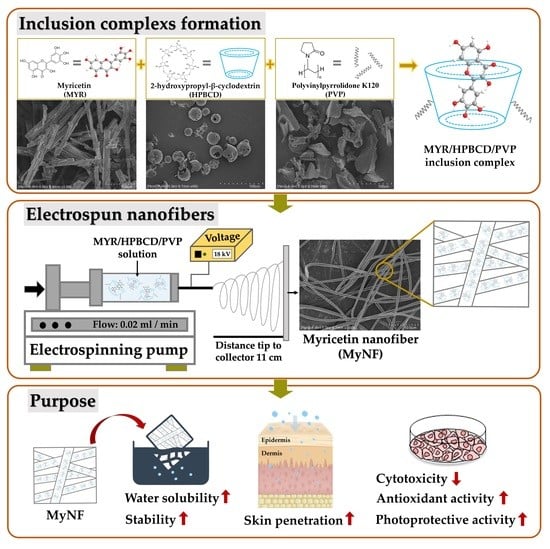
Myricetin Nanofibers Enhanced Water Solubility and Skin Penetration for Increasing Antioxidant and Photoprotective Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC Analysis of MYR
2.3. Preparation of HPBCD/PVP-Loaded Myricetin Nanofibers (MyNF)
2.4. Water Solubility
2.5. Yield and Encapsulation Efficiency
2.6. Determination of Particle Size and Morphology by SEM, DLS and TEM
2.7. Determination of Crystalline-to-Amorphous Transformation by XRD
2.8. Determination of Hydrogen Bond Formation by FTIR and 1H-NMR
2.9. Stability of MYR and MyNF
2.10. In Vitro Skin Penetration Assay
2.11. DPPH and ABTS+ Free Radical Scavenging Activity
2.12. Ferric Reducing Antioxidant Power
2.13. Cell Culture and Cell Safety Assay
2.14. Photoprotective Effects of MYR and MyNF on HaCaT Cells
2.15. Effects of MYR and MyNF on UVB-Induced Reactive Oxygen Species (ROS) Assay
2.16. Statistical Analysis
3. Results
3.1. Water Solubility, Yield, and Encapsulation Efficiency of MYR and Its Nanofibers Formulation
3.2. Morphology and Size of MYR and MyNF
3.2.1. Surface Morphology of MYR and MyNF by SEM
3.2.2. Particle Size and Polydispersity Index of MYR and MyNF by DLS
3.2.3. Particles Morphology of MyNF under TEM Observation
3.3. Crystalline and Amorphous Structure of MYR and MyNF
3.4. Intermolecular Chemical Bond Formation between MYR and Its Excipients
3.4.1. Fourier-Transform Infrared Spectroscopy
3.4.2. 1H-Nuclear Magnetic Resonance Spectroscopy
3.5. Photostability, Thermostability, and Centrifuge Testing of MYR and MyNF
3.5.1. Thermostability Testing
3.5.2. Centrifuge Testing
3.6. In Vitro Percutaneous Penetration of MYR and MyNF
3.7. Antioxidant Activity of MYR and MyNF
3.8. Photoprotective Effect of MYR and MyNF
3.8.1. Cell Survival of MYR and MyNF in UVB-Induced Keratinocytes Damage
3.8.2. ROS Production of MYR and MyNF under UVB Irradiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raimondi, S.; Suppa, M.; Gandini, S. Melanoma Epidemiology and Sun Exposure. Acta Derm. Venereol. 2020, 100, adv00136. [Google Scholar] [CrossRef]
- Kawashima, S.; Funakoshi, T.; Sato, Y.; Saito, N.; Ohsawa, H.; Kurita, K.; Nagata, K.; Yoshida, M.; Ishigami, A. Protective effect of pre- and post-vitamin C treatments on UVB-irradiation-induced skin damage. Sci. Rep. 2018, 8, 16199. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Basli, A.; Soulet, S.; Chaher, N.; Mérillon, J.M.; Chibane, M.; Monti, J.P.; Richard, T. Wine polyphenols: Potential agents in neuroprotection. Oxidative Med. Cell. Longev. 2012, 2012, 805762. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.; Siddiqui, M.A.; Khan, M.M.; Ajmal, M.; Ahsan, R.; Rahaman, M.A.; Ahmad, M.A.; Arshad, M.; Khushtar, M. Current Pharmacological Trends on Myricetin. Drug Res. 2020, 70, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Charalabidis, A.; Sfouni, M.; Bergström, C.; Macheras, P. The Biopharmaceutics Classification System (BCS) and the Biopharmaceutics Drug Disposition Classification System (BDDCS): Beyond guidelines. Int. J. Pharm. 2019, 566, 264–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. Occupational Diseases in Workers Exposed to Organic Solvents. HMR 2010, 30, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Yang, C.Y.; Huang, P.H.; Tseng, C.H.; Yen, F.L. Topical Artocarpus communis Nanoparticles Improved the Water Solubility and Skin Permeation of Raw A. communis Extract, Improving Its Photoprotective Effect. Pharmaceutics 2021, 13, 1372. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hu, S.C.; Huang, P.-H.; Lin, T.-C.; Yen, F.-L. Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications. Pharmaceutics 2020, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Mihajlovic, T.; Kachrimanis, K.; Graovac, A.; Djuric, Z.; Ibric, S. Improvement of Aripiprazole Solubility by Complexation with (2-Hydroxy)propyl-β-cyclodextrin Using Spray Drying Technique. AAPS PharmSciTech 2012, 13, 623–631. [Google Scholar] [CrossRef]
- Huang, P.H.; Hu, S.C.S.; Yen, F.L.; Tseng, C.H. Improvement of Skin Penetration, Antipollutant Activity and Skin Hydration of 7,3′,4′-Trihydroxyisoflavone Cyclodextrin Inclusion Complex. Pharmaceutics 2019, 11, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teh, S.S.; Mah, S.H. Stability Evaluations of Different Types of Vegetable Oil-based Emulsions. J. Oleo Sci. 2018, 67, 1381–1387. [Google Scholar] [CrossRef] [Green Version]
- Teng, W.L.; Huang, P.H.; Wang, H.C.; Tseng, C.H.; Yen, F.L. Pterostilbene Attenuates Particulate Matter-Induced Oxidative Stress, Inflammation and Aging in Keratinocytes. Antioxidants 2021, 10, 1552. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Gumustas, M.; Sengel-Turk, C.T.; Gumustas, A.; Ozkan, S.A.; Uslu, B. Chapter 5—Effect of Polymer-Based Nanoparticles on the Assay of Antimicrobial Drug Delivery Systems. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–108. [Google Scholar] [CrossRef]
- Han, D.; Han, Z.; Liu, L.; Wang, Y.; Xin, S.; Zhang, H.; Yu, Z. Solubility Enhancement of Myricetin by Inclusion Complexation with Heptakis-O-(2-Hydroxypropyl)-β-Cyclodextrin: A Joint Experimental and Theoretical Study. Int. J. Mol. Sci. 2020, 21, 766. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Xie, Y.; Hong, C.; Li, G.; Shen, H.; Ji, G. Development of a myricetin/hydroxypropyl-β-cyclodextrin inclusion complex: Preparation, characterization, and evaluation. Carbohydr. Polym. 2014, 110, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lin, J.; Chen, Y.; Liu, Y. Artemether/hydroxypropyl-β-cyclodextrin host–guest system: Characterization, phase-solubility and inclusion mode. Bioorg. Med. Chem. 2009, 17, 6311–6317. [Google Scholar] [CrossRef] [PubMed]
- Ao, F.; Shen, W.; Ge, X.; Wang, L.; Ning, Y.; Ren, H.; Fan, G.; Huang, M. Effects of the crystallinity on quercetin loaded the Eudragit L-100 electrospun nanofibers. Colloids Surf. B Biointerfaces 2020, 195, 111264. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Ye, J.; Wang, W.; Yang, Y.; Wang, H.; Sun, T.; Gao, L.; Liu, Y. Self-Assembled Lecithin/Chitosan Nanoparticles Based on Phospholipid Complex: A Feasible Strategy to Improve Entrapment Efficiency and Transdermal Delivery of Poorly Lipophilic Drug. Int. J. Nanomed. 2020, 15, 5629–5643. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Servidio, C.; Laganà, A.S.; Conforti, F.; Marrelli, M.; Cassano, R. Viscosified Solid Lipidic Nanoparticles Based on Naringenin and Linolenic Acid for the Release of Cyclosporine A on the Skin. Molecules 2020, 25, 3535. [Google Scholar] [CrossRef]
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fast-acting clotrimazole composited PVP/HPβCD nanofibers for oral candidiasis application. Pharm. Res. 2014, 31, 1893–1906. [Google Scholar] [CrossRef]
- Samprasit, W.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fast releasing oral electrospun PVP/CD nanofiber mats of taste-masked meloxicam. Int. J. Pharm. 2015, 487, 213–222. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast-dissolving antiviral drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111514. [Google Scholar] [CrossRef]
- Karagianni, A.; Kachrimanis, K.; Nikolakakis, I. Co-Amorphous Solid Dispersions for Solubility and Absorption Improvement of Drugs: Composition, Preparation, Characterization and Formulations for Oral Delivery. Pharmaceutics 2018, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Kubo, A.; Ishizaki, I.; Kubo, A.; Kawasaki, H.; Nagao, K.; Ohashi, Y.; Amagai, M. The stratum corneum comprises three layers with distinct metal-ion barrier properties. Sci. Rep. 2013, 3, 1731. [Google Scholar] [CrossRef] [Green Version]
- Pünnel, L.C.; Lunter, D.J. Film-Forming Systems for Dermal Drug Delivery. Pharmaceutics 2021, 13, 932. [Google Scholar] [CrossRef] [PubMed]
- Cal, K.; Centkowska, K. Use of cyclodextrins in topical formulations: Practical aspects. Eur. J. Pharm. Biopharm. 2008, 68, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Wu, Z.; Shao, W.; Guo, P.; Lin, Y.; Pan, W.; Zeng, W.; Zhang, G.; Wu, C.; Xu, Y. Synergetic skin targeting effect of hydroxypropyl-β-cyclodextrin combined with microemulsion for ketoconazole. Eur. J. Pharm. Biopharm. 2015, 93, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Antioxidant Vitamin E/Cyclodextrin Inclusion Complex Electrospun Nanofibers: Enhanced Water Solubility, Prolonged Shelf Life, and Photostability of Vitamin E. J. Agric. Food Chem. 2017, 65, 5404–5412. [Google Scholar] [CrossRef]
- Lin, H.S.; Chean, C.S.; Ng, Y.Y.; Chan, S.Y.; Ho, P.C. 2-hydroxypropyl-beta-cyclodextrin increases aqueous solubility and photostability of all-trans-retinoic acid. J. Clin. Pharm. Ther. 2000, 25, 265–269. [Google Scholar] [CrossRef] [PubMed]









| Ratio (MYR:HPβCD:PVP, w/w/w) | Water Solubility (µg/mL) | Yield (%) | Encapsulation Efficiency (%) |
|---|---|---|---|
| MYR | 0.32 ± 0.16 | - | - |
| 1:20:4 | 852.83 ± 21.38 *,# | 79.14 ± 2.89 *,# | 99.54 ± 0.05 * |
| 1:20:8 | 892.01 ± 10.11 * | 86.30 ± 1.99 * | 99.18 ± 0.02 * |
| 1:20:12 | 914.67 ± 13.18 * | 90.15 ± 5.04 * | 98.63 ± 0.16 * |
| Ratio (MYR:HPβCD:PVP, w/w/w) | Particle Size (nm) | Polydispersity Index (PDI) |
|---|---|---|
| MYR | 4065.27 ± 327.11 | 1.37 ± 0.10 |
| 1:20:4 | 137.33 ± 6.79 * | 0.10 ± 0.01 * |
| 1:20:8 | 88.73 ± 3.07 * | 0.07 ± 0.00 * |
| 1:20:12 | 38.27 ± 1.02 * | 0.24 ± 0.02 * |
| Group | DPPH Free Radical | ABTS+ Free Radical | FRAP |
|---|---|---|---|
| SC50 (μg/mL) a | EC50 (μg/mL) b | ||
| MYR in water | NE c | NE c | NE c |
| MYR in methanol | 10.70 ± 0.19 *,# | 11.93 ± 0.66 *,# | 3.79 ± 0.17 *,# |
| MyNF (1:20:12) in water | 9.61 ± 0.10 *,# | 11.31 ± 0.44 *,# | 1.74 ± 0.64 *,# |
| Vitamin C in water d | 14.35 ± 0.35 *,# | 21.84 ± 0.80 *,# | 5.95 ± 0.72 *,# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-C.; Yang, C.-Y.; Wu, T.-H.; Tseng, C.-H.; Yen, F.-L. Myricetin Nanofibers Enhanced Water Solubility and Skin Penetration for Increasing Antioxidant and Photoprotective Activities. Pharmaceutics 2023, 15, 906. https://doi.org/10.3390/pharmaceutics15030906
Lin T-C, Yang C-Y, Wu T-H, Tseng C-H, Yen F-L. Myricetin Nanofibers Enhanced Water Solubility and Skin Penetration for Increasing Antioxidant and Photoprotective Activities. Pharmaceutics. 2023; 15(3):906. https://doi.org/10.3390/pharmaceutics15030906
Chicago/Turabian StyleLin, Tzu-Ching, Chun-Yin Yang, Tzu-Hui Wu, Chih-Hua Tseng, and Feng-Lin Yen. 2023. "Myricetin Nanofibers Enhanced Water Solubility and Skin Penetration for Increasing Antioxidant and Photoprotective Activities" Pharmaceutics 15, no. 3: 906. https://doi.org/10.3390/pharmaceutics15030906