Research Advances in Nucleic Acid Delivery System for Rheumatoid Arthritis Therapy
Abstract
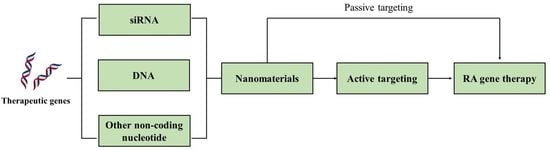
:1. Introduction
2. Nanomaterials for RA Gene Therapy
2.1. Polyethylenimine
2.2. Lipid-Based Nanoparticles
2.3. Chitosan
2.4. Others
3. Active Targeting for RA Gene Therapy
4. siRNA Delivery
4.1. siRNA against TNF-α
4.2. siRNA against Other Cytokines
4.3. siRNA Blocking Pathways
5. DNA Delivery
6. Strategies Targeting cfDNA
7. Challenges and Perspectives on the Nucleic Acid Delivery System for Rheumatoid Arthritis Therapy
- Limited tissue specificity: One of the challenges of nucleic acid delivery is achieving specific targeting to the inflammatory tissues in rheumatoid arthritis, which can limit the therapeutic effects of the nucleic acids. Furthermore, the complexity of the joint environment and the rapid clearance from the body may increase the difficulty of precise targeting.
- Limited cell entry: One of the challenges of nucleic acid delivery is getting the genetic material into the cells where it can have an effect. Many cells have mechanisms to protect against foreign nucleic acids, which can make it difficult for the therapeutic nucleic acids to enter the cells. The efficiency of cell entry can be improved by constructing biomimetic nanoparticles or modifying the surface of the delivery systems, such as changing the surface charges or adding active targeting ligands.
- Short-lived effects: As the nucleic acids used for gene therapy do not integrate into the cell genome, their therapeutic efficacy will weaken along with the division and death of cells. Therefore, the effects of gene therapy may be short-lived, particularly if the cells receiving the nucleic acid are rapidly dividing. This can limit the effectiveness of the therapy and require multiple treatments over time. Since RA is a chronic disease that needs long-term treatment, novel delivery systems, such as hydrogel systems, with sustained release capabilities can prolong the efficacy of therapeutic genes [84].
- Immune response: The body’s immune system may react to the therapeutic nucleic acids as foreign substances, potentially leading to an immune response that reduces the effectiveness of the therapy or causes side effects.
- Off-target effects: Although active targeting strategies are applied to nucleic acid delivery systems, they may still have off-target effects, leading to unintended changes in gene expression or other cellular processes. This could potentially cause harm or limit the effectiveness of the therapy.
- Patient individuality: As the etiology of RA is complex and the cause in each patient is different, the response to the same gene therapy drug varies among each patient. More clinical research is needed to optimize the therapeutic approach and determine which patients may benefit most from gene therapy.
- Long-term effects: The long-term effects of gene therapy for rheumatoid arthritis are not yet fully understood. As with any new therapy, there is a need for ongoing monitoring of safety and efficacy over time to determine the potential benefits and risks of treatment.
- Cost and accessibility: Gene therapy can be expensive and may not be accessible to all patients due to cost or availability issues.
- Improved targeting: The development of more targeted delivery systems could improve the specificity and efficiency of gene delivery to cells and tissues involved in rheumatoid arthritis.
- Combination therapy: Nucleic acid delivery systems may be used in combination with other rheumatoid arthritis therapies, such as DMARDs or biologics, to enhance efficacy and reduce side effects. Combining nucleic acid delivery systems with immunotherapies could enhance the immune response to the therapeutic gene and improve the overall effectiveness of rheumatoid arthritis treatment.
- Localized delivery: The use of localized delivery systems, such as injectable hydrogels, could enable targeted delivery of therapeutic nucleic acids to specific joint sites affected by rheumatoid arthritis.
- Personalized medicine: Gene therapy has the potential to be tailored to each individual patient based on their specific genetic and disease characteristics. This could lead to more effective and targeted treatments with fewer side effects.
- Safety: The long-term effects of gene therapy for rheumatoid arthritis are not yet fully understood. As with any new therapy, there is a need for ongoing monitoring of safety and efficacy over time to determine the potential benefits and risks of treatment.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, N.; Guo, F. Nanoparticle-siRNA: A potential strategy for rheumatoid arthritis therapy? J. Control. Release 2020, 325, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Pirmardvand Chegini, S.; Varshosaz, J.; Taymouri, S. Recent approaches for targeted drug delivery in rheumatoid arthritis diagnosis and treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 502–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, C.; Sun, M.; Guo, Y.; Xia, W.; Qiao, S.; Tao, Y.; Fang, Y.; Zhang, Q.; Zhu, Y.; Yalikun, Y.; et al. Cholinergic dysfunction-induced insufficient activation of alpha7 nicotinic acetylcholine receptor drives the development of rheumatoid arthritis through promoting protein citrullination via the SP3/PAD4 pathway. Acta Pharm. Sin. B 2023. [Google Scholar] [CrossRef]
- Song, Y.; Huang, Y.; Zhou, F.; Ding, J.; Zhou, W. Macrophage-targeted nanomedicine for chronic diseases immunotherapy. Chin. Chem. Lett. 2022, 33, 597–612. [Google Scholar] [CrossRef]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, X.Y.; Fang, J.Y.; Sun, X. Nanomedicines for the treatment of rheumatoid arthritis: State of art and potential therapeutic strategies. Acta Pharm. Sin. B 2021, 11, 1158–1174. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-B.; Hong, H.; Lim, H.-J.; Choi, J.H.; Choi, Y.P.; Seo, S.W.; Lee, H.W.; Chae, C.H.; Park, W.-K.; Kim, H.Y.; et al. A novel IRAK4/PIM1 inhibitor ameliorates rheumatoid arthritis and lymphoid malignancy by blocking the TLR/MYD88-mediated NF-κB pathway. Acta Pharm. Sin. B 2022, 13, 1093–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, X.; Ho, K.-H.; Cai, C.; Li, C.-W.; Yang, M.; Yi, C. Nanotechnology for diagnosis and therapy of rheumatoid arthritis: Evolution towards theranostic approaches. Chin. Chem. Lett. 2021, 32, 66–86. [Google Scholar] [CrossRef]
- Kesharwani, D.; Paliwal, R.; Satapathy, T.; Das Paul, S. Rheumatiod Arthritis: An Updated Overview of Latest Therapy and Drug Delivery. J. Pharmacopunct. 2019, 22, 210–224. [Google Scholar] [CrossRef]
- Deviatkin, A.A.; Vakulenko, Y.A.; Akhmadishina, L.V.; Tarasov, V.V.; Beloukhova, M.I.; Zamyatnin, A.A., Jr.; Lukashev, A.N. Emerging Concepts and Challenges in Rheumatoid Arthritis Gene Therapy. Biomedicines 2020, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cring, M.R.; Sheffield, V.C. Gene therapy and gene correction: Targets, progress, and challenges for treating human diseases. Gene Ther. 2022, 29, 3–12. [Google Scholar] [CrossRef]
- Gao, J.; Xia, Z.; Vohidova, D.; Joseph, J.; Luo, J.N.; Joshi, N. Progress in non-viral localized delivery of siRNA therapeutics for pulmonary diseases. Acta Pharm. Sin. B 2022. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salahpour Anarjan, F. Active targeting drug delivery nanocarriers: Ligands. Nano-Struct. Nano-Objects 2019, 19, 100370. [Google Scholar] [CrossRef]
- Son, S.; Singha, K.; Kim, W.J. Bioreducible BPEI-SS-PEG-cNGR polymer as a tumor targeted nonviral gene carrier. Biomaterials 2010, 31, 6344–6354. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Hwang, D.W.; Singha, K.; Jeong, J.H.; Park, T.G.; Lee, D.S.; Kim, W.J. RVG peptide tethered bioreducible polyethylenimine for gene delivery to brain. J. Control. Release 2011, 155, 18–25. [Google Scholar] [CrossRef]
- Yin, N.; Tan, X.; Liu, H.; He, F.; Ding, N.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A novel indomethacin/methotrexate/MMP-9 siRNA in situ hydrogel with dual effects of anti-inflammatory activity and reversal of cartilage disruption for the synergistic treatment of rheumatoid arthritis. Nanoscale 2020, 12, 8546–8562. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, H.; Li, Y.; Chen, W.; Li, H.; Peng, K.; Zhang, Z.; Sun, X. Targeting NF-kB signaling with polymeric hybrid micelles that co-deliver siRNA and dexamethasone for arthritis therapy. Biomaterials 2017, 122, 10–22. [Google Scholar] [CrossRef]
- Khoury, M.; Louis-Plence, P.; Escriou, V.; Noel, D.; Largeau, C.; Cantos, C.; Scherman, D.; Jorgensen, C.; Apparailly, F. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor alpha in experimental arthritis. Arthritis Rheum. 2006, 54, 1867–1877. [Google Scholar] [CrossRef]
- Jansen, M.A.A.; Klausen, L.H.; Thanki, K.; Lyngsø, J.; Skov Pedersen, J.; Franzyk, H.; Nielsen, H.M.; van Eden, W.; Dong, M.; Broere, F.; et al. Lipidoid-polymer hybrid nanoparticles loaded with TNF siRNA suppress inflammation after intra-articular administration in a murine experimental arthritis model. Eur. J. Pharm. Biopharm. 2019, 142, 38–48. [Google Scholar] [CrossRef]
- Song, P.; Yang, C.; Thomsen, J.S.; Dagnæs-Hansen, F.; Jakobsen, M.; Brüel, A.; Deleuran, B.; Kjems, J. Lipidoid-siRNA Nanoparticle-Mediated IL-1β Gene Silencing for Systemic Arthritis Therapy in a Mouse Model. Mol. Ther. 2019, 27, 1424–1435. [Google Scholar] [CrossRef]
- Shi, Q.; Rondon-Cavanzo, E.P.; Dalla Picola, I.P.; Tiera, M.J.; Zhang, X.; Dai, K.; Benabdoune, H.A.; Benderdour, M.; Fernandes, J.C. In vivo therapeutic efficacy of TNFα silencing by folate-PEG-chitosan-DEAE/siRNA nanoparticles in arthritic mice. Int. J. Nanomed. 2018, 13, 387–402. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Lee, A.; Hwang, S.R.; Park, J.S.; Jang, J.; Huh, M.S.; Jo, D.G.; Yoon, S.Y.; Byun, Y.; Kim, S.H.; et al. TNF-α gene silencing using polymerized siRNA/thiolated glycol chitosan nanoparticles for rheumatoid arthritis. Mol. Ther. 2014, 22, 397–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, C.; Bhan, A.K.; Deshpande, V.; Shankar, P.; Manjunath, N. Silencing TNF-α in macrophages and dendritic cells for arthritis treatment. Scand. J. Rheumatol. 2013, 42, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Wu, H.; McBride, J.L.; Jung, K.E.; Kim, M.H.; Davidson, B.L.; Lee, S.K.; Shankar, P.; Manjunath, N. Transvascular delivery of small interfering RNA to the central nervous system. Nature 2007, 448, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-S.; Ye, C.; Kumar, P.; Chiu, I.; Subramanya, S.; Wu, H.; Shankar, P.; Manjunath, N. Targeted Delivery of siRNA to Macrophages for Anti-inflammatory Treatment. Mol. Ther. 2010, 18, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, Y.; Zheng, X.; Jia, M.; Mei, Z.; Wang, Y.; Zhang, Z.; Zhou, M.; Li, C. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J. Control. Release 2022, 341, 16–30. [Google Scholar] [CrossRef]
- Shi, B.; Zheng, M.; Tao, W.; Chung, R.; Jin, D.; Ghaffari, D.; Farokhzad, O.C. Challenges in DNA Delivery and Recent Advances in Multifunctional Polymeric DNA Delivery Systems. Biomacromolecules 2017, 18, 2231–2246. [Google Scholar] [CrossRef]
- Wang, F.; Shen, Y.; Zhang, W.; Li, M.; Wang, Y.; Zhou, D.; Guo, S. Efficient, dual-stimuli responsive cytosolic gene delivery using a RGD modified disulfide-linked polyethylenimine functionalized gold nanorod. J. Control. Release 2014, 196, 37–51. [Google Scholar] [CrossRef]
- Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Glutathione responsive polymers and their application in drug delivery systems. Polym. Chem. 2017, 8, 97–126. [Google Scholar] [CrossRef]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Z.; Yang, H.; Xu, Q. Tailoring combinatorial lipid nanoparticles for intracellular delivery of nucleic acids, proteins, and drugs. Acta Pharm. Sin. B 2022, 12, 2624–2639. [Google Scholar] [CrossRef] [PubMed]
- Wahane, A.; Waghmode, A.; Kapphahn, A.; Dhuri, K.; Gupta, A.; Bahal, R. Role of Lipid-Based and Polymer-Based Non-Viral Vectors in Nucleic Acid Delivery for Next-Generation Gene Therapy. Molecules 2020, 25, 2866. [Google Scholar] [CrossRef]
- Cun, D.; Jensen, D.K.; Maltesen, M.J.; Bunker, M.; Whiteside, P.; Scurr, D.; Foged, C.; Nielsen, H.M. High loading efficiency and sustained release of siRNA encapsulated in PLGA nanoparticles: Quality by design optimization and characterization. Eur. J. Pharm. Biopharm. 2011, 77, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Giri, T.K.; Thakur, A.; Alexander, A.; Ajazuddin; Badwaik, H.; Tripathi, D.K. Modified chitosan hydrogels as drug delivery and tissue engineering systems: Present status and applications. Acta Pharm. Sin. B 2012, 2, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.; Venkatraman, S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, J.; Dong, J.; Zhang, T.; Su, Z.; Ding, J.; Zhang, Y.; Mao, X. Polyethyleneimine-functionalized iron oxide nanoparticles for systemic siRNA delivery in experimental arthritis. Nanomedicine 2014, 9, 789–801. [Google Scholar] [CrossRef]
- Zhou, H.F.; Yan, H.; Pan, H.; Hou, K.K.; Akk, A.; Springer, L.E.; Hu, Y.; Allen, J.S.; Wickline, S.A.; Pham, C.T. Peptide-siRNA nanocomplexes targeting NF-κB subunit p65 suppress nascent experimental arthritis. J. Clin. Investig. 2014, 124, 4363–4374. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Jo, S.; Chung, J.Y.; Oh, Y.; Yoon, S.; Lee, Y.L.; Kim, S.S.; Yang, J.H.; Jang, K.; Yang, C.S.; et al. RNA interference-mediated suppression of TNF-α converting enzyme as an alternative anti-TNF-α therapy for rheumatoid arthritis. J. Control. Release 2021, 330, 1300–1312. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, X.; Wang, C.; Liu, Y.; Jia, M.; Lei, F.; Tian, J.; Li, C. Targeted co-delivery biomimetic nanoparticles reverse macrophage polarization for enhanced rheumatoid arthritis therapy. Drug Deliv. 2022, 29, 1025–1037. [Google Scholar] [CrossRef]
- Feng, X.; Chen, Y. Drug delivery targets and systems for targeted treatment of rheumatoid arthritis. J. Drug Target. 2018, 26, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Li, H. Combination of NF-kB targeted siRNA and methotrexate in a hybrid nanocarrier towards the effective treatment in rheumatoid arthritis. J. Nanobiotechnology 2018, 16, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, F.; Lee, R.J.; Zhong, L.; Dong, S.; Yang, C.; Teng, L.; Meng, Q.; Lu, J.; Xie, J.; Teng, L. Hybrid micelles containing methotrexate-conjugated polymer and co-loaded with microRNA-124 for rheumatoid arthritis therapy. Theranostics 2019, 9, 5282–5297. [Google Scholar] [CrossRef]
- Li, X.; Yu, C.; Meng, X.; Hou, Y.; Cui, Y.; Zhu, T.; Li, Y.; Teng, L.; Sun, F.; Li, Y. Study of double-targeting nanoparticles loaded with MCL-1 siRNA and dexamethasone for adjuvant-induced arthritis therapy. Eur. J. Pharm. Biopharm. 2020, 154, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Escriou, V.; Courties, G.; Galy, A.; Yao, R.; Largeau, C.; Scherman, D.; Jorgensen, C.; Apparailly, F. Efficient suppression of murine arthritis by combined anticytokine small interfering RNA lipoplexes. Arthritis Rheum. 2008, 58, 2356–2367. [Google Scholar] [CrossRef]
- Duvvuri, B.; Lood, C. Cell-Free DNA as a Biomarker in Autoimmune Rheumatic Diseases. Front. Immunol. 2019, 10, 502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozaki, Y.; Ri, J.; Sakai, K.; Niki, K.; Kinoshita, K.; Funauchi, M.; Matsumura, I. Inhibition of the IL-18 Receptor Signaling Pathway Ameliorates Disease in a Murine Model of Rheumatoid Arthritis. Cells 2020, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheinman, R.I.; Trivedi, R.; Vermillion, S.; Kompella, U.B. Functionalized STAT1 siRNA nanoparticles regress rheumatoid arthritis in a mouse model. Nanomedicine 2011, 6, 1669–1682. [Google Scholar] [CrossRef]
- Rai, M.F.; Pan, H.; Yan, H.; Sandell, L.J.; Pham, C.T.N.; Wickline, S.A. Applications of RNA interference in the treatment of arthritis. Transl. Res. 2019, 214, 1–16. [Google Scholar] [CrossRef]
- Sioud, M. RNA Interference: Story and Mechanisms. Methods Mol. Biol. 2021, 2282, 1–15. [Google Scholar] [CrossRef]
- Jang, D.-i.; Lee, A.H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Vasanthi, P.; Nalini, G.; Rajasekhar, G. Role of tumor necrosis factor-alpha in rheumatoid arthritis: A review. APLAR J. Rheumatol. 2007, 10, 270–274. [Google Scholar] [CrossRef]
- Moreland, L.W.; Misischia, R.J. Rheumatoid arthritis: Developing pharmacological therapies. Expert Opin. Investig. Drugs 2002, 11, 927–935. [Google Scholar] [CrossRef]
- Aldayel, A.M.; O’Mary, H.L.; Valdes, S.A.; Li, X.; Thakkar, S.G.; Mustafa, B.E.; Cui, Z. Lipid nanoparticles with minimum burst release of TNF-α siRNA show strong activity against rheumatoid arthritis unresponsive to methotrexate. J. Control. Release 2018, 283, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hardie, J.; Liu, Y.; Ray, M.; Luo, X.; Das, R.; Landis, R.F.; Farkas, M.E.; Rotello, V.M. Nanocapsule-mediated cytosolic siRNA delivery for anti-inflammatory treatment. J. Control. Release 2018, 283, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Howard, K.A.; Paludan, S.R.; Behlke, M.A.; Besenbacher, F.; Deleuran, B.; Kjems, J. Chitosan/siRNA nanoparticle-mediated TNF-alpha knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model. Mol. Ther. 2009, 17, 162–168. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef]
- Brzustewicz, E.; Bryl, E. The role of cytokines in the pathogenesis of rheumatoid arthriti—Practical and potential application of cytokines as biomarkers and targets of personalized therapy. Cytokine 2015, 76, 527–536. [Google Scholar] [CrossRef]
- Wang, D.; Deng, X.; Leng, X.; Mao, X. Interleukin-15 receptor-directed immunotoxins atteunuate disease severity in rat adjuvant arthritis. Mol. Immunol. 2010, 47, 1535–1543. [Google Scholar] [CrossRef]
- Zhang, T.; Bai, X.; Mao, X. Systemic delivery of small interfering RNA targeting the interleukin-2/15 receptor β chain prevents disease progression in experimental arthritis. PLoS ONE 2013, 8, e78619. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.E.; Hyam, S.R.; Han, M.J.; Kim, S.Y.; Lee, B.G.; Kim, D.H. Lactobacillus brevis G-101 ameliorates colitis in mice by inhibiting NF-κB, MAPK and AKT pathways and by polarizing M1 macrophages to M2-like macrophages. J. Appl. Microbiol. 2013, 115, 888–896. [Google Scholar] [CrossRef]
- Kanazawa, T.; Endo, T.; Arima, N.; Ibaraki, H.; Takashima, Y.; Seta, Y. Systemic delivery of small interfering RNA targeting nuclear factor κB in mice with collagen-induced arthritis using arginine-histidine-cysteine based oligopeptide-modified polymer nanomicelles. Int. J. Pharm. 2016, 515, 315–323. [Google Scholar] [CrossRef]
- Liu, X.; Guo, R.; Huo, S.; Chen, H.; Song, Q.; Jiang, G.; Yu, Y.; Huang, J.; Xie, S.; Gao, X.; et al. CaP-based anti-inflammatory HIF-1α siRNA-encapsulating nanoparticle for rheumatoid arthritis therapy. J. Control. Release 2022, 343, 314–325. [Google Scholar] [CrossRef]
- Feng, N.; Liang, L.; Fan, M.; Du, Y.; Chen, C.; Jiang, R.; Yu, D.; Yang, Y.; Zhang, M.; Deng, L.; et al. Treating Autoimmune Inflammatory Diseases with an siERN1-Nanoprodrug That Mediates Macrophage Polarization and Blocks Toll-like Receptor Signaling. ACS Nano 2021, 15, 15874–15891. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Yang, H.N.; Jeon, S.Y.; Woo, D.G.; Kim, M.S.; Park, K.H. The use of anti-COX2 siRNA coated onto PLGA nanoparticles loading dexamethasone in the treatment of rheumatoid arthritis. Biomaterials 2012, 33, 8600–8612. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, A.; Zhang, Y.; Zuo, Z.Q.; Cao, Z.T.; Zhang, H.B.; Xu, C.F.; Wang, J. Nanoparticle-delivered siRNA targeting Bruton’s tyrosine kinase for rheumatoid arthritis therapy. Biomater. Sci. 2019, 7, 4698–4707. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, J.S.; Lee, S.J.; Jang, J.; Park, J.S.; Back, S.H.; Bahn, G.; Park, J.H.; Kang, Y.M.; Kim, S.H.; et al. Notch1 targeting siRNA delivery nanoparticles for rheumatoid arthritis therapy. J. Control. Release 2015, 216, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Sun, J.; Fu, S. An enhanced RRM2 siRNA delivery to rheumatoid arthritis fibroblast-like synoviocytes through a liposome-protamine-DNA-siRNA complex with cell permeable peptides. Int. J. Mol. Med. 2018, 42, 2393–2402. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Dong, S.; Li, X.; Yu, K.; Sun, F.; Lee, R.J.; Li, Y.; Teng, L. Delivery of siRNA using folate receptor-targeted pH-sensitive polymeric nanoparticles for rheumatoid arthritis therapy. Nanomedicine 2019, 20, 102017. [Google Scholar] [CrossRef]
- te Boekhorst, B.C.; Jensen, L.B.; Colombo, S.; Varkouhi, A.K.; Schiffelers, R.M.; Lammers, T.; Storm, G.; Nielsen, H.M.; Strijkers, G.J.; Foged, C.; et al. MRI-assessed therapeutic effects of locally administered PLGA nanoparticles loaded with anti-inflammatory siRNA in a murine arthritis model. J. Control. Release 2012, 161, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Komano, Y.; Yagi, N.; Onoue, I.; Kaneko, K.; Miyasaka, N.; Nanki, T. Arthritic joint-targeting small interfering RNA-encapsulated liposome: Implication for treatment strategy for rheumatoid arthritis. J. Pharmacol. Exp. Ther. 2012, 340, 109–113. [Google Scholar] [CrossRef]
- Jain, S.; Tran, T.H.; Amiji, M. Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials 2015, 61, 162–177. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tang, X.; Xiang, X.; Tang, Y.; Qiu, L. Experimental study of TNF-α receptor gene transfection by ultrasound-targeted microbubble destruction to treat collagen-induced arthritis in rats in vivo. Exp. Ther. Med. 2019, 17, 1601–1610. [Google Scholar] [CrossRef] [Green Version]
- Hu, N.; Zhu, L.; Zhang, L.; Wang, J.; Wang, Y.; Luo, J.; He, L.; Hao, Z.; Zhang, L. Immunomodulatory effect and safety of TNF-α RNAi mediated by oral yeast microcapsules in rheumatoid arthritis therapy. Mater. Today Bio. 2022, 16, 100384. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Li, J.; Liu, D.; Pan, H.; Zhu, R.; Yang, Y.; Pan, W. Amino acids functionalized dendrimers with nucleus accumulation for efficient gene delivery. Int. J. Pharm. 2021, 602, 120641. [Google Scholar] [CrossRef] [PubMed]
- St Clair, E.W. Interleukin 10 treatment for rheumatoid arthritis. Ann. Rheum. Dis. 1999, 58 (Suppl. 1), i99–i102. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Amiji, M. Tuftsin-modified alginate nanoparticles as a noncondensing macrophage-targeted DNA delivery system. Biomacromolecules 2012, 13, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, T.; Liu, M.; Wang, S.; Liu, S.; Yang, Y.; Yang, Y.; Nan, Y.; Huang, Q.; Ai, K. Rheumatoid arthritis microenvironment insights into treatment effect of nanomaterials. Nano Today 2022, 42, 101358. [Google Scholar] [CrossRef]
- Tu, Z.X.; Zhong, Y.L.; Hu, H.Z.; Shao, D.; Haag, R.N.; Schirner, M.; Lee, J.; Sullenger, B.; Leong, K.W. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022, 7, 557–574. [Google Scholar] [CrossRef]
- Peng, B.; Liang, H.; Li, Y.; Dong, C.; Shen, J.; Mao, H.Q.; Leong, K.W.; Chen, Y.; Liu, L. Tuned Cationic Dendronized Polymer: Molecular Scavenger for Rheumatoid Arthritis Treatment. Angew. Chem. Int. Ed. Engl. 2019, 58, 4254–4258. [Google Scholar] [CrossRef]
- Liang, H.; Peng, B.; Dong, C.; Liu, L.; Mao, J.; Wei, S.; Wang, X.; Xu, H.; Shen, J.; Mao, H.Q.; et al. Cationic nanoparticle as an inhibitor of cell-free DNA-induced inflammation. Nat. Commun. 2018, 9, 4291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.L.; Chen, S.; Yan, Y.Z.; Liu, L.X.; Chen, Y.M. Nanoparticulate DNA scavenger loading methotrexate targets articular inflammation to enhance rheumatoid arthritis treatment. Biomaterials 2022, 286, 121594. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.L.; Jiang, X.F.; Cai, J.H.; Chen, Y.T.; Huang, H.J.; Yang, Y.; Zheng, L.; Zhao, J.M.; Gao, M. Dimethylamino group modified polydopamine nanoparticles with positive charges to scavenge cell-free DNA for rheumatoid arthritis therapy. Bioact. Mater. 2022, 18, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Madry, H. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nat. Rev. Rheumatol. 2019, 15, 18–29. [Google Scholar] [CrossRef]








| Nanomaterials | Advantages | Limitations | Delivery System | Refs. |
|---|---|---|---|---|
| Polyethylenimine | High transfection efficiency; pH buffering capacity; Endosomal escape capacity; Ability to transfect non-dividing cells | Potential toxicity; Inflammatory response | A in situ hydrogel loaded with disulfide-crosslinked polyethyleneimine nanoparticles | [17] |
| A hybrid micelle system consisting of PCL-PEI and PCL-PEG | [18] | |||
| Lipids | Low immunogenicity; Good biocompatibility; Versatility | Low stability; Short half-time in the bloodstream; Potential toxicity | Liposomes consisting of RPR209120 and DOPE | [19] |
| Lipid-polymer hybrid nanoparticles consisting of DOTAP and PLGA | [20] | |||
| Lipidoid-polymer hybrid nanoparticles consisting of Pluronic F127 and spermidine-based lipidoid (S14) | [21] | |||
| Chitosan | Good biocompatibility; Low toxicity; Cationic charge Ability to be modified with active targeting ligands | Poor water solubility; Poor targeting ability; Charge deduction under physiological condition; Premature release in the cytoplasm | Nanoparticles consisting of a chitosan derivative containing diethylethylamine (DEAE) | [22] |
| A nanocomplex consisting of thiolated glycol chitosan | [23] | |||
| Iron oxide nanoparticles | Biocompatibility; Magnetic property; High surface area; Biodegradability; Imaging capability | Limited loading capacity; Instability; Limited specificity | Polyethyleneimine-functionalized iron oxide nanoparticles | [23] |
| Cationic peptides | High delivery efficiency; Low immunogenicity; Specificity; Good biocompatibility; Ease of synthesis | Potential toxicity; Instability; Limited loading capacity | Anti-TNF-α siRNA complexed to RVG-9R | [24,25,26] |
| Biomimetic nanoparticles | Enhanced targeting; Good biocompatibility; Versatility; Reduced immunogenicity | Complexity; Limited loading capacity; High cost | M2 exosomes | [27] |
| Type | Receptor | Refs. |
|---|---|---|
| Folic acid | Folate receptor β | [32,38,45,46] |
| Methotrexate | Folate receptor β | [39] |
| RGD | αvβ3 integrins | [47] |
| Tuftsin peptide | Fc and neuropilin-1 receptors | [48] |
| D-Asp8 | Bone resorption surfaces | [35] |
| Human serum albumin | Synovial tissues | [37] |
| Hyaluronic acid | CD44 | [25] |
| Type | Nucleic Acids | Delivery System | Co-Delivery | Administration | Ref. |
|---|---|---|---|---|---|
| siRNA | MMP9 siRNA | In situ hydrogel loaded with PEI-SS-IND-MTX-MMP-9 siRNA NPs | Indomethacin and methotrexate | i.a. | [17] |
| siRNA | IL-2/15Rβ siRNA | PEI/siRNA complexes | - | i.v. | [60] |
| siRNA | IL-2/15Rβ siRNA | Polyethyleneimine -superparamagnetic iron oxide nanoparticle | - | i.v. | [37] |
| siRNA | anti-COX2 siRNA | Drug-loaded PLGA nanoparticles complexed with poly (-ethyleneimine) (PEI)/siRNA | Dexamethasone | - | [65] |
| siRNA | NF-kB-targeted siRNA (p65 siRNA) | Calcium phosphate/liposome | Methotrexate | i.v. | [42] |
| siRNA | NF-kB-targeted siRNA (p65 siRNA) | Polymeric hybrid micelle (98% PCL-PEG and 2% PCL-PEI) | Dexamethasone | i.v. | [18] |
| siRNA | NF-kB-targeted siRNA (p65 siRNA) | Arginine–histidine–cysteine-based oligopeptide-modified polymer micelles | - | i.v. | [62] |
| siRNA | NF-kB-targeted siRNA (p65 siRNA) | Melittin-derived cationic amphipathic peptide-siRNA nanocomplexes | - | i.v. | [38] |
| siRNA | siRNA targeting Bruton’s tyrosine kinase | Cationic lipid-assisted PEG-b-PLGA nanoparticles | - | i.v. | [66] |
| siRNA | Several anti-cytokine siRNA (anti-IL-1, anti-IL-6, anti-IL-18) | Lipoplexes | - | i.v. | [45] |
| siRNA | STAT1 siRNA | RGD-PLGA nanoparticles | - | i.v. | [48] |
| siRNA | Notch1 targeting siRNA | Thiolated glycol chitosan (tGC) nanoparticles | - | i.v. | [67] |
| siRNA | IL-1β siRNA | Lipidoid-polymer hybrid nanoparticle | - | i.v. | [21] |
| siRNA | HIF-1α siRNA | HIF-1α siRNA-loaded calcium phosphate nanoparticles encapsulated in apolipoprotein E3-reconstituted high-density lipoprotein | - | i.v. | [63] |
| siRNA | Ribonucleotide reductase M2 (RRM2) siRNA | Cell permeable peptide-conjugated liposome/protamine/DNA/RRM2 siRNA complex | - | - | [68] |
| siRNA | siERN1 | FA(folic acid)−PEG−R(RKKRRQRRR)−NPs(ss−PBAA−PEI)@siERN1 | - | i.v. | [64] |
| siRNA | Myeloid cell leukemia-1 (Mcl-1) siRNA | Polymeric nanoparticles composed of PK3 as a pH-sensitive polymer, folate-polyethyleneglycol-poly(lactide-co-glycolide) as a targeting ligand and a DOTAP/siRNA core | - | i.v. | [69] |
| siRNA | Mcl-1 (siRNA) | HA-coated and pH-responsive nanoparticles | Dexamethasone | i.v. | [44] |
| siRNA | TNF-α siRNA | Nanoparticle-stabilized nanocapsules | - | i.v. | [55] |
| siRNA | TNF-α siRNA | Acid-sensitive sheddable PEGylated solid-lipid nanoparticle | - | i.v. | [54] |
| siRNA | TNF-α siRNA | Lipidpolymer hybrid nanoparticles | - | i.a. | [20] |
| siRNA | TNF-α siRNA | RVG-9R/siRNA complexes | - | i.v. | [24] |
| siRNA | TNF-α siRNA | PLGA nanoparticles (DOTAP-modified) | - | i.a. | [70] |
| siRNA | TNF-α siRNA | Nanocomplexes of polymerized siRNA (poly-siRNA) targeting TNF-α with thiolated glycol chitosan (tGC) polymers | - | i.v. | [23] |
| siRNA | TNF-α siRNA | siRNA/WS complex | i.v. | [71] | |
| siRNA | TNF-α siRNA | Folate-PEG-chitosan-DEAE/siRNA nanoparticles | - | i.p. | [22] |
| siRNA | TNF siRNA | Lipid-polymer hybrid nanoparticles (LPNs) composed of lipidoid and poly (DLlactic-co-glycolic acid) | - | i.a. | [20] |
| siRNA | anti-TNF-α Dicer-substrate siRNA (2′-OMe-modified) | Chitosan/siRNA nanoparticles | - | i.p. | [56] |
| DNA | IL-10 plasmid DNA | M2-type exosomes nanoparticles | Betamethasone sodium phosphate | i.v. | [27] |
| DNA | IL-10 plasmid DNA | Tuftsin-modified alginate nanoparticles | - | i.p. | [72] |
| DNA | IL-10 plasmid DNA | Human serum albumin (HSA) preparing pDNA/DSP-NPs | Dexamethasone sodium phosphate | i.v. | [40] |
| DNA | TNF-α receptor (TNFR) gene | Ultrasound-targeted microbubble | - | Injected at the ankle joint and tibialis anterior muscle, respectively | [73] |
| Non-coding nucleotide | miRNA-124 | Methotrexate-conjugated polymer hybrid micelles | Methotrexate | i.v. | [43] |
| Non-coding nucleotide | TNF-α short hairpin RNA (shRNA) (Plasmids pIN27-hU6- TNF-α-shRNA) | Oral yeast microcapsules | - | Oral administration | [74] |
| Non-coding nucleotide | TNF-α converting enzyme (TACE) (shRNA) in the psi-U6.1 vector driven by the U6 promoter | shTACE/peptide (8D-16R) carrier complex | - | i.v. | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, Y.; Xiao, C.; Guan, Y.; Gao, Z.; Huang, W. Research Advances in Nucleic Acid Delivery System for Rheumatoid Arthritis Therapy. Pharmaceutics 2023, 15, 1237. https://doi.org/10.3390/pharmaceutics15041237
Zhang X, Liu Y, Xiao C, Guan Y, Gao Z, Huang W. Research Advances in Nucleic Acid Delivery System for Rheumatoid Arthritis Therapy. Pharmaceutics. 2023; 15(4):1237. https://doi.org/10.3390/pharmaceutics15041237
Chicago/Turabian StyleZhang, Xintong, Yanhong Liu, Congcong Xiao, Youyan Guan, Zhonggao Gao, and Wei Huang. 2023. "Research Advances in Nucleic Acid Delivery System for Rheumatoid Arthritis Therapy" Pharmaceutics 15, no. 4: 1237. https://doi.org/10.3390/pharmaceutics15041237