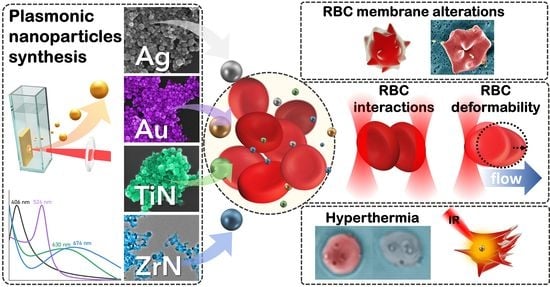
Impact of Plasmonic Nanoparticles on Poikilocytosis and Microrheological Properties of Erythrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles Synthesis and Characterization
2.2. Blood Samples
2.3. Hemolytic Activity
2.4. Optical Microscopy
2.5. Optical Tweezers
2.6. Scanning Electron Microscopy of RBC
3. Results
3.1. Plasmonic Nanoparticles
3.2. Hemolytic Activity of NP
3.3. RBC Poikilocytosis
3.4. RBC Aggregation and Deformability
3.5. NP-Induced Hyperthermia of RBC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NP | Nanoparticles |
| RBC | Red blood cells |
| LAL | Laser ablation in liquid |
| OT | Optical tweezers |
References
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.; Sershen, S.R.; Rivera, B.; Price, R.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, S.; Albanese, S.; Mancini, M. State-of-the-art preclinical photoacoustic imaging in oncology: Recent advances in cancer theranostics. Contrast Media Mol. Imaging 2019, 2019, 5080267. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, J.; Kneipp, H.; McLaughlin, M.; Brown, D.; Kneipp, K. In vivo molecular probing of cellular compartments with gold nanoparticles and nanoaggregates. Nano Lett. 2006, 6, 2225–2231. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Gao, M.; Sun, L.; Wang, Z.; Zhao, Y. Controlled synthesis of Ag nanoparticles with different morphologies and their antibacterial properties. Mater. Sci. Eng. C. 2013, 33, 397–404. [Google Scholar] [CrossRef]
- Guler, U.; Shalaev, V.M.; Boltasseva, A. Nanoparticle plasmonics: Going practical with transition metal nitrides. Mater. Today 2015, 18, 227–237. [Google Scholar] [CrossRef]
- Lalisse, A.; Tessier, G.; Plain, J.; Baffou, G. Plasmonic efficiencies of nanoparticles made of metal nitrides (TiN, ZrN) compared with gold. Sci. Rep. 2016, 6, 38647. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Tian, Z.; Dong, Y.; Wang, X.; Zhan, L. Current advances in nanomaterials affecting morphology, structure, and function of erythrocytes. RSC Adv. 2021, 11, 6958–6971. [Google Scholar] [CrossRef]
- Zhu, R.; Avsievich, T.; Popov, A.; Bykov, A.; Meglinski, I. In vivo nano-biosensing element of red blood cell-mediated delivery. Biosens. Bioelectron. 2020, 175, 112845. [Google Scholar] [CrossRef]
- Avsievich, T.; Zhu, R.; Popov, A.; Bykov, A.; Meglinski, I. Blood–nanomaterials interactions. In Nanotechnology for Hematology, Blood Transfusion, and Artificial Blood; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–40. [Google Scholar]
- Danielczok, J.G.; Terriac, E.; Hertz, L.; Petkova-Kirova, P.; Lautenschläger, F.; Laschke, M.W.; Kaestner, L. Red blood cell passage of small capillaries is associated with transient Ca2+-mediated adaptations. Front. Physiol. 2017, 8, 979. [Google Scholar] [CrossRef] [Green Version]
- Wadhwa, R.; Aggarwal, T.; Thapliyal, N.; Kumar, A.; Yadav, P.; Kumari, V.; Reddy, B.S.C.; Chandra, P.; Maurya, P.K. Red blood cells as an efficient in vitro model for evaluating the efficacy of metallic nanoparticles. 3 Biotech 2019, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Lomanovskaya, T.; Piavchenko, G.; Venediktov, A.; Kustavinova, E.; Kartashkina, N.; Mukhamedova, S.; Boronikhina, T.; Markov, A.; Telyshev, D.; Meglinski, I.; et al. Structural changes of erythrocyte membrane revealed by 3D confocal optical profilometer. J. Biophoton. 2022, 10, e202200222. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.C.; Myerson, J.W.; Brenner, J.S.; Patel, P.N.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Nanoparticle properties modulate their attachment and effect on carrier red blood cells. Sci. Rep. 2018, 8, 1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, Y.; Zheng, Y.; Wang, B. Mechanical characteristics of human red blood cell membrane change due to C 60 nanoparticle infiltration. Phys. Chem. Chem. Phys. 2013, 15, 2473–2481. [Google Scholar] [CrossRef]
- Avsievich, T.; Popov, A.; Bykov, A.; Meglinski, I. Mutual interaction of red blood cells influenced by nanoparticles. Sci. Rep. 2019, 9, 5147. [Google Scholar] [CrossRef] [Green Version]
- Bian, Y.; Kim, K.; Ngo, T.; Kim, I.; Bae, O.N.; Lim, K.M.; Chung, J.H. Silver nanoparticles promote procoagulant activity of red blood cells: A potential risk of thrombosis in susceptible population. Part. Fibre Toxicol. 2019, 16, 9. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, S. Toxic effects of silver nanoparticles and nanowires on erythrocyte rheology. Food Chem. Toxicol. 2014, 67, 80–86. [Google Scholar] [CrossRef]
- He, Z.; Liu, J.; Du, L. The unexpected effect of PEGylated gold nanoparticles on the primary function of erythrocytes. Nanoscale 2014, 6, 9017–9024. [Google Scholar] [CrossRef] [PubMed]
- Fojtik, A.; Henglein, A. Laser ablation of films and suspended particles in a solvent: Formation of cluster and colloid solutions. Ber. Bunsenges. Phys. Chem. 1993, 97, 252. [Google Scholar]
- Kabashin, A.V.; Delaporte, P.; Pereira, A.; Grojo, D.; Torres, R.; Sarnet, T.; Sentis, M. Nanofabrication with pulsed lasers. Nanoscale Res. Lett. 2010, 5, 454–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcikowski, S.; Compagnini, G. Advanced nanoparticle generation and excitation by lasers in liquids. Phys. Chem. Chem. Phys. 2013, 15, 3022–3026. [Google Scholar] [CrossRef]
- Maximova, K.; Aristov, A.; Sentis, M.; Kabashin, A.V. Size-controllable synthesis of bare gold nanoparticles by femtosecond laser fragmentation in water. Nanotechnology 2015, 26, 065601. [Google Scholar] [CrossRef]
- Hebie, S.; Holade, Y.; Maximova, K.; Sentis, M.; Delaporte, P.; Kokoh, K.B.; Napporn, T.W.; Kabashin, A.V. Advanced electrocatalysts on the basis of bare Au nanomaterials for biofuel cell applications. ACS Catal. 2015, 5, 6489–6496. [Google Scholar] [CrossRef]
- Bailly, A.L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef] [Green Version]
- Kabashin, A.V.; Meunier, M. Femtosecond laser ablation in aqueous solutions: A novel method to synthesize non-toxic metal colloids with controllable size. J. Phys. Conf. Ser. 2007, 59, 074. [Google Scholar] [CrossRef]
- Popov, A.A.; Tselikov, G.; Dumas, N.; Berard, C.; Metwally, K.; Jones, N.; Al-Kattan, A.; Larrat, B.; Braguer, D.; Mensah, S.; et al. Laser-synthesized TiN nanoparticles as promising plasmonic alternative for biomedical applications. Sci. Rep. 2019, 9, 1194. [Google Scholar] [CrossRef] [Green Version]
- Zelepukin, I.V.; Popov, A.A.; Shipunova, V.O.; Tikhonowski, G.V.; Mirkasymov, A.B.; Popova-Kuznetsova, E.A.; Klimentov, S.M.; Kabashin, A.V.; Deyev, S.M. Laser-synthesized TiN nanoparticles for biomedical applications: Evaluation of safety, biodistribution and pharmacokinetics. Mater. Sci. Eng. C 2021, 120, 111717. [Google Scholar] [CrossRef]
- Maldonado, M.E.; Das, A.; Gomes, A.S.; Popov, A.A.; Klimentov, S.M.; Kabashin, A.V. Nonlinear photoacoustic response of suspensions of laser-synthesized plasmonic titanium nitride nanoparticles. Opt. Lett. 2020, 45, 6695–6698. [Google Scholar] [CrossRef]
- Uusitalo, S.; Popov, A.; Ryabchikov, Y.V.; Bibikova, O.; Alakomi, H.L.; Juvonen, R.; Kontturi, V.; Siitonen, S.; Kabashin, A.; Meglinski, I.; et al. Surface-enhanced Raman spectroscopy for identification and discrimination of beverage spoilage yeasts using patterned substrates and gold nanoparticles. J. Food Eng. 2017, 212, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Kögler, M.; Ryabchikov, Y.V.; Uusitalo, S.; Popov, A.; Popov, A.; Tselikov, G.; Välimaa, A.L.; Al-Kattan, A.; Hiltunen, J.; Laitinen, R.; et al. Bare laser-synthesized Au-based nanoparticles as nondisturbing surface-enhanced Raman scattering probes for bacteria identification. J. Biophoton. 2018, 11, e201700225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashkin, A. Acceleration and trapping of particles by radiation pressure. Phys. Rev. Lett. 1970, 24, 156. [Google Scholar] [CrossRef] [Green Version]
- Avsievich, T.; Tarakanchikova, Y.; Zhu, R.; Popov, A.; Bykov, A.; Skovorodkin, I.; Vainio, S.; Meglinski, I. Impact of nanocapsules on red blood cells interplay jointly assessed by optical tweezers and microscopy. Micromachines 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, R.; Avsievich, T.; Bykov, A.; Popov, A.; Meglinski, I. Influence of pulsed He–Ne Laser irradiation on the red blood cell interaction studied by optical tweezers. Micromachines 2019, 10, 853. [Google Scholar] [CrossRef] [Green Version]
- ASTM E2524-22; Standard Test Method for Analysis of Hemolytic Properties of Nanoparticles. 2022. Available online: https://www.astm.org/e2524-22.html (accessed on 16 March 2022).
- He, Z.; Li, C.; Zhang, X.; Zhong, R.; Wang, H.; Liu, J.; Du, L. The effects of gold nanoparticles on the human blood functions. Artif. Cells. Nanomed. Bbiotechnol. 2018, 46, 720–726. [Google Scholar] [CrossRef] [Green Version]
- Piety, N.Z.; Reinhart, W.H.; Pourreau, P.H.; Abidi, R.; Shevkoplyas, S.S. Shape matters: The effect of red blood cell shape on perfusion of an artificial microvascular network. Transfusion 2016, 56, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Reinhart, W.; Singh-Marchetti, M.; Straub, P. The influence of erythrocyte shape on suspension viscosities. Eur. J. Clin. Invest. 1992, 22, 38–44. [Google Scholar] [CrossRef]
- Seol, Y.; Carpenter, A.E.; Perkins, T.T. Gold nanoparticles: Enhanced optical trapping and sensitivity coupled with significant heating. Opt. Lett. 2006, 31, 2429–2431. [Google Scholar] [CrossRef] [Green Version]
- Heger, M. Thrombosis versus thermal coagulum formation as a result of endovenous laser treatment: Biochemistry versus photophysics. Phlebology 2014, 29, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Urban, A.S.; Carretero-Palacios, S.; Lutich, A.A.; Lohmüller, T.; Feldmann, J.; Jäckel, F. Optical trapping and manipulation of plasmonic nanoparticles: Fundamentals, applications, and perspectives. Nanoscale 2014, 6, 4458–4474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, C.H.; Pan, D.C.; Johnston, I.H.; Greineder, C.F.; Walsh, L.R.; Hood, E.D.; Cines, D.B.; Poncz, M.; Siegel, D.L.; Muzykantov, V.R. Biocompatible coupling of therapeutic fusion proteins to human erythrocytes. Blood Adv. 2018, 2, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Glassman, P.M.; Hood, E.D.; Ferguson, L.T.; Zhao, Z.; Siegel, D.L.; Mitragotri, S.; Brenner, J.S.; Muzykantov, V.R. Red blood cells: The metamorphosis of a neglected carrier into the natural mothership for artificial nanocarriers. Adv. Drug Deliv. Rev. 2021, 178, 113992. [Google Scholar] [CrossRef]
- Antonelli, A.; Magnani, M. Red blood cells as carriers of iron oxide-based contrast agents for diagnostic applications. J. Biomed. Nanotechnol. 2014, 10, 1732–1750. [Google Scholar] [CrossRef]
- RB, M.; Kirch, H. Enhanced biological activity of human recombinant interleukin 2 coupled to mouse red blood cells as evaluated using the mouse Meth A sarcoma model. Biotechnol. Appl. Biochem. 1996, 23, 29–36. [Google Scholar]
- Glassman, P.M.; Villa, C.H.; Marcos-Contreras, O.A.; Hood, E.D.; Walsh, L.R.; Greineder, C.F.; Myerson, J.W.; Shuvaeva, T.; Puentes, L.; Brenner, J.S.; et al. Targeted In Vivo Loading of Red Blood Cells Markedly Prolongs Nanocarrier Circulation. Bioconjug. Chem. 2022, 33, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, S.; Spitzer, D.; Murciano, J.C.; Ding, B.S.; Tliba, S.; Kowalska, M.A.; Marcos-Contreras, O.A.; Kuo, A.; Stepanova, V.; Atkinson, J.P.; et al. Sustained thromboprophylaxis mediated by an RBC-targeted pro-urokinase zymogen activated at the site of clot formation. Blood 2010, 115, 5241–5248. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, K.; Krasik, T.; Medinilla, S.; Bdeir, K.; Cines, D.B.; Muzykantov, V.R.; Murciano, J.C. Blood clearance and activity of erythrocyte-coupled fibrinolytics. J. Pharmacol. Exp. Ther. 2005, 312, 1106–1113. [Google Scholar] [CrossRef]
- Smirnov, V.; Domogatsky, S.; Dolgov, V.; Hvatov, V.; Klibanov, A.; Koteliansky, V.; Muzykantov, V.; Repin, V.; Samokhin, G.; Shekhonin, B. Carrier-directed targeting of liposomes and erythrocytes to denuded areas of vessel wall. Proc. Natl. Acad. Sci. USA 1986, 83, 6603–6607. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, L.T.; Hood, E.D.; Shuvaeva, T.; Shuvaev, V.V.; Basil, M.C.; Wang, Z.; Nong, J.; Ma, X.; Wu, J.; Myerson, J.W.; et al. Dual Affinity to RBCs and Target Cells (DART) Enhances Both Organ-and Cell Type-Targeting of Intravascular Nanocarriers. ACS Nano 2022, 16, 4666–4683. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avsievich, T.; Zhu, R.; Popov, A.P.; Yatskovskiy, A.; Popov, A.A.; Tikhonowsky, G.; Pastukhov, A.I.; Klimentov, S.; Bykov, A.; Kabashin, A.; et al. Impact of Plasmonic Nanoparticles on Poikilocytosis and Microrheological Properties of Erythrocytes. Pharmaceutics 2023, 15, 1046. https://doi.org/10.3390/pharmaceutics15041046
Avsievich T, Zhu R, Popov AP, Yatskovskiy A, Popov AA, Tikhonowsky G, Pastukhov AI, Klimentov S, Bykov A, Kabashin A, et al. Impact of Plasmonic Nanoparticles on Poikilocytosis and Microrheological Properties of Erythrocytes. Pharmaceutics. 2023; 15(4):1046. https://doi.org/10.3390/pharmaceutics15041046
Chicago/Turabian StyleAvsievich, Tatiana, Ruixue Zhu, Alexey P. Popov, Alexander Yatskovskiy, Anton A. Popov, Gleb Tikhonowsky, Andrei I. Pastukhov, Sergei Klimentov, Alexander Bykov, Andrei Kabashin, and et al. 2023. "Impact of Plasmonic Nanoparticles on Poikilocytosis and Microrheological Properties of Erythrocytes" Pharmaceutics 15, no. 4: 1046. https://doi.org/10.3390/pharmaceutics15041046