Insights into the Safety and Versatility of 4D Printed Intravesical Drug Delivery Systems
Abstract
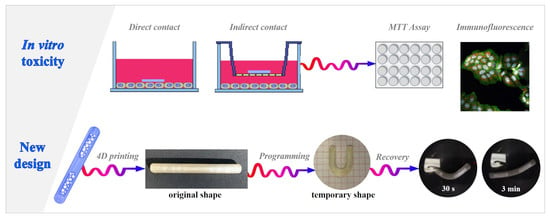
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of PVA-Based Formulations
2.2.2. HME
2.2.3. 3D Printing
2.2.4. Film-Coating
2.2.5. Physio-Technological Characterization
2.2.6. In vitro Toxicity Studies
Cell Culture
Cell Viability, Proliferation and Death
Cytokine Analysis by Real-Time PCR
Statistical Analysis
3. Results and Discussion
3.1. Cytotoxic Evaluation of PVA-Based Samples
3.2. New Configuration of the PVA-Based DDS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farokhzad, O.C.; Dimitrakov, J.D.; Karp, J.M.; Khademhosseini, A.; Freeman, M.R.; Langer, R. Drug delivery systems in urology-getting “smarter”. Urology 2006, 68, 463–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GuhaSarkar, S.; Banerjee, R. Intravesical drug delivery: Challenges, current status, opportunities and novel strategies. J. Control. Release 2010, 148, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choy, Y.B. Implantable devices for sustained, intravesical drug delivery. Int. Neurourol. J. 2016, 20, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarfraz, M.; Qamar, S.; Rehman, M.U.; Tahir, M.A.; Ijaz, M.; Ahsan, A.; Asim, M.H.; Nazir, I. Nano-formulation based intravesical drug delivery systems: An overview of versatile approaches to improve urinary bladder diseases. Pharmaceutics 2022, 14, 1909. [Google Scholar] [CrossRef]
- Zacchè, M.M.; Srikrishna, S.; Cardozo, L. Novel targeted bladder drug-delivery systems: A review. Res. Rep. Urol. 2015, 7, 169–178. [Google Scholar] [CrossRef] [Green Version]
- de Lima, C.S.A.; Varca, J.P.R.O.; Alves, V.M.; Nogueira, K.M.; Cruz, C.P.C.; Rial-Hermida, M.I.; Kadłubowski, S.S.; Varca, G.H.C.; Lugão, A.B. Mucoadhesive polymers and their applications in drug delivery systems for the treatment of bladder cancer. Gels 2022, 8, 587. [Google Scholar] [CrossRef]
- Gugleva, V.; Michailova, V.; Mihaylova, R.; Momekov, G.; Zaharieva, M.M.; Najdenski, H.; Petrov, P.; Rangelov, S.; Forys, A.; Trzebicka, B.; et al. Formulation and evaluation of hybrid niosomal in situ gel for intravesical co-delivery of curcumin and gentamicin sulfate. Pharmaceutics 2022, 14, 747. [Google Scholar] [CrossRef]
- Guo, P.; Wang, L.; Shang, W.; Chen, J.; Chen, Z.; Xiong, F.; Wang, Z.; Tong, Z.; Wang, K.; Yang, L.; et al. Intravesical in situ immunostimulatory gel for triple therapy of bladder cancer. ACS ACS Appl. Mater. Interfaces 2020, 12, 54367–54377. [Google Scholar] [CrossRef]
- Cima, M.J.; Lee, H.; Daniel, K.; Tanenbauma, L.M.; Mantzavinou, A.; Spencer, K.C.; Ong, Q.; Sy, J.C.; Santini, J., Jr.; Schoellhammer, C.M.; et al. Single compartment drug delivery. J. Control. Release 2014, 190, 157–171. [Google Scholar] [CrossRef]
- Wang, L.H.; Shang, L.; Shan, D.Y.; Che, X. Long-term floating control-released intravesical preparation of 5-fluorouracil for the local treatment of bladder cancer. Drug Dev. Ind. Pharm. 2017, 43, 1343–1350. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, Y.; Wang, K.; Zhao, X.; Lian, H.; Wang, W.; Wang, H.; Wu, J.; Hu, Y.; Guo, H. Visualized intravesical floating hydrogel encapsulating vaporized perfluoropentane for controlled drug release. Drug Deliv. 2016, 23, 2820–2826. [Google Scholar] [CrossRef] [Green Version]
- Maroni, A.; Melocchi, A.; Zema, L.; Foppoli, A.; Gazzaniga, A. Retentive drug delivery systems based on shape memory materials. J. Appl. Polym. Sci. 2020, 137, 48798. [Google Scholar] [CrossRef]
- Palugan, L.; Cerea, M.; Cirilli, M.; Moutaharrik, A.; Maroni, A.; Zema, L.; Melocchi, A.; Uboldi, M.; Filippin, I.; Foppoli, A.; et al. Intravesical drug delivery approaches for improved therapy of urinary bladder diseases. Int. J. Pharm. X 2021, 3, 100100. [Google Scholar] [CrossRef] [PubMed]
- Behl, M.; Lendlein, A. Shape memory polymers. Mater. Today 2007, 10, 20–28. [Google Scholar]
- Melocchi, A.; Uboldi, M.; Cerea, M.; Foppoli, A.; Maroni, A.; Moutaharrik, S.; Palugan, L.; Zema, L.; Gazzaniga, A. Shape memory materials and 4D printing in pharmaceutics. Adv. Drug Deliv. Rev. 2021, 173, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, L.; Zhang, F.; Leng, J.; Liu, Y. Shape memory polymers and their composites in biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 864–883. [Google Scholar] [CrossRef]
- Wischke, C.; Behl, M.; Lendlein, A. Drug-releasing shape-memory polymers-the role of morphology, processing effects, and matrix degradation. Expert Opin. Drug Deliv. 2013, 10, 1193–1205. [Google Scholar] [CrossRef]
- Giesing, D.; Lee, H.; Daniel, K.D. Drug Delivery Systems and Methods for Treatment of Bladder Cancer with Gemcitabine. U.S. Patent US 2015/0250717 A1, 10 September 2015. [Google Scholar]
- Lee, H.; Daniel, K.D. Intravesical drug delivery devices and methods including elastic polymer-drug matrix systems. U.S. Patent WO 2015/200752 Al, 30 December 2015. [Google Scholar]
- Nickel, J.C.; Jain, P.; Shore, N.; Anderson, J.; Giesing, D.; Lee, H.; Kim, G.; Daniel, K.; White, S.; Larrivee-Elkins, C.; et al. Continuous intravesical lidocaine treatment for interstitial cystitis/bladder pain syndrome: Safety and efficacy of a new drug delivery device. Sci. Transl. Med. 2012, 4, 143a100. [Google Scholar] [CrossRef]
- Xu, X.; Goyanes, A.; Trenfield, S.J.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Gaisford, S.; Basit, A.W. Stereolithography (SLA) 3D printing of a bladder device for intravesical drug delivery. Mater. Sci. Eng. C 2021, 120, 111773. [Google Scholar] [CrossRef]
- Peterson, G.I.; Dobrynin, A.V.; Becker, M.L. Biodegradable shape memory polymers in medicine. Adv. Healthc. Mater. 2017, 6, 1700694. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Huang, W.M. Thermo/moisture responsive shape-memory polymer for possible surgery/operation inside living cells in future. Mat. Des. 2010, 31, 2684–2689. [Google Scholar] [CrossRef]
- Wong, Y.S.; Salvekar, A.V.; Zhuang, K.D.; Liu, H.; Birch, W.R.; Tay, K.H.; Huang, W.M.; Venkatraman, S.S. Bioabsorbable radiopaque water-responsive shape memory embolization plug for temporary vascular occlusion. Biomaterials 2016, 102, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Huang, W.M. Heating/solvent responsive shape-memory polymers for implant biomedical devices in minimally invasive surgery: Current status and challenge. Macromol. Biosci. 2020, 20, 2000108. [Google Scholar] [CrossRef] [PubMed]
- Rahmatabadi, D.; Aberoumand, M.; Soltanmohammadi, K.; Soleyman, E.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Zolfagharian, A.; Bodaghi, M.; Baghani, M. A New strategy for achieving shape memory effects in 4D printed two-layer composite structures. Polymers 2022, 14, 5446. [Google Scholar] [CrossRef] [PubMed]
- Rahmatabadi, D.; Aberoumand, M.; Soltanmohammadi, K.; Soleyman, E.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Bodaghi, M.; Baghani, M. 4D Printing-encapsulated polycaprolactone–thermoplastic polyurethane with high shape memory performances. Adv. Eng. Mater. 2023, 2022, 2201309. [Google Scholar] [CrossRef]
- Soleyman, E.; Aberoumand, M.; Soltanmohammadi, K.; Rahmatabadi, D.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Baghani, M. 4D printing of PET-G via FDM including tailormade excess third shape. Manuf. Lett. 2022, 33, 1–4. [Google Scholar] [CrossRef]
- Soleyman, E.; Aberoumand, M.; Rahmatabadi, D.; Soltanmohammadi, K.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Baghani, M. Assessment of controllable shape transformation, potential applications, and tensile shape memory properties of 3D printed PETG. J. Mater. Res. Technol. 2022, 18, 4201–4215. [Google Scholar] [CrossRef]
- Elkasabgy, N.A.; Mahmoud, A.A.; Maged, A. 3D printing: An appealing route for customized drug delivery systems. Int. J. Pharm. 2020, 588, 119732. [Google Scholar] [CrossRef]
- Patel, S.K.; Khoder, M.; Peak, M.; Alhnan, M.A. Controlling drug release with additive manufacturing-based solutions. Adv. Drug Deliv. Rev. 2021, 174, 369–386. [Google Scholar] [CrossRef]
- Bandari, S.; Nyavanandi, D.; Dumpa, N.; Repka, M.A. Coupling hot melt extrusion and fused deposition modeling: Critical properties for successful performance. Adv. Drug Deliv. Rev. 2021, 172, 52–63. [Google Scholar] [CrossRef]
- Krueger, L.; Miles, J.A.; Popat, A. 3D printing hybrid materials using fused deposition modelling for solid oral dosage forms. J. Control. Rel. 2022, 351, 444–455. [Google Scholar] [CrossRef]
- Parulski, C.; Jennotte, O.; Lechanteur, A.; Evrard, B. Challenges of fused deposition modeling 3D printing in pharmaceutical applications: Where are we now? Adv. Drug Deliv. Rev. 2021, 175, 113810. [Google Scholar] [CrossRef]
- Melocchi, A.; Inverardi, N.; Uboldi, M.; Baldi, F.; Maroni APandini, S.; Briatico-Vangosa, F.; Zema, L.; Gazzaniga, A. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly(vinyl alcohol): Design concept and 4D printing feasibility. Int. J. Pharm. 2019, 559, 299–311. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Inverardi, N.; Briatico-Vangosa, F.; Baldi, F.; Pandini, S.; Scalet, G.; Auricchio, F.; Cerea, M.; Foppoli, A.; et al. Expandable drug delivery system for gastric retention based on shape memory polymers: Development via 4D printing and extrusion. Int. J. Pharm. 2019, 571, 118700. [Google Scholar] [CrossRef]
- Fang, Z.Q.; Kuang, Y.D.; Zhou, P.P.; Ming, S.Y.; Zhu, P.H.; Liu, Y.; Ning, H.L.; Chen, G. Programmable shape recovery process of water-responsive shape memory poly(vinyl alcohol) by wettability contrast strategy. ACS Appl. Mater. Interfaces 2017, 9, 5495–5502. [Google Scholar] [CrossRef] [PubMed]
- Inverardi, N.; Scalet, G.; Melocchi, A.; Uboldi, M.; Maroni, A.; Zema, L.; Gazzaniga, A.; Auricchio, F.; Briatico-Vangosa, F.; Baldi, F.; et al. Experimental and computational analysis of a pharmaceutical-grade shape memory polymer applied to the development of gastroretentive drug delivery systems. J. Mech. Behav. Biomed Mater. 2021, 124, 104814. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Guo, Y. Enhanced shape memory property and mechanical property of polyvinyl alcohol by carbon black. J. Biomater. Tissue Eng. 2019, 9, 76–81. [Google Scholar] [CrossRef]
- Uboldi, M.; Melocchi, A.; Moutaharrik, S.; Palugan, L.; Cerea, M.; Foppoli, A.; Maroni, A.; Gazzaniga, A.; Zema, L. Administration strategies and smart devices for drug release in specific sites of the upper GI tract. J. Control. Release 2022, 348, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lai, H.; Cheng, Z.; Kang, H.; Wang, Y.; Zhang, H.; Wang, J.; Liu, Y. Water-induced poly(vinyl alcohol)/carbon quantum dot nanocomposites with tunable shape recovery performance and fluorescence. J. Mater. Chem. B 2018, 6, 7444–7450. [Google Scholar] [CrossRef] [PubMed]
- Afzali Naniz, M.; Askari, M.; Zolfagharian, A.; Afzali Naniz, M.; Bodaghi, M. 4D printing: A cutting-edge platform for biomedical applications. Biomed. Mat. 2022, 17, 062001. [Google Scholar] [CrossRef]
- Pingale, P.; Dawre, S.; Dhapte-Pawar, V.; Dhas, N.; Rajput, A. Advances in 4D printing: From stimulation to simulation. Drug Deliv. Transl. Res. 2023, 13, 164–188. [Google Scholar] [CrossRef] [PubMed]
- Pourmasoumi, P.; Moghaddam, A.; Nemati Mahand, S.; Heidari, F.; Salehi Moghaddam, Z.; Arjmand, M.; Kühnert, I.; Kruppke, B.; Wiesmann, H.-P.; Khonakdar, H.A. A review on the recent progress, opportunities, and challenges of 4D printing and bioprinting in regenerative medicine. J. Biomater. Sci. Polym. Ed. 2023, 34, 108–146. [Google Scholar] [CrossRef]
- Uboldi, M.; Melocchi, A.; Moutaharrik, S.; Cerea, M.; Gazzaniga, A.; Zema, L. Dataset on a small-scale film-coating process developed for self-expanding 4D printed drug delivery devices. Coatings 2021, 11, 1252. [Google Scholar] [CrossRef]
- Uboldi, M.; Pasini, C.; Pandini, S.; Baldi, F.; Briatico-Vangosa, F.; Inverardi, N.; Maroni, A.; Moutaharrik, S.; Melocchi, A.; Gazzaniga, A.; et al. Expandable drug delivery systems based on shape memory polymers: Impact of film coating on mechanical properties and release and recovery performance. Pharmaceutics 2022, 14, 2814. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Brambilla, L.; Sartori, P.; Moscheni, C.; Perrotta, C.; Zema, L.; Bertarelli, C.; Castiglioni, C. Development of tailored graphene nanoparticles: Preparation, sorting and structure assessment by complementary techniques. Molecules 2023, 28, 565. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef]
- Sherif, A.Y.; Mahrou, G.M.; Alanazi, F.K. Novel in-situ gel for intravesical administration of ketorolac. Saudi Pharm. J. 2018, 26, 845–851. [Google Scholar] [CrossRef]
- Auwerx, J. The human leukemia cell line, THP-1: A multifacetted model for the study of monocyte-macrophage differentiation. Experientia 1991, 47, 22–31. [Google Scholar] [CrossRef]
- Liu, X.; Yin, S.; Chen, Y.; Wu, Y.; Zheng, W.; Dong, H.; Bai, Y.; Qin, Y.; Li, J.; Feng, S.; et al. 1LPS-induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF-κB, STAT3 or AP-1 activation. Mol. Med. Rep. 2018, 17, 5484–5491. [Google Scholar]
- Bizzozero, L.; Cazzato, D.; Cervia, D.; Assi, E.; Simbari, F.; Pagni, F.; De Palma, C.; Monno, A.; Verdelli, C.; Querini, P.R.; et al. Acid sphingomyelinase determines melanoma progression and metastatic behaviour via the microphtalmia-associated transcription factor signalling pathway. Cell Death Differ. 2014, 21, 507–520. [Google Scholar] [CrossRef] [Green Version]
- Perrotta, C.; Buonanno, F.; Zecchini, S.; Giavazzi, A.; Proietti Serafini, F.; Catalani, E.; Guerra, L.; Belardinelli, M.C.; Picchietti, S.; Fausto, A.M.; et al. Climacostol reduces tumour progression in a mouse model of melanoma via the p53-dependent intrinsic apoptotic programme. Sci. Rep. 2016, 6, 7281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrotta, C.; Cervia, D.; Di Renzo, I.; Moscheni, C.; Bassi, M.T.; Campana, L.; Martelli, C.; Catalani, E.; Giovarelli, M.; Zecchini, S.; et al. Nitric oxide generated by tumor-associated macrophages is responsible for cancer resistance to cisplatin and correlated with syntaxin 4 and acid sphingomyelinase inhibition. Front. Immunol. 2018, 9, 1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervia, D.; Assi, E.; De Palma, C.; Giovarelli, M.; Bizzozero, L.; Pambianco, S.; Di Renzo, I.; Zecchini, S.; Moscheni, C.; Vantaggiato, C.; et al. Essential role for acid sphingomyelinase-inhibited autophagy in melanoma response to cisplatin. Oncotarget 2016, 7, 24995–25009. [Google Scholar] [CrossRef] [Green Version]
- Coazzoli, M.; Napoli, A.; Roux-Biejat, P.; De Palma, C.; Moscheni, C.; Catalani, E.; Zecchini, S.; Conte, V.; Giovarelli, M.; Caccia, S.; et al. Acid sphingomyelinase downregulation enhances mitochondrial fusion and promotes oxidative metabolism in a mouse model of melanoma. Cells 2020, 9, 848. [Google Scholar] [CrossRef] [Green Version]
- Roux-Biejat, P.; Coazzoli, M.; Marrazzo, P.; Zecchini, S.; Di Renzo, I.; Prata, C.; Napoli, A.; Moscheni, C.; Giovarelli, M.; Barbalace, M.C.; et al. Acid sphingomyelinase controls early phases of skeletal muscle regeneration by shaping the macrophage phenotype. Cells 2021, 10, 3028. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, C.; Costa, C.; Palmeira, C.; Pinto-Leite, R.; Oliveira, P.; Freitas, R.; Amado, F.; Santos, L.L. What we have learned from urinary bladder cancer models. J. Cancer. Metastasis Treat. 2016, 2, 51–58. [Google Scholar]
- Baumgart, E.; Cohen, M.S.; Silva Neto, B.; Jacobs, M.A.; Wotkowicz, C.; Rieger-Christ, K.M.; Biolo, A.; Zeheb, R.; Loda, M.; Libertino, J.A.; et al. Identification and prognostic significance of an epithelial-mesenchymal transition expression profile in human bladder tumors. Clin. Cancer. Res. 2007, 13, 1685–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onrust, S.V.; Wiseman, L.R.; Goa, K.L. Epirubicin: A review of its intravesical use in superficial bladder cancer. Drugs Aging 1999, 15, 307–333. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, Z.; Chen, Z.; Li, M.; Wu, Z. Clinicopathological and prognostic value of Ki-67 expression in bladder cancer: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158891. [Google Scholar] [CrossRef] [Green Version]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.; Mohamad, A.B.; Al-Amiery, A.A. Properties and Applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra Ch, N.; Priya, R.; Swain, S.; Jena, G.K.; Panigrahi, K.C.; Ghose, D. Pharmaceutical significance of Eudragit: A review. Future J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Rehman, S.; Ranjha, N.M.; Shoukat, H.; Madni, A.; Ahmad, F.; Raza MRJameel, Q.A.; Majeed, A.; Ramzan, N. Fabrication, evaluation, in vivo pharmacokinetic and toxicological analysis of pH-sensitive Eudragit S-100-coated hydrogel beads: A promising strategy for colon targeting. AAPS Pharm. Sci. Tech. 2021, 22, 209. [Google Scholar] [CrossRef] [PubMed]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional profiling of the LPS induced NF-κB response in macrophages. BMC Immunol. 2007, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omata, S.; Sawae, Y.; Murakami, T. Effect of poly (vinyl alcohol) (PVA) wear particles generated in water lubricant on immune response of macrophage. Biosurf. Biotribol. 2015, 1, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Strehl, C.; Gaber, T.; Maurizi, L.; Hahne, M.; Rauch, R.; Hoff, P.; Häupl, T.; Hofmann-Amtenbrink, M.; Poole, A.R.; Hofmann, H.; et al. Effects of PVA coated nanoparticles on human immune cells. Int. J. Nanomed. 2015, 10, 3429–3445. [Google Scholar] [CrossRef] [Green Version]
- Chikaura, H.; Nakashima, Y.; Fujiwara, Y.; Komohara, Y.; Takeya, M.; Nakanishi, Y. Effect of particle size on biological response by human monocyte-derived macrophages. Biosurf. Biotribol. 2016, 2, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Green, T.R.; Fisher, J.; Stone, M.; Wroblewski, B.M.; Ingham, E. Polyethylene particles of a ‘critical size’ are necessary for the induction of cytokines by macrophages in vitro. Biomaterials 1998, 19, 2297–2302. [Google Scholar] [CrossRef]
- Lévi, F.; Okyar, A. Circadian clocks and drug delivery systems: Impact and opportunities in chronotherapeutics. Expert Opin. Drug Deliv. 2011, 8, 1535–1541. [Google Scholar] [CrossRef]
- Maroni, A.; Zema, L.; Curto, M.D.D.; Loreti, G.; Gazzaniga, A. Oral pulsatile delivery: Rationale and chronopharmaceutical formulations. Int. J. Pharm. 2010, 398, 1–8. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Briatico-Vangosa, F.; Moutaharrik, S.; Cerea, M.; Foppoli, A.; Maroni, A.; Palugan, L.; Zema, L.; Gazzaniga, A. The Chronotopic™ system for pulsatile and colonic delivery of active molecules in the era of precision medicine: Feasibility by 3D printing via fused deposition modeling (FDM). Pharmaceutics 2021, 13, 759. [Google Scholar] [CrossRef] [PubMed]










| Parameter | Value |
|---|---|
| Nozzle diameter | 0.5 mm |
| Printing temperature | 200 °C |
| Build plate temperature | 50 °C |
| Extrusion flow | 100% of the maximum flow |
| Printing speed | 23 mm/s |
| Retraction length | 2.00 mm |
| Retraction speed | 20 mm/s |
| Layer height | 0.10 mm |
| Infill | 100% or 50%, |
| Infill geometry | Rectilinear |
| Number of top/bottom | 2 |
| Number of perimeters | 1 |
| Parameter | Value |
|---|---|
| Nozzle diameter | 0.5 mm |
| Printing temperature | 200 °C |
| Build plate temperature | 40 °C |
| Extrusion flow | 100% of the maximum flow |
| Printing speed | 65 mm/s |
| Retraction length | 2.40 mm |
| Retraction speed | 45 mm/s |
| Layer height | 0.20 mm |
| Infill | 75% |
| Infill geometry | Honeycomb |
| Number of top/bottom | 3 |
| Number of perimeters | 2 |
| Gene | Primer Sequences |
|---|---|
| IL-6 | F: 5′-GGCACTGGCAGAAAACAACC-3′ R: 5′-GCAAGTCTCCTCATTGAATCC-3′ |
| IL-1β | F: 5′-TTCGACACATGGGATAACGAGG-3′ R: 5′-TTTTTGCTGTGAGTCCCGGAG-3′ |
| TNF | F: 5′-CCCAGGGACCTCTCTCTAATCA-3′ R: 5′-GCTACAGGCTTGTCACTCGG-3′ |
| GAPDH | F: 5′-TGAGGTCAATGAAGGGGTC-3′ R: 5′-GTGAAGGTCGGAGTCAACG 3′ |
| RPL32 | R: 5′-TTAAGCGTAACTGGCGGAAAC-3′ F: 5′-AAACATTGTGAGCGATCTCGG-3′ |
| Weight, mg (CV) | ||
|---|---|---|
| Manually Filled | Trapdoor-Filled | |
| 100% Infill | 841.85 (9.52) | 838.63 (5.02) |
| 50% Infill | 723.44 (10.11) | 736.11 (5.61) |
| Thickness, μm (CV) | Overall Thickness, μm (CV) | Weight Gain, % (CV) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| 100% Infill | 56.43 (6.36) | 55.37 (7.78) | 55.75 (6.61) | 51.70 (8.72) | 53.27 (9.25) | 55.98 (6.37) | 54.75 (7.64) | 6.73 (6.84) |
| 50% Infill | 54.71 (7.35) | 58.03 (9.06) | 52.30 (6.23) | 57.05 (7.59) | 54.85 (9.63) | 54.54 (9.93) | 55.24 (8.53) | 6.22 (7.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uboldi, M.; Perrotta, C.; Moscheni, C.; Zecchini, S.; Napoli, A.; Castiglioni, C.; Gazzaniga, A.; Melocchi, A.; Zema, L. Insights into the Safety and Versatility of 4D Printed Intravesical Drug Delivery Systems. Pharmaceutics 2023, 15, 757. https://doi.org/10.3390/pharmaceutics15030757
Uboldi M, Perrotta C, Moscheni C, Zecchini S, Napoli A, Castiglioni C, Gazzaniga A, Melocchi A, Zema L. Insights into the Safety and Versatility of 4D Printed Intravesical Drug Delivery Systems. Pharmaceutics. 2023; 15(3):757. https://doi.org/10.3390/pharmaceutics15030757
Chicago/Turabian StyleUboldi, Marco, Cristiana Perrotta, Claudia Moscheni, Silvia Zecchini, Alessandra Napoli, Chiara Castiglioni, Andrea Gazzaniga, Alice Melocchi, and Lucia Zema. 2023. "Insights into the Safety and Versatility of 4D Printed Intravesical Drug Delivery Systems" Pharmaceutics 15, no. 3: 757. https://doi.org/10.3390/pharmaceutics15030757