Derivatives of L-Ascorbic Acid in Emulgel: Development and Comprehensive Evaluation of the Topical Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Emulgel Preparation
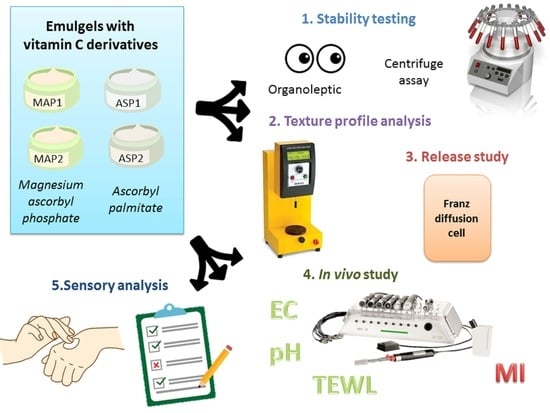
2.2.2. Physico-Chemical Testing of Emulgels
2.2.3. Texture Profile Analysis (TPA)
2.2.4. Release Study of L-Ascorbic Acid Derivatives from the Emulgels
2.2.5. Electrical Capacitance (EC), Trans-Epidermal Water Loss (TEWL) and pH Measurements
2.2.6. Melanin Index (MI) Measurements
2.2.7. Examination of Sensory Properties
2.2.8. Ethical Standards
2.2.9. Statistical Analysis
3. Results and Discussion
- electrical capacitance (EC) was an indicator of the effect of tested emulgels on skin moisture;
- transepidermal water loss (TEWL) was measured to determine whether the tested emulgels influence skin barrier function;
- skin pH to assess the effect of tested emulgels on skin damage;
- melanin index (MI) portrayed the effects of the tested emulgels on skin color and the possibility of the lightening of the skin.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tadić, V.M.; Žugić, A.; Martinović, M.; Stanković, M.; Maksimović, S.; Frank, A.; Nešić, I. Enhanced Skin Performance of Emulgel vs. Cream as Systems for Topical Delivery of Herbal Actives (Immortelle Extract and Hemp Oil). Pharmaceutics 2021, 13, 1919. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Saini, S.; Joshi, B.; Rana, A.C. Emulgel: A new platform for topical drug delivery. Int. J. Pharm. Biol. Sci. 2012, 3, 485–498. [Google Scholar]
- Dhawas, V.; Dhabarde, D.; Patil, S. Emulgel: A comprehensive review for novel topical drug delivery system. Int. J. Recent. Sci. Res. 2020, 11, 38135–38136. [Google Scholar]
- Panwar, A.; Upadhyay, N.; Bairagi, M.; Gujar, S.; Darwhekar, G.; Jain, D. Emulgel: A review. Asian J. Pharm. Life Sci. 2011, 2231, 4423. [Google Scholar]
- Haneefa, K.M.; Mohanta, G.P.; Nayar, C. Emulgel: An Advanced Review. J. Pharm. Sci. Res. 2013, 5, 254–258. [Google Scholar]
- Asija, R.; Sharma, R.; Gupta, A. Emulgel: A novel approach to topical drug delivery. J. Biomed. Pharm. Res. 2013, 2, 91–94. [Google Scholar]
- Sushma, G.; Pravalika, T.; Sri, B.R.; Priyanaka, P.; Priya, P.V.; Sharma, J.V.C. Emulgels-A Novel Approach for Topical Drug Delivery. Int. J. Pharm. Sci. Rev. Res. 2021, 67, 142–147. [Google Scholar] [CrossRef]
- Panchal, B.; Rathi, S. Topical emulgel: A review on state of art. PSM 2018, 9, 253–264. [Google Scholar]
- Alexander, A.; Khichariya, A.; Gupta, S.; Patel, R.J.; Giri, T.K.; Tripathi, D.K. Recent expansions in an emergent novel drug delivery technology: Emulgel. J. Control. Release 2013, 171, 122–132. [Google Scholar] [CrossRef]
- Rehman, K.; Zulfakar, M.H. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 433–440. Available online: http://informahealthcare.com/ddi (accessed on 1 December 2022). [CrossRef]
- Caritá, A.C.; Santos, B.F.; Shultz, J.D.; Kohn, B.M.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomedicine 2020, 24, 102117. [Google Scholar] [CrossRef]
- Telang, P.S. Vitamin C in dermatology. Indian Dermatol. Online J. 2013, 4, 143–146. [Google Scholar] [CrossRef]
- Farris, P.K. Topical Vitamin C: A Useful Agent for Treating Photoaging and Other Dermatologic Conditions. Dermatol. Surg. 2005, 31, 814–818. [Google Scholar] [CrossRef]
- Hwang, S.W.; Oh, D.J.; Lee, D.; Kim, J.W.; Park, S.W. Clinical efficacy of 25% L-ascorbic acid (C’ensil) in the treatment of melisma. J. Cutan. Med. Surg. 2009, 13, 74–81. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, F. Degradation of Ascorbic Acid in Aqueous Solution. J. Agric. Food Chem. 1998, 46, 5078–5082. [Google Scholar] [CrossRef]
- Touitou, E.; Alkabes, M.; Memoli, A.; Alhaique, F. Glutathione stabilizes ascorbic acid in aqueous solution. Int. J. Pharm. 1996, 133, 85–88. [Google Scholar] [CrossRef]
- Austria, R.; Semenzato, A.; Bettero, A. Stability of vitamin C derivatives in solution and topical formulations. J. Pharm. Biomed. Anal. 1997, 15, 795–801. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Huang, J.; Xia, N.; Li, T.; Xia, Q. Self-double-emulsifying drug delivery system incorporated in natural hydrogels: A new way for topical application of vitamin C. J. Microencapsul. 2018, 35, 90–101. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, W.; Zou, L.; Liu, W.; Liu, C.; Liang, R.; Chen, J. Storage stability and skin permeation of vitamin C liposomes improved by pectin coating. Colloids Surf. B Biointerfaces 2014, 117, 330–337. [Google Scholar] [CrossRef]
- Kim, S.; Lee, T.G. Stabilization of L-ascorbic acid in cosmetic emulsions. J. Ind. Eng. Chem. 2018, 57, 193–198. [Google Scholar] [CrossRef]
- Dini, I. Contribution of Nanoscience Research in Antioxidants Delivery Used in Nutricosmetic Sector. Antioxidants 2022, 11, 563. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Ramasamy, M.; Willis, N.C.; Kim, D.S.; Anderson, W.A.; Tam, K.C. Encapsulation and controlled release of vitamin C in modified cellulose nanocrystal/chitosan nanocapsules. Curr. Res. Food Sci. 2021, 4, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Fraj, J.; Petrović, L.; Đekić, L.; Budinčić, J.M.; Bučko, S.; Katona, J. Encapsulation and release of vitamin C in double W/O/W emulsions followed by complex coacervation in gelatin-sodium caseinate system. J. Food Eng. 2021, 292, 110353. [Google Scholar] [CrossRef]
- Jurkovič, P.; Šentjurc, M.; Kristl, J.; Pečar, S.; Gašperlin, M. Comparison of two ascorbic acid derivatives effectiveness for scavenging ultraviolet-induced free radicals in the skin. J. Drug Deliv. Sci. Technol. 2004, 14, 229–233. [Google Scholar] [CrossRef]
- Perioli, L.; Pagano, C.; Mazzitelli, S.; Rossi, C.; Nastruzzi, C. Rheological and functional characterization of new antiinflammatory delivery systems designed for buccal administration. Int. J. Pharm. 2008, 356, 19–28. [Google Scholar] [CrossRef]
- Mohamed, M.I. Optimization of chlorphenesin emulgel formulation. AAPS J. 2004, 6, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Sintov, A.C.; Hofmann, M.A. A novel thermo-mechanical system enhanced transdermal delivery of hydrophilic active agents by fractional ablation. Int. J. Pharm. 2016, 511, 821–830. [Google Scholar] [CrossRef]
- Nováková, L.; Solich, P.; Solichová, D. HPLC methods for simultaneous determination of ascorbic and dehydroascorbic acids. Trac Trends Anal. Chem. 2008, 27, 942–958. [Google Scholar] [CrossRef]
- Đurašević, S.; Ružičić, A.; Lakić, I.; Tosti, T.; Đurović, S.; Glumac, S.; Pejić, S.; Todorović, A.; Drakulić, D.; Stanković, S.; et al. The Effects of a Meldonium Pre-Treatment on the Course of the LPS-Induced Sepsis in Rats. Int. J. Mol. Sci. 2022, 23, 2395. [Google Scholar] [CrossRef]
- Arsić, I.; Žugić, A.; Tadić, V.; Tasić-Kostov, M.; Mišić, D.; Primorac, M.; Runjaić-Antić, D. Estimation of Dermatological Application of Creams with St. John’s Wort Oil Extracts. Molecules 2011, 17, 275–294. [Google Scholar] [CrossRef] [Green Version]
- UNION, P. Regulation (EC) No 1223/2009 of the european parliament and of the council. Off. J. Eur. Union L 2009, 342, 59–209. [Google Scholar]
- Colipa, C. Guidelines for the Evaluation of the Efficacy of Cosmetic Products, 3rd ed.; European Economicand Social Committee: Brussels, Belgium, 2008. [Google Scholar]
- Clarys, P.; Alewaeters, K.; Lambrecht, R.; Barel, A.O. Skin color measurements: Comparison between three instruments: The Chromameter®, the DermaSpectrometer® and the Mexameter®. Ski. Res. Technol. 2000, 6, 230–238. [Google Scholar] [CrossRef]
- Martinović, M.; Stojanović, N.; Nešić, I. Textural and Sensory Characterization of Carbomeric Gels with Panthenol. Acta Fac. Medicae Naissensis 2022, 39, 232–243. [Google Scholar] [CrossRef]
- Leonardi, G.R.; Gaspar, L.R.; Campos, P.M.B.G.M. Application of a non-invasive method to study the moisturizing effect of formulations containing vitamins A or E or ceramide on human skin. J. Cosmet. Sci. 2002, 53, 263–268. [Google Scholar]
- Campos, P.M.S.G.M.; Goncalves, G.M.S.; Gaspar, L.R. In vitro antioxidant activity and in vivo efficacy of topical formulations containing vitamin C and its derivatives studied by non-invasive methods. Ski. Res. Technol. 2008, 14, 376–380. [Google Scholar] [CrossRef]
- Špiclin, P.; Gašperlin, M.; Kmetec, V. Stability of ascorbyl palmitate in topical microemulsions. Int. J. Pharm. 2001, 222, 271–279. [Google Scholar] [CrossRef]
- Stolić-Jovanović, A.; Martinović, M.; Nešić, I. The Influence of Selected Thickeners on the Textural Properties of Oil-In-Water Emulsions. Acta Fac. Medicae Naissensis 2022, 39, 57–65. [Google Scholar] [CrossRef]
- Vasiljević, D.; Savić, S.; Đorđević, L.J.; Krajišnik, L.J.; Krajišnik, D. Priručnik iz kozmetologije, 2nd ed.; Nauka: Beograd, Srbija, 2009. [Google Scholar]
- Lodén, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Dermatol. 2003, 4, 771–778. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. Int. J. Pharm. 1997, 151, 223–233. [Google Scholar] [CrossRef]
- Trinh, K.T.; Glasgow, S. On the texture profile analysis test. In Proceedings of the Chemeca 2012, Wellington, New Zealand, 23–26 September 2012; pp. 23–26. [Google Scholar]
- Estanqueiro, M.; Amaral, M.H.; Lobo, J.M.S. Comparison between sensory and instrumental characterization of topical formulations: Impact of thickening agents. Int. J. Cosmet. Sci. 2016, 38, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Szczesniak, A.S.; Brandt, M.A.; Friedman, H.H. Development of standard rating scales for mechanical parameters of texture and correlation between the objective and the sensory methods of texture evaluation. J. Food Sci. 1963, 28, 397–403. [Google Scholar] [CrossRef]
- Savary, G.; Gilbert, L.; Grisel, M.; Picard, C. Instrumental and sensory methodologies to characterize the residual film of topical products applied to skin. Ski. Res. Technol. 2019, 25, 415–423. [Google Scholar] [CrossRef]
- Szczesniak, A.S. Texture is a sensory property. Food Qual. Prefer. 2002, 13, 215–225. [Google Scholar] [CrossRef]
- Paarakh, M.P.; Jose, P.A.; Setty, C.M.; Christoper, G.P. Release kinetics–concepts and applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar] [CrossRef]
- Binder, L.; Mazál, J.; Petz, R.; Klang, V.; Valenta, C. The role of viscosity on skin penetration from cellulose ether-based hydrogels. Ski. Res. Technol. 2019, 25, 725–734. [Google Scholar] [CrossRef] [Green Version]
- Batheja, P.; Sheihet, L.; Kohn, J.; Singer, A.J.; Michniak-Kohn, B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimi-zation and in vitro and in vivo skin distribution studies. J. Control. Release 2011, 149, 159–167. [Google Scholar] [CrossRef]
- Jurkovič, P.; Šentjurc, M.; Gašperlin, M.; Kristl, J.; Pečar, S. Skin protection against ultraviolet induced free radicals with ascorbyl palmitate in microemulsions. Eur. J. Pharm. Biopharm. 2003, 56, 59–66. [Google Scholar] [CrossRef]
- Gosenca, M.; Gašperlin, M. Dermal delivery of ascorbyl palmitate: The potential of colloidal delivery systems. J. Drug Deliv. Sci. Technol. 2011, 21, 535–537. [Google Scholar] [CrossRef]
- Ćorović, M.; Milivojević, A.; Simović, M.; Banjanac, K.; Pjanović, R.; Bezbradica, D. Enzymatically derived oil-based L-ascorbyl esters: Synthesis, antioxidant properties and controlled release from cosmetic formulations. Sustain. Chem. Pharm. 2020, 15, 100231. [Google Scholar] [CrossRef]
- Gosenca, M.; Bešter-Rogač, M.; Gašperlin, M. Lecithin based lamellar liquid crystals as a physiologically acceptable dermal delivery system for ascorbyl palmitate. Eur. J. Pharm. Sci. 2013, 50, 114–122. [Google Scholar] [CrossRef]
- Rozman, B.; Zvonar, A.; Falson, F.; Gasperlin, M. Temperature-Sensitive Microemulsion Gel: An Effective Topsical Delivery System for Simultaneous Delivery of Vitamins C and E. AAPS Pharmscitech 2009, 10, 54–61. [Google Scholar] [CrossRef]
- Iliopoulos, F.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. 3-O-ethyl-l-ascorbic acid: Characterisation and investigation of single solvent systems for delivery to the skin. Int. J. Pharm. X 2019, 1, 100025. [Google Scholar] [CrossRef]
- Farahmand, S.; Tajerzadeh, H.; Farboud, E.S. Formulation and Evaluation of a Vitamin C Multiple Emulsion. Pharm. Dev. Technol. 2006, 11, 255–261. [Google Scholar] [CrossRef]
- Duarah, S.; Durai, R.D.; Narayanan, V.B. Nanoparticle-in-gel system for delivery of vitamin C for topical application. Drug Deliv. Transl. Res. 2017, 7, 750–760. [Google Scholar] [CrossRef]
- Khan, H.; Akhtar, N.; Ali, A.; Khan, H.M.S.; Sohail, M.; Naeem, M.; Nawaz, Z. Physical and chemical stability analysis of cosmetic multiple emulsions loaded with ascorbyl palmitate and sodium ascorbyl phosphate salts. Acta Pol. Pharm. 2016, 73, 1339–1349. [Google Scholar]
- Huang, H.C.; Chang, T.M. Ceramide 1 and ceramide 3 act synergistically on skin hydration and the transepidermal water loss of sodium lauryl sulfate-irritated skin. Int. J. Dermatol. 2008, 47, 812–819. [Google Scholar] [CrossRef]
- Khan, H.; Akhtar, N. Synergistic effects of ascorbyl palmitate and sodium ascorbyl phosphate loaded in multiple emulsions on facial skin melanin and erythema content. Biomed. Res. J. 2016, 27, 570–576. [Google Scholar]
- Kameyama, K.; Saka, C.; Kondoh, S.; Yonemoto, K.; Nishiyama, S.; Tagawa, M.; Murata, T.; Ohnuma, T.; Quigley, J.; Dorsky, A.; et al. Inhibitory effect of magnesium L-ascorbyl-2-phosphate (VC-PMG) on melanogenesis in vitro and in vivo. J. Am. Acad. Dermatol. 1996, 34, 29–33. [Google Scholar] [CrossRef]
- Gilbert, L.; Savary, G.; Grisel, M.; Picard, C. Predicting sensory texture properties of cosmetic emulsions by physical measurements. Chemom. Intell. Lab. Syst. 2013, 124, 21–31. [Google Scholar] [CrossRef]
- Yadav, S.K.; Mishra, M.K.; Tiwari, A.; Shukla, A. Emulgel: A new approach for enhanced topical drug delivery. Int. J. Curr. Pharm. Res. 2016, 9, 15–19. [Google Scholar]








| Ingredients (INCI Name) | Function in the Formulation | Content [% m/m] | ||||
|---|---|---|---|---|---|---|
| ASP1 | ASP2 | MAP1 | MAP2 | PE | ||
| Oil phase | ||||||
| Caprylic/capric triglycerides | Emollient | 11.00 | 11.00 | 11.00 | 11.00 | 11.00 |
| Isopropyl myristate | Emollient | 7.50 | 7.50 | 7.50 | 7.50 | 7.50 |
| Olive oil | Emollient | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Cetearyl alcohol (and) Coco-glucoside | o/w emulsifier | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 |
| Myristyl alcohol (and) Myristyl glucoside | o/w emulsifier | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Active substance | ||||||
| Ascorbyl palmitate | 1.00 | 2.00 | - | - | - | |
| Magnesium ascorbyl phosphate | - | - | 1.00 | 2.00 | - | |
| Water phase | ||||||
| Hydroxyethyl cellulose (HEC) | Thickener/Gelling agent | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Propylene glycol | Humectant | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Phenoxyethanol (and) Ethylhexylglycerin | Preservative | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Aqua (Water) | Water phase | q.s. 100.00 | q.s. 100.00 | q.s. 100.00 | q.s. 100.00 | q.s. 100.00 |
| Prior to Application | |||||||
|---|---|---|---|---|---|---|---|
| Consistency | Gloss Level | ||||||
| liquid | matte | gloss | |||||
| semi-solid | pearl gloss | very glossy | |||||
| slightly glossy | |||||||
| In the Course of Application | |||||||
| Spreadability | Adhesion | Density | Grease | Gloss | Absorption rate | ||
| very difficult to spread | not sticky | rare | not greasy | not shiny | slow | ||
| difficult to spread | slightly sticky | slightly dense | slightly greasy | slightly shiny | moderate | ||
| easy to spread | sticky | dense | greasy | shiny | fast | ||
| very sticky | very dense | very greasy | very shiny | ||||
| Following Application | |||||||
| Residual film | Stickiness | Grease | Gloss | ||||
| no film | not sticky | not greasy | not shiny | ||||
| moderate film | slightly sticky | slightly greasy | slightly shiny | ||||
| expressive film | sticky | greasy | shiny | ||||
| very sticky | very greasy | very shiny | |||||
| pH | ||||
|---|---|---|---|---|
| Before Centrifuge Assay | After Centrifuge Assay | After Accelerated Stability Test | After Long-Term Stability Test | |
| MAP 1 | 7.16 | 7.17 | 7.14 | 7.16 |
| MAP 2 | 7.17 | 7.20 | 7.15 | 7.16 |
| ASP 1 | 4.21 | 4.23 | 4.24 | 4.20 |
| ASP 2 | 3.99 | 3.94 | 3.98 | 4.00 |
| Electrical conductivity (μS/cm) | ||||
| Before centrifuge assay | After centrifuge assay | After accelerated stability test | After long-term stability test | |
| MAP 1 | −4.4 | −5.5 | −4.9 | −4.4 |
| MAP 2 | −5.4 | −7.2 | −6.8 | −5.9 |
| ASP 1 | 166.5 | 164.9 | 165.9 | 165.4 |
| ASP 2 | 178.7 | 182.1 | 184.2 | 179.0 |
| Adhesiveness (mJ) | Cohesiveness | Hardness Cycle 1 (g) | Hardness Cycle 2 (g) | |
|---|---|---|---|---|
| PE | 0.60 ± 0.10 a * | 0.80 ± 0.19 a | 23.33 ± 1.15 b | 21.67 ± 1.53 b |
| MAP1 | 0.45 ± 0.07 ab | 0.89 ± 0.02 a | 36.00 ± 5.66 a | 35.50 ± 6.36 a |
| MAP2 | 0.50 ± 0.00 ab | 0.86 ± 0.05 a | 33.67 ± 4.16 a | 31.67 ± 4.04 a |
| ASP1 | 0.47 ± 0.06 ab | 0.87 ± 0.14 a | 21.67 ± 1.53 b | 20.67 ± 1.53 b |
| ASP2 | 0.43 ± 0.06 b | 1.07 ± 0.11 a | 21.67 ± 0.58 b | 21.00 ± 0.00 b |
| Before Application | |||||
|---|---|---|---|---|---|
| PE | ASP1 | ASP2 | MAP1 | MAP2 | |
| Consistency | 8.32 | 9.17 | 9.17 | 6.64 | 10.00 |
| Gloss level | 5.83 | 5.67 | 6.33 | 5.66 | 5.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolić Jovanović, A.; Martinović, M.; Žugić, A.; Nešić, I.; Tosti, T.; Blagojević, S.; Tadić, V.M. Derivatives of L-Ascorbic Acid in Emulgel: Development and Comprehensive Evaluation of the Topical Delivery System. Pharmaceutics 2023, 15, 813. https://doi.org/10.3390/pharmaceutics15030813
Stolić Jovanović A, Martinović M, Žugić A, Nešić I, Tosti T, Blagojević S, Tadić VM. Derivatives of L-Ascorbic Acid in Emulgel: Development and Comprehensive Evaluation of the Topical Delivery System. Pharmaceutics. 2023; 15(3):813. https://doi.org/10.3390/pharmaceutics15030813
Chicago/Turabian StyleStolić Jovanović, Aleksandra, Milica Martinović, Ana Žugić, Ivana Nešić, Tomislav Tosti, Stevan Blagojević, and Vanja M. Tadić. 2023. "Derivatives of L-Ascorbic Acid in Emulgel: Development and Comprehensive Evaluation of the Topical Delivery System" Pharmaceutics 15, no. 3: 813. https://doi.org/10.3390/pharmaceutics15030813