Microneedles: An Emerging Vaccine Delivery Tool and a Prospective Solution to the Challenges of SARS-CoV-2 Mass Vaccination
Abstract
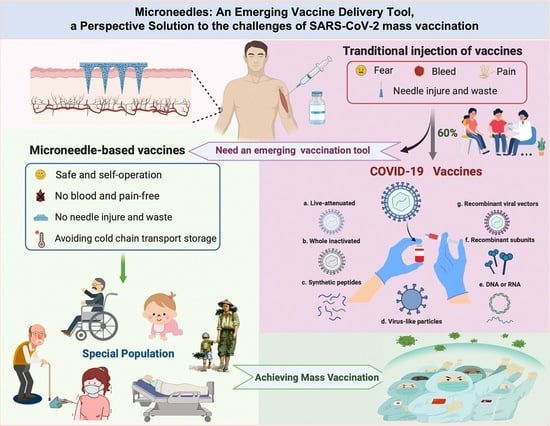
:1. Introduction
2. Development and Selection of MNs
2.1. Classification of MNs
2.2. Materials for the Preparation of MNs
3. Development of MNs Delivery of Vaccines
3.1. Classification of Vaccines
3.2. MNs Deliver Different Types of Vaccines
3.2.1. Live-Attenuated Vaccine
3.2.2. Inactive Vaccine
3.2.3. Pathogen Component Vaccine
DNA Vaccine
RNA Vaccine
Protein Vaccine
VLP Vaccine
3.2.4. Toxoid Vaccines
4. COVID-19 Vaccines and Their MNs Delivery
4.1. SARS-CoV-2
4.2. Types of COVID-19 Vaccines
4.3. MN Delivery of COVID-19 Vaccine
4.3.1. Whole-Virus Vaccine
4.3.2. Recombinant Subunit Vaccine
4.3.3. Viral Vector Vaccines
4.3.4. Nucleic Acid Vaccines
| Time | Vaccine Type | MNs Type | Advantages | Drawbacks | Ref. |
|---|---|---|---|---|---|
| 18 March 2020 | Recombinant protein | dMN | 1: Produced higher levels of neutralizing antibodies. 2: Reduced vaccine doses required to substantially reduce costs. 3: Polymer matrix helps stabilize vaccine for at least 1 month. 4: Potential for self-management. | 1: Uncertainty in neutralizing antibody test results: only four weeks (reliable test is after six weeks). 2: Immunogenicity also needs to be evaluated in clinical trials. | [30] |
| 12 October 2020 | Recombinant protein | dMN | 1: minimally invasive. 2: Significant antibody responses can be maintained for up to 97 days. 3: Will not cause any damage to the environment. 4: Facilitates rapid vaccination. | 1: This platform is not suitable for delivering mRNA. 2: The titers of specific antibodies produced by the MN method vary widely. 3: Sterility is difficult to guarantee. | [111] |
| 27 February 2021 | Viral vector vaccine | dMN | 1: Incorporation of vaccine into patch significantly improves its thermal stability. 2: MN delivery of vaccines enables. low-dose priming of immune response. 3: Allow storage and distribution without cold chain. | 1: Clinical data are difficult to obtain and lack robust statistical analysis. | [116] |
| 17 August 2021 | Nucleic acid vaccines | cMN | 1: Non-intrusive and self-applying. 2: Dose savings achieved. 3: Realized the combination of rapid 3D printing technology and MN vaccine formulation, providing a versatile platform to improve global immunization and healthcare. | 1: Potential safety hazards caused by needle tip breakage. 2: Coated vaccine doses are limited. | [119] |
| 26 August 2021 | DNA vaccine | dMN | 1: Enhanced thermal stability of the vaccine and ease of handling. 2: Did not have any noticeable side effects during in vivo vaccination. | 1: Stability needs further investigation because DNA vaccine itself is relatively stable. 2: Nanovaccine needs more clinical research. | [120] |
| 13 September 2021 | DNA vaccine | sMN | 1: Ultra-low-cost (<$1), handheld (<50 g), battery-free electroporation microneedle vaccination system. 2: Can save at least 10 times the vaccine dose. 3: Vaccination was well tolerated with mild, transient skin effects. | 1: The electric field generated between the electrodes may cause skin and nerve irritation. 2: The immune response is only characterized by pseudoviruses, and more detailed immunological characterization is required. | [20] |
| 29 October 2021 | recombinant protein | dMN | 1: Ease of self-administration, reduced cold chain dependency, and improved thermal stability. 2: Facilitates improved vaccine transportation and delivery to patients in low- and middle-income countries 3: Protection with just a single dose of the vaccine. | 1: Antibody and/or cellular immunity induced by the vaccination regime needs further validation and may not be sufficient to suppress viral replication. | [81] |
| 12 January 2022 | mRNA Vaccine | dMN | 1: Helping lower barriers to vaccine access in resource-poor settings 2: For contributions to the development of biomaterials for vaccine applications. | 1: mRNA vaccines are prone to degradation 2: Antibody titers of MN vaccines vary, and precise dosing is challenging. | [121] |
| 15 April 2022 | inactivated virus | dMN | 1: Helping vaccinators accurately record vaccination data. 2: Easily self-administered by individuals. 3: Patch base can be easily removed from the skin surface. | 1: There are currently no large-scale trials of vaccine delivery in large animals or humans. | [16] |
4.4. Advantages and Challenges of MN Delivery of COVID-19 Vaccine
5. Challenges and Prospects of MNs Delivery of Vaccines
5.1. Vaccine Waste
5.2. Vaccine Safety
5.3. Vaccine Degradation
5.4. Vaccine Loading
5.5. Prospects
5.5.1. Practical Application of MNs Vaccines
5.5.2. An Alternative Solution of Mass Vaccination in Special Population
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilleman, M.R. Vaccines in Historic Evolution and Perspective: A Narrative of Vaccine Discoveries. Vaccine 1999, 18, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- WHO. Vaccines and Immunization. 2022. Available online: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1 (accessed on 1 December 2022).
- SDGs. World Health Statistics 2022. Available online: https://repository.gheli.harvard.edu/repository/11008/ (accessed on 1 December 2022).
- Chong, I.; Shin, S.D.J.; Sanoj Rejinold, N.; Yeu-Chun, K. Microneedles for vaccine delivery: Challenges and future perspectives. Ther. Deliv. 2017, 8, 447–460. [Google Scholar]
- Portnoy, A.; Ozawa, S.; Grewal, S.; Norman, B.A.; Rajgopal, J.; Gorham, K.M.; Haidari, L.A.; Brown, S.T.; Lee, B.Y. Costs of vaccine programs across 94 low- and middle-income countries. Vaccine 2015, 33 (Suppl. S1), A99–A108. [Google Scholar] [CrossRef]
- Love, A.S.; Love, R.J. Considering Needle Phobia among Adult Patients During Mass COVID-19 Vaccinations. J. Prim. Care Community Health 2021, 12, 21501327211007393. [Google Scholar] [CrossRef] [PubMed]
- Weniger, B.G.; Papania, M.J. Alternative vaccine delivery methods. Vaccines 2013, 1200–1231. [Google Scholar]
- Levin, C.; Perrin, H.; Combadiere, B. Tailored immunity by skin antigen-presenting cells. Hum. Vaccines Immunother. 2015, 11, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Engelke, L.; Winter, G.; Hook, S.; Engert, J. Recent insights into cutaneous immunization: How to vaccinate via the skin. Vaccine 2015, 33, 4663–4674. [Google Scholar] [CrossRef] [PubMed]
- Waltz, E. How nasal-spray vaccines could change the pandemic. Nature 2022, 609, 240–242. [Google Scholar] [CrossRef]
- Liang, F.; Loré, K. Local innate immune responses in the vaccine adjuvant-injected muscle. Clin. Transl. Immunol. 2016, 5, e74. [Google Scholar] [CrossRef]
- Niu, L.; Chu, L.Y.; Burton, S.A.; Hansen, K.J.; Panyam, J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J. Control. Release 2019, 294, 268–278. [Google Scholar] [CrossRef]
- Patel, S.M.; Atmar, R.L.; El Sahly, H.M.; Guo, K.; Hill, H.; Keitel, W.A. Direct Comparison of an Inactivated Subvirion Influenza A Virus Subtype H5N1 Vaccine Administered by the Intradermal and Intramuscular Routes. J. Infect. Dis. 2012, 206, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, Y.; Nandakumar, S.; Choi, S.-O.; Lee, J.W.; Kim, Y.-C.; Posey, J.E.; Sable, S.B.; Prausnitz, M.R. Bacillus Calmette-Guérin vaccination using a microneedle patch. Vaccine 2011, 29, 2626–2636. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Srivastava, V.; Baindara, P.; Ahmad, A. Thermostable vaccines: An innovative concept in vaccine development. Expert Rev. Vaccines 2022, 21, 811–824. [Google Scholar] [CrossRef]
- Li, Q.; Xu, R.; Fan, H.; Xu, J.; Xu, Y.; Cao, P.; Zhang, Y.; Liang, T.; Zhang, Y.; Chen, W.; et al. Smart Mushroom-Inspired Imprintable and Lightly Detachable (MILD) Microneedle Patterns for Effective COVID-19 Vaccination and Decentralized Information Storage. ACS Nano 2022, 16, 7512–7524. [Google Scholar] [CrossRef] [PubMed]
- Manzoli, L.; Ioannidis, J.P.; Flacco, M.E.; De Vito, C.; Villari, P. Effectiveness and harms of seasonal and pandemic influenza vaccines in children, adults and elderly: A critical review and re-analysis of 15 meta-analyses. Hum. Vaccin. Immunother. 2012, 8, 851–862. [Google Scholar] [CrossRef]
- Zuckerman, J.N. Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J. Med. Virol. 2006, 78, 169–177. [Google Scholar] [CrossRef]
- Darrah, P.A.; Zeppa, J.J.; Maiello, P.; Hackney, J.A.; Wadsworth, M.H., 2nd; Hughes, T.K.; Pokkali, S.; Swanson, P.A., 2nd; Grant, N.L.; Rodgers, M.A.; et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020, 577, 95–102. [Google Scholar] [CrossRef]
- Xia, D.; Jin, R.; Byagathvalli, G.; Yu, H.; Ye, L.; Lu, C.Y.; Bhamla, M.S.; Yang, C.; Prausnitz, M.R. An ultra-low-cost electroporator with microneedle electrodes (ePatch) for SARS-CoV-2 vaccination. Proc. Natl. Acad. Sci. USA 2021, 118, e2110817118. [Google Scholar] [CrossRef]
- Jin, Z.; Zhao, B.; Wan, W.; Li, Y.; Bai, X.; Hu, C.; Wang, J.; Li, Y.; Xin, W.; Kang, L.; et al. Application of microneedle-based vaccines in biosecurity. J. Biosaf. Biosecur. 2022, 4, 75–83. [Google Scholar] [CrossRef]
- van der Maaden, K.; Trietsch, S.J.; Kraan, H.; Varypataki, E.M.; Romeijn, S.; Zwier, R.; van der Linden, H.J.; Kersten, G.; Hankemeier, T.; Jiskoot, W.; et al. Novel hollow microneedle technology for depth-controlled microinjection-mediated dermal vaccination: A study with polio vaccine in rats. Pharm. Res. 2014, 31, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Wirth, D.M.; Ortega-Rivera, O.A.; Steinmetz, N.F.; Pokorski, J.K. Dissolving Microneedle Delivery of a Prophylactic HPV Vaccine. Biomacromolecules 2022, 23, 903–912. [Google Scholar] [CrossRef]
- Courtenay, A.J.; Rodgers, A.M.; McCrudden, M.T.C.; McCarthy, H.O.; Donnelly, R.F. Novel Hydrogel-Forming Microneedle Array for Intradermal Vaccination in Mice Using Ovalbumin as a Model Protein Antigen. Mol. Pharm. 2019, 16, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Mikszta, J.A.; Alarcon, J.B.; Brittingham, J.M.; Sutter, D.E.; Pettis, R.J.; Harvey, N.G. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat. Med. 2002, 8, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Kim, Y.C.; Vunnava, A.; Yoo, D.G.; Song, J.M.; Prausnitz, M.R.; Compans, R.W.; Kang, S.M. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J. Virol. 2010, 84, 7760–7769. [Google Scholar] [CrossRef]
- Edens, C.; Collins, M.L.; Goodson, J.L.; Rota, P.A.; Prausnitz, M.R. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine 2015, 33, 4712–4718. [Google Scholar] [CrossRef]
- Arya, J.M.; Dewitt, K.; Scott-Garrard, M.; Chiang, Y.W.; Prausnitz, M.R. Rabies vaccination in dogs using a dissolving microneedle patch. J. Control. Release 2016, 239, 19–26. [Google Scholar] [CrossRef]
- Kines, R.C.; Zarnitsyn, V.; Johnson, T.R.; Pang, Y.Y.; Corbett, K.S.; Nicewonger, J.D.; Gangopadhyay, A.; Chen, M.; Liu, J.; Prausnitz, M.R.; et al. Vaccination with human papillomavirus pseudovirus-encapsidated plasmids targeted to skin using microneedles. PLoS ONE 2015, 10, e0120797. [Google Scholar] [CrossRef]
- Kim, E.; Erdos, G.; Huang, S.; Kenniston, T.W.; Balmert, S.C.; Carey, C.D.; Raj, V.S.; Epperly, M.W.; Klimstra, W.B.; Haagmans, B.L.; et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine 2020, 55, 102743. [Google Scholar] [CrossRef]
- WHO. WHO Coronavirus (COVID-19). 2022. Available online: https://covid19.who.int/ (accessed on 1 December 2022).
- McDermott, A. Core Concept: Herd immunity is an important-and often misunderstood-public health phenomenon. Proc. Natl. Acad. Sci. USA 2021, 118, e2107692118. [Google Scholar] [CrossRef]
- Forman, R.; Shah, S.; Jeurissen, P.; Jit, M.; Mossialos, E. COVID-19 vaccine challenges: What have we learned so far and what remains to be done? Health Policy 2021, 125, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Vescovo, P.; Rettby, N.; Ramaniraka, N.; Liberman, J.; Hart, K.; Cachemaille, A.; Piveteau, L.D.; Zanoni, R.; Bart, P.A.; Pantaleo, G. Safety, tolerability and efficacy of intradermal rabies immunization with DebioJect. Vaccine 2017, 35, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- DebioJect Micro-Needles Intradermal Injections. Available online: https://www.debiotech.com/debioject/ (accessed on 1 December 2022).
- Pierre, M.B.; Rossetti, F.C. Microneedle-based drug delivery systems for transdermal route. Curr. Drug Targets 2014, 15, 281–291. [Google Scholar] [CrossRef]
- Wagner, A.R. Article of Manufacture For Intracutaneous Injections. U.S. Patent 2,893,392, 7 July 1959. [Google Scholar]
- Gerstel, M.S.; Place, V.A. Drug Delivery Device Background of the Invention. US3964482A, 22 June 1976. [Google Scholar]
- Hashmi, S.; Hashmi, G.; Gaugler, R. Genetic Transformation of an Entomopathogenic Nematode by Microinjection. J. Invertebr. Pathol. 1994, 66, 293–296. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mikszta, J.A.; Cormier, M.; Andrianov, A.K. Microneedle-based vaccines. Curr. Top. Microbiol. Immunol. 2009, 333, 369–393. [Google Scholar] [CrossRef]
- Holly Korschun, J.T. Disappearing Needles: Vaccine-Delivery Patch with Dissolving Microneedles Eliminates “Sharps” Waste and Improves Protection. Fierce Biotech, 20 July 2010. [Google Scholar]
- Hyung, I.I.; Jung, K.L. A Solid Type Microneedle and Methods for Preparing It; World Intellectual Proprety Organisation: Geneva, Switzerland, 2006. [Google Scholar]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, B.Z.; Wang, Q.L.; Jin, X.; Guo, X.D. Fabrication of coated polymer microneedles for transdermal drug delivery. J. Control. Release 2017, 265, 14–21. [Google Scholar] [CrossRef]
- Choi, I.J.; Cha, H.R.; Hwang, S.J.; Baek, S.K.; Lee, J.M.; Choi, S.O. Live Vaccinia Virus-Coated Microneedle Array Patches for Smallpox Vaccination and Stockpiling. Pharmaceutics 2021, 13, 209. [Google Scholar] [CrossRef]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef] [PubMed]
- Lahiji, S.F.; Dangol, M.; Jung, H. A patchless dissolving microneedle delivery system enabling rapid and efficient transdermal drug delivery. Sci. Rep. 2015, 5, 7914. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. Macromol. Biosci. 2021, 21, e2000307. [Google Scholar] [CrossRef] [PubMed]
- Pradeep Narayanan, S.; Raghavan, S. Solid silicon microneedles for drug delivery applications. Int. J. Adv. Manuf. Technol. 2016, 93, 407–422. [Google Scholar] [CrossRef]
- Gholami, S.; Mohebi, M.M.; Hajizadeh-Saffar, E.; Ghanian, M.H.; Zarkesh, I.; Baharvand, H. Fabrication of microporous inorganic microneedles by centrifugal casting method for transdermal extraction and delivery. Int. J. Pharm. 2019, 558, 299–310. [Google Scholar] [CrossRef]
- Soltani-Arabshahi, R.; Wong, J.W.; Duffy, K.L.; Powell, D.L. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014, 150, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Vallhov, H.; Xia, W.; Engqvist, H.; Scheynius, A. Bioceramic microneedle arrays are able to deliver OVA to dendritic cells in human skin. J. Mater. Chem. B 2018, 6, 6808–6816. [Google Scholar] [CrossRef]
- Mugo, S.M.; Lu, W. Modified stainless steel microneedle electrode for polyphenolics detection. Anal.Bioanal. Chem. 2020, 412, 7063–7072. [Google Scholar] [CrossRef]
- Parker, E.R.; Rao, M.P.; Turner, K.L.; Meinhart, C.D.; MacDonald, N.C. Bulk Micromachined Titanium Microneedles. J. Microelectromech. Syst. 2007, 16, 289–295. [Google Scholar] [CrossRef]
- Yadav, S.; Dogra, S. A Cutaneous Reaction to Microneedling for Postacne Scarring Caused by Nickel Hypersensitivity. Aesthetic Surg. J. 2016, 36, NP168–NP170. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Carrier, A.; Chen, Y.; Lin, S.; Wang, J.; Cui, S.; Zhang, X. Polymeric microneedles for controlled transdermal drug delivery. J. Control. Release 2019, 315, 97–113. [Google Scholar] [CrossRef]
- Mbituyimana, B.; Ma, G.; Shi, Z.; Yang, G. Polymer-based microneedle composites for enhanced non-transdermal drug delivery. Appl. Mater. Today 2022, 29, 659. [Google Scholar] [CrossRef]
- Hansen, L.J.J.; Daoussi, R.; Vervaet, C.; Remon, J.P.; De Beer, T.R.M. Freeze-drying of live virus vaccines: A review. Vaccine 2015, 33, 5507–5519. [Google Scholar] [CrossRef] [PubMed]
- Elveborg, S.; Monteil, V.M.; Mirazimi, A. Methods of Inactivation of Highly Pathogenic Viruses for Molecular, Serology or Vaccine Development Purposes. Pathogens 2022, 11, 271. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Goodson, J.L.; Rota, P.A.; Orenstein, W.A. A microneedle patch for measles and rubella vaccination: A game changer for achieving elimination. Curr. Opin. Virol. 2020, 41, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, H.J.; van de Wijdeven, G.G.; Kraan, H.; Amorij, J.P.; Kersten, G.F. Bioneedles as alternative delivery system for hepatitis B vaccine. J. Control. Release 2010, 147, 211–217. [Google Scholar] [CrossRef]
- Frew, P.M.; Paine, M.B.; Rouphael, N.; Schamel, J.; Chung, Y.; Mulligan, M.J.; Prausnitz, M.R. Acceptability of an inactivated influenza vaccine delivered by microneedle patch: Results from a phase I clinical trial of safety, reactogenicity, and immunogenicity. Vaccine 2020, 38, 7175–7181. [Google Scholar] [CrossRef]
- Rodgers, A.M.; McCrudden, M.T.C.; Vincente-Perez, E.M.; Dubois, A.V.; Ingram, R.J.; Larraneta, E.; Kissenpfennig, A.; Donnelly, R.F. Design and characterisation of a dissolving microneedle patch for intradermal vaccination with heat-inactivated bacteria: A proof of concept study. Int. J. Pharm. 2018, 549, 87–95. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, B.; Xu, J.; Shou, D.; Liu, E.; Gao, J.; Liang, W.; Huang, Y. Microneedle-assisted dendritic cell-targeted nanoparticles for transcutaneous DNA immunization. Polym. Chem. 2015, 6, 373–379. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, L.; Zhang, S.; Xu, B.; Gao, Y.; Hu, Y.; Hou, J.; Bai, B.; Shen, H.; Mao, P. DNA-based vaccination against hepatitis B virus using dissolving microneedle arrays adjuvanted by cationic liposomes and CpG ODN. Drug Deliv. 2016, 23, 2391–2398. [Google Scholar] [CrossRef]
- WHO. Comirnaty, COVID 19 mRNA Vaccine. Available online: https://cdn.who.int/media/docs/default-source/immunization/covid-19/21040_chinese_vaccine-explainer_comirnaty-2.pdf?sfvrsn=271a9940_8 (accessed on 1 December 2022).
- Chen, Y.-C.; Chen, S.-J.; Cheng, H.-F.; Yeh, M.-K. Development of Yersinia pestis F1 antigen-loaded liposome vaccine against plague using microneedles as a delivery system. J. Drug Deliv. Sci. Technol. 2020, 55. [Google Scholar] [CrossRef]
- Weldon, W.C.; Martin, M.P.; Zarnitsyn, V.; Wang, B.; Koutsonanos, D.; Skountzou, I.; Prausnitz, M.R.; Compans, R.W. Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clin. Vaccine Immunol. 2011, 18, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Pattarabhiran, S.P.; Saju, A.; Sonawane, K.R.; Manimaran, R.; Bhatnagar, S.; Roy, G.; Kulkarni, R.B.; Venuganti, V.V.K. Dissolvable Microneedle-Mediated Transcutaneous Delivery of Tetanus Toxoid Elicits Effective Immune Response. AAPS Pharm. Sci. Tech. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Romeijn, S.; Du, G.; Le Devedec, S.E.; Vrieling, H.; O’Mahony, C.; Bouwstra, J.A.; Kersten, G. Diphtheria toxoid dissolving microneedle vaccination: Adjuvant screening and effect of repeated-fractional dose administration. Int. J. Pharm. 2020, 580, 119182. [Google Scholar] [CrossRef]
- Dean, C.H.; Alarcon, J.B.; Waterston, A.M.; Draper, K.; Early, R.; Guirakhoo, F.; Monath, T.P.; Mikszta, J.A. Cutaneous delivery of a live, attenuated chimeric flavivirus vaccine against Japanese encephalitis (ChimeriVax)-JE) in non-human primates. Hum. Vaccin. 2005, 1, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.D.; Murtadak, V.B.; Kale, S.D.; Shinde, P.V.; Parkhi, S.S. Evaluation of different inactivation methods for high and low pathogenic avian influenza viruses in egg-fluids for antigen preparation. J. Virol. Methods 2015, 222, 28–33. [Google Scholar] [CrossRef]
- Koutsonanos, D.G.; Esser, E.S.; McMaster, S.R.; Kalluri, P.; Lee, J.W.; Prausnitz, M.R.; Skountzou, I.; Denning, T.L.; Kohlmeier, J.E.; Compans, R.W. Enhanced immune responses by skin vaccination with influenza subunit vaccine in young hosts. Vaccine 2015, 33, 4675–4682. [Google Scholar] [CrossRef]
- Sharon, E.; Frey, C.H.; Robert, F.; Yang, E.; Boken, D.; Sekulovich, R.E.; Percell, S.A.E.I.; Hirabayashi, S.; Burke, R.L. Effects of Antigen Dose and Immunization Regimens on Antibody Responses to a Cytomegalovirus Glycoprotein B Subunit Vaccine. J. Infect. Dis. 1999, 180, 1700–1703. [Google Scholar]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef]
- Maripat, C.; Lee, D.J.; Dennis, A. Carson, and Helen Tighe. Gene Vaccination with Naked Plasmid DNA: Mechanism of CTL Priming. J. Exp. Med. 1996, 184, 1555–1560. [Google Scholar]
- Prather, K.J.; Sagar, S.; Murphy, J.; Chartrain, M. Industrial scale production of plasmid DNA for vaccine and gene therapy: Plasmid design, production, and purification. Enzym. Microb. Technol. 2003, 33, 865–883. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Weiner, D.B. Developing DNA vaccines that call to dendritic cells. J. Clin. Investig. 2004, 114, 1241–1244. [Google Scholar] [CrossRef]
- McMillan, C.L.; Choo, J.J.; Idris, A.; Supramaniam, A.; Modhiran, N.; Amarilla, A.A.; Isaacs, A.; Cheung, S.T.; Liang, B.; Bielefeldt-Ohmann, H.; et al. Complete protection by a single-dose skin patch–delivered SARS-CoV-2 spike vaccine. Sci. Adv. 2021, 7, eabj8065. [Google Scholar] [CrossRef]
- Cole, G.; McCaffrey, J.; Ali, A.A.; McBride, J.W.; McCrudden, C.M.; Vincente-Perez, E.M.; Donnelly, R.F.; McCarthy, H.O. Dissolving microneedles for DNA vaccination: Improving functionality via polymer characterization and RALA complexation. Hum. Vaccines Immunother. 2017, 13, 50–62. [Google Scholar] [CrossRef]
- Liao, J.F.; Lee, J.C.; Lin, C.K.; Wei, K.C.; Chen, P.Y.; Yang, H.W. Self-Assembly DNA Polyplex Vaccine inside Dissolving Microneedles for High-Potency Intradermal Vaccination. Theranostics 2017, 7, 2593–2605. [Google Scholar] [CrossRef]
- Park, K.S.; Sun, X.; Aikins, M.E.; Moon, J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021, 169, 137–151. [Google Scholar] [CrossRef]
- Blackburn, L. RNA Vaccines: An Introduction. Policy Briefing. 2018. Available online: https://www.grove-park.org/covid/800-VACCINE-INFO/mRNA-vaccines-an-introduction-briefing-note.pdf (accessed on 1 December 2022).
- Rein Verbeke, I.L.; De Smedt, S.; Dewitte, H. Three decades of messenger RNA vaccine development. Nano Today 2019, 28, 10766. [Google Scholar] [CrossRef]
- Boczkowski, D.; Nair, S.K.; Snyder, D.; Gilboa, E. Dendritic Cells Pulsed with RNA are Potent Antigen-presenting Cells In Vitro and In Vivo. J. Exp. Med. 1996, 184, 465–472. [Google Scholar] [CrossRef]
- Koh, K.J.; Liu, Y.; Lim, S.H.; Loh, X.J.; Kang, L.; Lim, C.Y.; Phua, K.K.L. Formulation, characterization and evaluation of mRNA-loaded dissolvable polymeric microneedles (RNApatch). Sci. Rep. 2018, 8, 11842. [Google Scholar] [CrossRef]
- Golombek, S.; Pilz, M.; Steinle, H.; Kochba, E.; Levin, Y.; Lunter, D.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Intradermal Delivery of Synthetic mRNA Using Hollow Microneedles for Efficient and Rapid Production of Exogenous Proteins in Skin. Mol. Ther. Nucleic Acids 2018, 11, 382–392. [Google Scholar] [CrossRef]
- Available online: https://www.gavi.org/vaccineswork/what-are-protein-subunit-vaccines-and-how-could-they-be-used-against-covid-19 (accessed on 1 December 2022).
- Wang, Y.; Li, S.; Dong, C.; Ma, Y.; Song, Y.; Zhu, W.; Kim, J.; Deng, L.; Denning, T.L.; Kang, S.M.; et al. Skin vaccination with dissolvable microneedle patches incorporating influenza neuraminidase and flagellin protein nanoparticles induces broad immune protection against multiple influenza viruses. ACS Appl. Bio. Mater. 2021, 4, 4953–4961. [Google Scholar] [CrossRef]
- Song, J.M.; Kim, Y.C.; Barlow, P.G.; Hossain, M.J.; Park, K.M.; Donis, R.O.; Prausnitz, M.R.; Compans, R.W.; Kang, S.M. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antivir. Res. 2010, 88, 244–247. [Google Scholar] [CrossRef]
- Kim, Y.C.; Quan, F.S.; Compans, R.W.; Kang, S.M.; Prausnitz, M.R. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS Pharm. Sci. Tech. 2010, 11, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, C.; Zhang, Q.; Cheng, K.; Shan, W.; Wang, X.; Yang, J.; Wang, Y.; Ren, L. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of HBc VLPs based cancer vaccine. Appl. Mater. Today 2021, 24, 110. [Google Scholar] [CrossRef]
- Shabir, O. What Is a Toxoid Vaccine? 2021. Available online: https://www.news-medical.net/health/What-is-a-Toxoid-Vaccine.aspx (accessed on 1 December 2022).
- Zhu, D.D.; Wang, X.L.; Zhang, X.P.; Ma, J.J.; Kong, L.; Zhang, M.M.; Guo, X.D.; Wang, C. A Dissolvable Microneedle Formulation of Bordetella pertussis Subunit Vaccine: Translational Development and Immunological Evaluation in Mice. ACS Appl. Bio. Mater. 2019, 2, 5053–5061. [Google Scholar] [CrossRef] [PubMed]
- de Groot, A.M.; Platteel, A.C.M.; Kuijt, N.; van Kooten, P.J.S.; Vos, P.J.; Sijts, A.; van der Maaden, K. Nanoporous Microneedle Arrays Effectively Induce Antibody Responses against Diphtheria and Tetanus Toxoid. Front. Immunol. 2017, 8, 1789. [Google Scholar] [CrossRef]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses—A statement of the Coronavirus Study Group. Nat. Microbiol. 2020, 5, 862. [Google Scholar] [CrossRef]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef]
- Oberfeld, B.; Achanta, A.; Carpenter, K.; Chen, P.; Gilette, N.M.; Langat, P.; Said, J.T.; Schiff, A.E.; Zhou, A.S.; Barczak, A.K.; et al. SnapShot: COVID-19. Cell 2020, 181, 954. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv, 2020; in print. [Google Scholar] [CrossRef]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Cohen, J. Wuhan seafood market may not be source of novel virus spreading globally. Science 2020, 10, 1126. [Google Scholar] [CrossRef]
- Billah, M.A.; Miah, M.M.; Khan, M.N. Reproductive number of coronavirus: A systematic review and meta-analysis based on global level evidence. PLoS ONE 2020, 15, e0242128. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271.e278–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Alimohamadi, Y.; Sepandi, M.; Taghdir, M.; Hosamirudsari, H. Determine the most common clinical symptoms in COVID-19 patients: A systematic review and meta-analysis. J. Prev. Med. Hyg. 2020, 61, E304–E312. [Google Scholar] [CrossRef]
- Available online: http://www.xinhuanet.com/world/2021-05/07/c_1127419114.htm (accessed on 1 December 2022).
- WHO. WHO Recommendation of Sinovac COVID-19 vaccine (Vero Cell [Inactivated])—CoronaVac. 2021. Available online: https://extranet.who.int/pqweb/vaccines/who-recommendation-sinovac-covid-19-vaccine-vero-cell-inactivated-coronavac (accessed on 1 December 2022).
- Available online: https://news.un.org/zh/story/2021/11/1093862 (accessed on 1 December 2022).
- COVID-19 Vaccine Tracker. Vaccines Candidates in Clinical Trials. 2022. Available online: https://covid19.trackvaccines.org/vaccines/#phase-3 (accessed on 1 December 2022).
- Kuwentrai, C.; Yu, J.; Rong, L.; Zhang, B.Z.; Hu, Y.F.; Gong, H.R.; Dou, Y.; Deng, J.; Huang, J.D.; Xu, C. Intradermal delivery of receptor-binding domain of SARS-CoV-2 spike protein with dissolvable microneedles to induce humoral and cellular responses in mice. Bioeng. Transl. Med. 2021, 6, e10202. [Google Scholar] [CrossRef]
- COVID-19 Vaccine AstraZeneca. 2021. Available online: https://www.tga.gov.au/apm-summary/covid-19-vaccine-astrazeneca (accessed on 1 December 2022).
- Interim Recommendations for Use of the ChAdOx1-S [Recombinant] Vaccine against COVID-19 (AstraZeneca COVID-19 Vaccine AZD1222 Vaxzevria™, SII COVISHIELD™) 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1/ (accessed on 1 December 2022).
- Available online: https://www.bjnews.com.cn/detail/161434174815958.html (accessed on 1 December 2022).
- Halperin, S.A.; Ye, L.; MacKinnon-Cameron, D.; Smith, B.; Cahn, P.E.; Ruiz-Palacios, G.M.; Ikram, A.; Lanas, F.; Lourdes Guerrero, M.; Muñoz Navarro, S.R.; et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: An international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet 2022, 399, 237–248. [Google Scholar] [CrossRef]
- Flynn, O.; Dillane, K.; Lanza, J.S.; Marshall, J.M.; Jin, J.; Silk, S.E.; Draper, S.J.; Moore, A.C. Low Adenovirus Vaccine Doses Administered to Skin Using Microneedle Patches Induce Better Functional Antibody Immunogenicity as Compared to Systemic Injection. Vaccines 2021, 9, 299. [Google Scholar] [CrossRef]
- BBC. 2020. Available online: https://web.archive.org/web/20210428215106/https://www.bbc.com/zhongwen/trad/world-55375185 (accessed on 1 December 2022).
- Available online: https://web.archive.org/web/20220705190758/https://www.cna.com.tw/news/aopl/202206180003.aspx (accessed on 1 December 2022).
- Caudill, C.; Perry, J.L.; Iliadis, K.; Tessema, A.T.; Lee, B.J.; Mecham, B.S.; Tian, S.; DeSimone, J.M. Transdermal vaccination via 3D-printed microneedles induces potent humoral and cellular immunity. Proc. Natl. Acad. Sci. USA 2021, 118, e2102595118. [Google Scholar] [CrossRef]
- Yin, Y.; Su, W.; Zhang, J.; Huang, W.; Li, X.; Ma, H.; Tan, M.; Song, H.; Cao, G.; Yu, S.; et al. Separable Microneedle Patch to Protect and Deliver DNA Nanovaccines Against COVID-19. ACS Nano 2021, 15, 14347–14359. [Google Scholar] [CrossRef]
- Kapnick, S.M. The Nanoparticle-Enabled Success of COVID-19 mRNA Vaccines and the Promise of Microneedle Platforms for Pandemic Vaccine Response. DNA Cell Biol. 2022, 41, 25–29. [Google Scholar] [CrossRef]
- Chen, M.C.; Huang, S.F.; Lai, K.Y.; Ling, M.H. Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination. Biomaterials 2013, 34, 3077–3086. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Gadeela, P.R.; Thathireddy, P.; Venuganti, V.V.K. Microneedle-based drug delivery: Materials of construction. J. Chem. Sci. 2019, 131, 1–28. [Google Scholar] [CrossRef]
- McCrudden, M.T.; Alkilani, A.Z.; Courtenay, A.J.; McCrudden, C.M.; McCloskey, B.; Walker, C.; Alshraiedeh, N.; Lutton, R.E.; Gilmore, B.F.; Woolfson, A.D.; et al. Considerations in the sterile manufacture of polymeric microneedle arrays. Drug Deliv. Transl. Res. 2015, 5, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.; Shayan, F.L.; Kim, S.; Huh, I.; Ma, Y.; Yang, H.; Kang, G.; Jung, H. Physicochemical study of ascorbic acid 2-glucoside loaded hyaluronic acid dissolving microneedles irradiated by electron beam and gamma ray. Carbohydr. Polym. 2018, 180, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.D.; Zhang, X.P.; Zhang, B.L.; Hao, Y.Y.; Guo, X.D. Safety Assessment of Microneedle Technology for Transdermal Drug Delivery: A Review. Adv. Ther. 2020, 3, 33. [Google Scholar] [CrossRef]
- Kathuria, H.; Kang, K.; Cai, J.; Kang, L. Rapid microneedle fabrication by heating and photolithography. Int. J. Pharm. 2020, 575, 118992. [Google Scholar] [CrossRef]
- Zhu, Q.; Zarnitsyn, V.G.; Ye, L.; Wen, Z.; Gao, Y.; Pan, L.; Skountzou, I.; Gill, H.S.; Prausnitz, M.R.; Yang, C.; et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl. Acad. Sci. USA 2009, 106, 7968–7973. [Google Scholar] [CrossRef]
- Gumel, A.B.; Iboi, E.A.; Ngonghala, C.N.; Ngwa, G.A. Toward Achieving a Vaccine-Derived Herd Immunity Threshold for COVID-19 in the U.S. Front. Public Health 2021, 9, 709369. [Google Scholar] [CrossRef]
- The Vaccine Innovation Prioritisation Strategy (VIPS). 2021. Available online: https://www.gavi.org/our-alliance/market-shaping/vaccine-innovation-prioritisation-strategy (accessed on 1 December 2022).
- Medical Countermeasures.gov. BARDA Establishes Four New Partnerships to Explore Innovative Vaccine Delivery Technologies. 2020. Available online: https://www.medicalcountermeasures.gov/newsroom/2020/barda-new-partnerships/ (accessed on 1 December 2022).
- Forster, A.; Junger, M. Opportunities and challenges for commercializing microarray patches for vaccination from a MAP developer’s perspective. Hum. Vaccines Immunother. 2022, 18, 2050123. [Google Scholar] [CrossRef]
- Forster, A.H.; Witham, K.; Depelsenaire, A.C.I.; Veitch, M.; Wells, J.W.; Wheatley, A.; Pryor, M.; Lickliter, J.D.; Francis, B.; Rockman, S.; et al. Safety, tolerability, and immunogenicity of influenza vaccination with a high-density microarray patch: Results from a randomized, controlled phase I clinical trial. PLoS Med. 2020, 17, e1003024. [Google Scholar] [CrossRef]
- Emergex COVID-19. Vaccine Candidate Successfully Coated onto Zosano Micro-Needle Patch. 2022. Available online: https://www.contractpharma.com/contents/view_breaking-news/2022-05-17/emergex-covid-19-vaccine-candidate-successfully-coated-onto-zosano-micro-needle-patch (accessed on 1 December 2022).
- Emergex Vaccines Announces the Successful Coating of its Novel CD8+ T cell Adaptive COVID-19 Vaccine onto Zosano Pharma’s Micro-Needle Patch. 2022. Available online: https://www.globenewswire.com/en/news-release/2022/05/17/2444730/0/en/Emergex-Vaccines-Announces-the-Successful-Coating-of-its-Novel-CD8-T-cell-Adaptive-COVID-19-Vaccine-onto-Zosano-Pharma-s-Micro-Needle-Patch.html (accessed on 1 December 2022).
- Taylor, N.P. In a first, Micron starts testing microneedle vaccine in kids. Firece Pharma. 2021. Available online: https://www.fiercepharma.com/drug-delivery/a-first-micron-starts-testing-microneedle-vaccine-kids (accessed on 1 December 2022).
- COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed on 1 December 2022).
- Sarmiento Suárez, R.; Local Burden of Disease Vaccine Coverage Collaborators. Mapping routine measles vaccination in low- and middle-income countries. Nature 2021, 589, 415–419. [Google Scholar] [CrossRef]
- Thomasy, H. Tiny Needles Make a Big Impact for Vaccine Delivery. 2023. Available online: https://www.drugdiscoverynews.com/tiny-needles-make-a-big-impact-for-vaccine-delivery-15615 (accessed on 1 March 2023).
- Andryukov, B.G.; Besednova, N.N. Older adults: Panoramic view on the COVID-19 vaccination. AIMS Public Health 2021, 8, 388–415. [Google Scholar] [CrossRef]
- Healthcare-Pharmaceuticals. Hong Kong Approves Baby Version of BioNTech Vaccine for Toddlers. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/hong-kong-approves-baby-version-biontech-vaccine-toddlers-2022-10-12/ (accessed on 1 December 2022).
- WHO. Measles-Rubella Microarray Patch (MR–MAP) Target Product Profile. UNICEF. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/330394/9789240000209-eng.pdf (accessed on 1 December 2022).









| Description | Traditional Injection Vaccines | MN-Based Vaccines | |||||
|---|---|---|---|---|---|---|---|
| Types | Intramuscular | Intradermal | sMN | cMN | hMN | dMN | Hyd MN |
| Vaccines | Influenza [17], HBV [18] | BCG [19] | COVID-19 [20] | Ebola [21] | Polio [22] | HPV [23] | Ovalbumin [24] |
| Sharps injury and contamination | Have risks | Needle Breakage Risk | No risks | ||||
| Stability | Refrigerated or frozen | Depending to the vaccine | Room temperature | ||||
| Cold chain | Need | Depending to the vaccine | No need | ||||
| Mechanism | Vaccines are injected into the muscle or intradermal multiple times through a syringe | Vaccines bypassing the stratum corneum and directly into epidermis or dermis | |||||
| Pain | Yes | Pain-free | |||||
| Patient compliance | Non-compliant | compliant | |||||
| Self-administration | Inoperable | Operable | |||||
| Type | Vaccine | Virus | Reference |
|---|---|---|---|
| Live-attenuated | Poliovirus vaccine | Poliomyelitis | [60] |
| Chimeric Flavivirus vaccine | Japanese encephalitis | [61] | |
| Measles vaccine | Measles virus | [27] | |
| Rubella vaccine | Rubella virus | [62] | |
| BCG | Mycobacterium tuberculosis | [14] | |
| Inactive vaccine | Hepatitis A | Hepatovirus A | [61] |
| Hepatitis B | Hepatitis B virus | [63] | |
| Influenza vaccines | Influenza virus | [64] | |
| Pseudomonas aeruginosa vaccine | Pseudomonas aeruginosa | [65] | |
| DNA vaccine | Rabies DNA vaccine | Rabies | [28] |
| Cancer vaccine | Malignant melanoma | [66] | |
| Hepatitis B | Hepatitis B virus | [67] | |
| RNA vaccine | COVID-19 vaccine | Sars-Cov-2 | [68] |
| Protein vaccine | F1 protein antigen of Yersinia pestis vaccine | Plague | [69] |
| Recombinant subunit Influenza vaccine | Influenza virus | [70] | |
| VLP vaccine | Influenza VLPs vaccine | Influenza virus | [26] |
| HPV vaccine | Human papillomavirus | [23] | |
| toxoid vaccines | Tetanus vaccine | Tetanus | [71] |
| Diphtheria vaccine | Corynebacterium diphtheriae | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.-X.; Hu, H.; Wong, Y.-Y.; Yao, X.; He, M.-L. Microneedles: An Emerging Vaccine Delivery Tool and a Prospective Solution to the Challenges of SARS-CoV-2 Mass Vaccination. Pharmaceutics 2023, 15, 1349. https://doi.org/10.3390/pharmaceutics15051349
Feng Y-X, Hu H, Wong Y-Y, Yao X, He M-L. Microneedles: An Emerging Vaccine Delivery Tool and a Prospective Solution to the Challenges of SARS-CoV-2 Mass Vaccination. Pharmaceutics. 2023; 15(5):1349. https://doi.org/10.3390/pharmaceutics15051349
Chicago/Turabian StyleFeng, Ya-Xiu, Huan Hu, Yu-Yuen Wong, Xi Yao, and Ming-Liang He. 2023. "Microneedles: An Emerging Vaccine Delivery Tool and a Prospective Solution to the Challenges of SARS-CoV-2 Mass Vaccination" Pharmaceutics 15, no. 5: 1349. https://doi.org/10.3390/pharmaceutics15051349