro and In Vivo. J. Pharm. Sci. 2020, 109, 1253–1260. [Google Scholar] [CrossRef]
- Pan-On, S.; Dilokthornsakul, P.; Tiyaboonchai, W. Trends in advanced oral drug delivery system for curcumin: A systematic review. J. Control. Release 2022, 348, 335–345. [Google Scholar] [CrossRef]
- Li, G.; Sun, B.; Li, Y.; Luo, C.; He, Z.; Sun, J. Small-Molecule Prodrug Nanoassemblies: An Emerging Nanoplatform for Anticancer Drug Delivery. Small 2021, 17, e2101460. [Google Scholar] [CrossRef]
- Fattahi, N.; Shahbazi, M.A.; Maleki, A.; Hamidi, M.; Ramazani, A.; Santos, H.A. Emerging insights on drug delivery by fatty acid mediated synthesis of lipophilic prodrugs as novel nanomedicines. J. Control. Release 2020, 326, 556–598. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, S.; Liu, S.; Wang, Y.; Liu, G. Emerging nanomedicines of paclitaxel for cancer treatment. J. Control. Release 2022, 342, 280–294. [Google Scholar] [CrossRef]
- Khuroo, T.; Mohamed, E.M.; Dharani, S.; Immadi, S.; Nutan, M.T.H.; Lu, D.; Ali, H.I.; Khan, M.A.; Rahman, Z. In-Situ Implant Formulation of Laurate and Myristate Prodrugs of Dolutegravir for Ultra-Long Delivery. J. Pharm. Sci. 2022, 111, 2312–2321. [Google Scholar] [CrossRef]
- Kulkarni, T.A.; Bade, A.N.; Sillman, B.; Shetty, B.L.D.; Wojtkiewicz, M.S.; Gautam, N.; Hilaire, J.R.; Sravanam, S.; Szlachetka, A.; Lamberty, B.G.; et al. A year-long extended release nanoformulated cabotegravir prodrug. Nat. Mater. 2020, 19, 910–920. [Google Scholar] [CrossRef]
- Yin, Q.; Shen, J.; Zhang, Z.; Yu, H.; Li, Y. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv. Drug Deliv. Rev. 2013, 65, 1699–1715. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Zheng, Q.; Zhao, Q.; Zhang, H.; Ma, Y.; Fallon, J.K.; Fu, Q.; Haynes, M.T.; Lin, G.; et al. Disulfide bond bridge insertion turns hydrophobic anticancer prodrugs into self-assembled nanomedicines. Nano Lett. 2014, 14, 5577–5583. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, L.; Zhang, T.; Shu, Y.; He, Z.; Ma, Y.; Liu, D.; Wang, Y. Redox-sensitive prodrug nanoassemblies based on linoleic acid-modified docetaxel to resist breast cancers. Acta Pharm. Sin. B 2019, 9, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Hou, B.; Xu, Z.; Saeed, M.; Yu, H.; Li, Y. Self-Amplified Drug Delivery with Light-Inducible Nanocargoes to Enhance Cancer Immunotherapy. Adv. Mater. 2019, 31, e1902960. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Sun, J.; Sun, B.; Liu, D.; Miao, L.; Goodwin, T.J.; Huang, L.; He, Z. Facile Fabrication of Tumor Redox-Sensitive Nanoassemblies of Small-Molecule Oleate Prodrug as Potent Chemotherapeutic Nanomedicine. Small 2016, 12, 6353–6362. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Luo, C.; Zhang, X.; Guo, M.; Sun, M.; Yu, H.; Chen, Q.; Yang, W.; Wang, M.; Zuo, S.; et al. Probing the impact of sulfur/selenium/carbon linkages on prodrug nanoassemblies for cancer therapy. Nat. Commun. 2019, 10, 3211. [Google Scholar] [CrossRef]
- Li, L.; Zuo, S.; Dong, F.; Liu, T.; Gao, Y.; Yang, Y.; Wang, X.; Sun, J.; Sun, B.; He, Z. Small changes in the length of diselenide bond-containing linkages exert great influences on the antitumor activity of docetaxel homodimeric prodrug nanoassemblies. Asian J. Pharm. Sci. 2021, 16, 337–349. [Google Scholar] [CrossRef]
- Allen, C.D.; Link, A.J. Self-Assembly of Catenanes from Lasso Peptides. J. Am. Chem. Soc. 2016, 138, 14214–14217. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, Z.; Wei, Z.; Tian, L. Hydrophobic Interaction: A Promising Driving Force for the Biomedical Applications of Nucleic Acids. Adv Sci. 2020, 7, 2001048. [Google Scholar] [CrossRef]
- Demangeat, C.; Dou, Y.; Hu, B.; Bretonniere, Y.; Andraud, C.; D’Aleo, A.; Wu, J.W.; Kim, E.; Le Bahers, T.; Attias, A.J. sigma-Conjugation and H-Bond-Directed Supramolecular Self-Assembly: Key Features for Efficient Long-Lived Room Temperature Phosphorescent Organic Molecular Crystals. Angew. Chem. Int. Ed. Engl. 2021, 60, 2446–2454. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.X.; Wang, D.P.; Zuo, S.Y.; Liu, T.; Dong, F.D.; Zhang, X.B.; He, Z.G.; Sun, B.J.; Sun, J. Minor change in the length of carbon chain has a great influence on the antitumor effect of paclitaxel-fatty alcohol prodrug nanoassemblies: Small roles, big impacts. Nano Res. 2022, 15, 3367–3375. [Google Scholar] [CrossRef]
- Liang, Y.; Li, S.; Wang, X.; Zhang, Y.; Sun, Y.; Wang, Y.; Wang, X.; He, B.; Dai, W.; Zhang, H.; et al. A comparative study of the antitumor efficacy of peptide-doxorubicin conjugates with different linkers. J. Control. Release 2018, 275, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Deng, F.; Zheng, N.; Liang, Y.; Zhang, H.; He, B.; Dai, W.; Wang, X.; Zhang, Q. The effect of linkers on the self-assembling and anti-tumor efficacy of disulfide-linked doxorubicin drug-drug conjugate nanoparticles. J. Control. Release 2018, 279, 136–146. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Wu, M.; Deng, Y.; Liu, Y.; Liu, Y.; Xu, J. Redox-Responsive Lipidic Prodrug Nano-Delivery System Improves Antitumor Effect of Curcumin Derivative C210. Pharmaceutics 2023, 15, 1546. https://doi.org/10.3390/pharmaceutics15051546
Guo X, Wu M, Deng Y, Liu Y, Liu Y, Xu J. Redox-Responsive Lipidic Prodrug Nano-Delivery System Improves Antitumor Effect of Curcumin Derivative C210. Pharmaceutics. 2023; 15(5):1546. https://doi.org/10.3390/pharmaceutics15051546
Chicago/Turabian StyleGuo, Xin, Min Wu, Yanping Deng, Yan Liu, Yanpeng Liu, and Jianhua Xu. 2023. "Redox-Responsive Lipidic Prodrug Nano-Delivery System Improves Antitumor Effect of Curcumin Derivative C210" Pharmaceutics 15, no. 5: 1546. https://doi.org/10.3390/pharmaceutics15051546