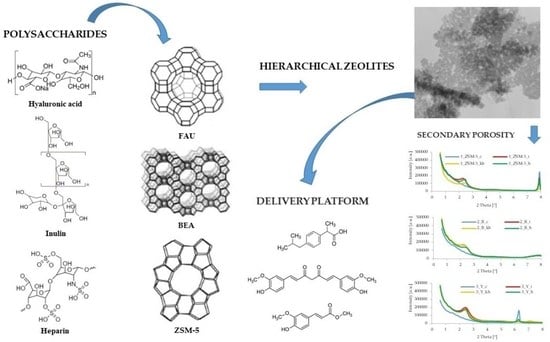
Synthesis and Characterization of Hierarchical Zeolites Modified with Polysaccharides and Its Potential Role as a Platform for Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Hierarchical Zeolites
2.1.1. Synthesis of Unmodified Hierarchical Zeolites Based on Zeolite MFI (ZSM-5), BEA (β) or FAU (Y)
2.1.2. Synthesis of Hierarchical Zeolites Modified with Inulin
2.1.3. Synthesis of Hierarchical Zeolites Modified with Hyaluronic Acid
2.1.4. Synthesis of Hierarchical Zeolites Modified with Heparin
2.2. Designation of Materials Used in the Work
2.3. Characterization of the Obtained Hierarchical Zeolites
- Elemental analysis;
- X-ray diffraction (XRD);
- Low-temperature nitrogen adsorption/desorption measurements;
- Transmission electron microscopy (TEM)
2.3.1. Elemental Analysis
2.3.2. XRD—X-ray Diffraction
2.3.3. Low-Temperature Nitrogen Adsorption/Desorption Measurements
2.3.4. Transmission Electron Microscopy (TEM)
2.4. Active Substances Loading
2.5. Release Profiles of Active Substances
2.6. Leaching Tests
3. Results
3.1. Elemental Analysis
3.2. XRD—X-ray Diffraction
3.3. Nitrogen Adsorption/Desorption Isotherms
- in the first one, there is a linear increase of adsorbed nitrogen while the pressure p/p0 has a low value; this correlates with monolayer adsorption deposited on the pore walls;
- in the second range, there is a rapid increase in adsorbed nitrogen for medium pressures p/p0, which is a capillary condensation effect occurring in the mesopores;
- in the third range, there is a gradual, linear increase in p/p0 in the high-pressure region, which results in nitrogen adsorption on the outer surface of the material, i.e., in the spaces between the pores.
- high specific surface area, which varies from about 400 to 830 [m2/g];
- high porosity, in which the total pore volume is as high as 0.66 [cm3/g];
- homogeneous pore size, where the pore width ranges from 3.0 to 3.5 nm.
3.4. Transmission Electron Microscopy (TEM)
3.5. Active Substances Loading and Release Profiles
- MFI-based hierarchical zeolite: 1_ZSM-5_i; 1_ZSM-5_h; 1_ZSM-5; 1_ZSM-5_kh
- BEA-based hierarchical zeolite: 2_B_h; 2_B_i; 2_B_kh; 2_B
- FAU-based hierarchical zeolite: 3_Y_h; 3_Y; 3_Y_kh; 3_Y_i
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serrano, D.P.; Escola, J.M.; Pizzaro, P. Synthesis strategies in the search for hierarchical zeolites. Chem. Soc. Rev. 2013, 9, 4004–4035. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Machoke, A.G.; Schwieger, W. Catalytic test reactions for the evaluation of hierarchical zeolites. Chem. Soc. Rev. 2016, 45, 3313–3330. [Google Scholar] [CrossRef] [PubMed]
- Feliczak-Guzik, A. Hierarchical zeolites: Synthesis and catalytic properties. Micropor. Mesopor. Mat. 2018, 259, 33–45. [Google Scholar] [CrossRef]
- Li, K.; Valla, J.; Garcıa-Martınez, J. Realizing the commercial potential of hierarchical zeolites: New opportunities in catalytic cracking. ChemCatChem 2014, 6, 46–66. [Google Scholar] [CrossRef]
- García-Martínez, J.; Li, K.; Davis, M.E. Mesoporous Zeolites: Preparation, Characterization and Applications; Wiley-VCH Verlag GmbH & Co., KGaA:: Weinheim, Germany, 2015. [Google Scholar]
- Jia, X.; Khan, W.; Wu, Z.; Choi, J.; Yip, A.C.K. Modern synthesis strategies for hierarchical zeolites: Bottom-up versus top-down strategies. Adv. Powder Technol. 2019, 30, 467–484. [Google Scholar] [CrossRef]
- Yadav, M. Synthesis of inorganic nanomaterials using carbohydrates. In Green Sustainable Process for Chemical and Environmental Engineering and Science, Green Inorganic Synthesis; Inamuddin Boddula, R., Ahamed, M.I., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 109–135. [Google Scholar] [CrossRef]
- Fakhari, A.; Berkland, C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013, 9, 7081–7092. [Google Scholar] [CrossRef]
- Caravaggi, C.; De Giglio, R.; Pritelli, C.; Sommaria, M.; Dalla Noce, S.; Faglia, E.; Mantero, M.; Clerici, G.; Fratino, P.; Dalla Paola, L.; et al. HYAFF 11-based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: A prospective, multicenter, controlled, randomized clinical trial. Diabets Care 2003, 26, 2853–2859. [Google Scholar] [CrossRef]
- Rügheimer, L. Hyaluronan: A matrix component. Proc. AIP Conf. 2008, 1049, 126–132. [Google Scholar] [CrossRef]
- Kablik, J.; Monheit, G.D.; Yu, L.; Chang, G.; Gershkovich, J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol. Surg. 2009, 35, 302–312. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. J. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Chong, B.F.; Blank, L.M.; Mclaughlin, R.; Nielsen, L.K. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005, 66, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Matarasso, S.L. Understanding and using hyaluronic acid. Aesthetic Surg. J. 2004, 24, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Blaauwhoed, J.P. Inulin. In Handbook of Hydrocolloids Woodhead Publishing Series in Food Science, Technology and Nutrition, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 829–848. [Google Scholar]
- Franck, A. Inulin. In Food Polysaccharides and Their Applications, 2nd ed.; Stephen, A.M., Phillips, G.O., Wiliams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 335–352. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; van Loo, J.; Rastall, R.A.; Roberfroid, M. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Panchev, I.; Delchev, N.; Kovacheva, D.; Slavov, A. Jerusalem artichoke flour as food ingredient and as source of fructooligosaccharides and inulin. Eur. Food Res. Technol. 2011, 233, 889–896. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Abdella, S.; Youssef, S.H.; Song, Y.; Garg, S. Inulin and its application in drug delivery. Pharmaceuticals 2021, 14, 855. [Google Scholar] [CrossRef]
- Koch, K.; Andersson, R.; Rydberg, I.; Åman, P. Influence of harvest date on inulin chain length distribution and sugar profile for six chicory (Cichorium intybus L.) cultivars. J. Sci. Food Agric. 1999, 79, 1503–1506. [Google Scholar] [CrossRef]
- Casu, B.; Lindahl, U. Structure and biological interactions of heparin and heparan sulfate. Adv. Carbohydr. Chem. Biochem. 2001, 57, 159–206. [Google Scholar] [CrossRef]
- Shriver, Z.; Capila, I.; Venkataraman, G.; Sasisekharan, R. Heparin and heparan sulfate: Analyzing structure and microheterogeneity. Handb. Exp. Pharmacol. 2012, 207, 159–176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Huang, X. Heparin mimetics as tools for modulation of biology and therapy. In Carbohydrates in Drug Discovery and Development, Synthesis and Application, 1st ed.; Tiwari, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–96. [Google Scholar] [CrossRef]
- Ojeda, M.; Budarin, V.; Shuttleworth, P.S.; Clark, J.H.; Pineda, A.; Balu, A.M.; Romero, A.A.; Luque, R. Simple Preparation of novel metal-containing mesoporous starches. Materials 2013, 6, 1891–1902. [Google Scholar] [CrossRef]
- Ari, B.; Sahiner, N. Biodegradable super porous inulin cryogels as potential drug carrier. Polym. Adv. Technol. 2020, 31, 2863–2873. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.C. Polysaccharide-based nanogels in drug delivery system: Application as a carrier for pharmaceuticals. Mater. Sci. Eng. C. 2016, 68, 964–981. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Opatz, T.; Landfester, K.; Wurm, F.R. Carbohydrate nanocarriers in biomedical applications: Functionalization and construction. Chem. Soc. Rev. 2015, 44, 8301–8325. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Chaudhary, J.P.; Meena, R. Anionic carboxymethylagarose-based pH-responsive smart superabsorbent hydrogels for controlled release of anticancer drugs. Int. J. Biol. Macromol. 2019, 124, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, X.; Yang, Y.; Wang, C.; Zhang, C.; Peng, G. Preparation and evaluation of novel hydrogel based on polysaccharide isolated from Bletilla striata. Pharm. Dev. Technol. 2017, 22, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, Y.; Yang, D.; Gao, X.; Wen, T.; Fu, J.; Wen, X.; Quan, G.; Pan, X.; Wu, C. Intelligent and spatiotemporal release drug based on multifunctional nanoparticle-integrated dissolving microneedle system for synergetic chemo-photothermal therapy to eradicate melanoma. Acta Biomater. 2021, 135, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Almurisi, S.H.; Al-Japairai, K.; Hilles, A.R.; Alelwani, W.; Bannunah, A.M.; Alshammari, F.; Alheibshy, F. Ibuprofen-loaded chitosan-lipid nanoconjugate hydrogel with gum arabic: Green synthesis, characterization, in vitro kinetics mechanistic release study and PGE2 production test. Gels 2021, 7, 254. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Mcoval, S.; Binkoski, E.; Hilpert, K.; Griel, A.E.; Etherton, T. Bioactive compounds in food: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71S–88S. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Nagahama, K.; Utsumi, T.; Kumano, T.; Maekawa, S.; Oyama, N.; Kawakami, J. Discovery of a new function of curcumin which enhances its anticancer therapeutic potency. Sci. Rep. 2016, 60, 30962–30976. [Google Scholar] [CrossRef]
- Park, H.J.; Cho, J.H.; Hong, S.H.; Kim, D.H.; Jung, H.Y.; Kang, I.K.; Cho, Y.J. Whitening and anti-wrinkle activities of ferulic acid isolated from Tetragonia tetragonioides in B16F10 melanoma and CCD-986sk fibroblast cells. J. Nat. Med. 2018, 72, 127–135. [Google Scholar] [CrossRef]
- Nile, S.H.; Ko, E.Y.; Kim, D.H.; Keum, Y.S. Screening of ferulic acid related compounds as inhibitors of xanthine oxidase and cyclooxygenase-2 with anti-inflammatory activity. Rev. Bras. Farmacogn. 2016, 26, 50–55. [Google Scholar] [CrossRef]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M. Ordered mesoporous materials in the context of drug delivery systems and bone tissue engineering. Chem. Eur. J. 2006, 12, 5934–5943. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial carbon-based nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef]
- Englert, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; Schubert, U.S. Pharmapolymers in the 21st century: Synthetic polymers in drug delivery applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar] [CrossRef]
- Rámila, A.; Muňoz, B.; Pérez-Pariente, J.; Vallet-Regí, M. Mesoporous MCM-41 as drug host system. J. Sol-Gel Sci. Technol. 2003, 26, 1199–1202. [Google Scholar] [CrossRef]
- Serati-Nouri, H.; Jafari, A.; Roshangar, L.; Dadashpour, M.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Biomedical applications of zeolite-based materials: A review. Mater. Sci. Eng. C 2020, 116, 111225. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A.; Sprynskyy, M.; Nowak, I.; Jaroniec, M.; Buszewski, B. Synthesis and physicochemical properties of hierarchical zeolites containing ruthenium oxide nanoparticles and their application in the reaction of dihydroxyacetone isomerization. J. Colloid Interface Sci. 2018, 516, 379–383. [Google Scholar] [CrossRef]
- Han, H.K. The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert Opin. Drug Metab. Toxicol. 2011, 7, 721–729. [Google Scholar] [CrossRef]
- Ramezani, H.; Naser Azizi, S.; Cravotto, G. Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method. Green Process Synth. 2019, 8, 730–741. [Google Scholar] [CrossRef]
- Qin, Z.; Lakiss, L.; Tosheva, L.; Gilson, J.-P.; Vicente, A.; Fernandez, C.; Valtchev, V. Comparative study of nano-ZSM-5 catalysts synthesized in OH− and F− media. Adv. Funct. Mater. 2014, 24, 257–264. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report Pure). Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Prouzet, E.; Pinnavaia, T.J. Assembly of mesoporous molecular sieves containing wormhole motifs by a nonionic surfactant pathway: Control of pore size by synthesis temperature. Angew. Chem. Int. Ed. 1997, 36, 516–518. [Google Scholar] [CrossRef]
- Nowak, I.; Kilos, B.; Ziolek, M.; Lewandowska, A. Epoxidation of cyclohexene on Nb-containing meso- and macroporous materials. Catal. Today 2003, 78, 487–498. [Google Scholar] [CrossRef]
- Schmid, M.H.; Korting, H.C. The concept of the acid mantle of the skin: Its relevance for the choice of skin cleansers. Dermatology 1995, 191, 276–280. [Google Scholar] [CrossRef]
- Sinha, V.R.; Kaur, M.P. Permeation enhancers for transdermal drug delivery. Drug Dev. Ind. Pharm. 2000, 26, 1131–1140. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Pathan, I.B.; Setty, C.M. Chemical penetration enhancers for transdermal drug delivery systems. Trop. J. Pharm. Res. 2009, 8, 173–179. [Google Scholar] [CrossRef]
- Jadach, B.; Feliczak-Guzik, A.; Nowak, I.; Milanowski, B.; Piotrowska-Kempisty, H.; Murias, M.; Lulek, J. Modifying release of poorly soluble active pharmaceutical ingredients with the amine functionalized SBA-16 type mesoporous materials. J. Biomater. Appl. 2019, 33, 1214–1231. [Google Scholar] [CrossRef]
- Goscianska, J.; Olejnik, A.; Nowak, I.; Marciniak, M.; Pietrzak, R. Ordered mesoporous silica modified with lanthanum for ibuprofen loading and release behavior. Eur. J. Pharm. Biopharm. 2015, 94, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Mellaerts, R.; Jammaer, J.A.G.; Van Speybroeck, M.; Chen, H.; Van Humbeeck, J.; Augustijns, P.; Van den Mooter, G.; Martens, J.A. Physical state of poorly water soluble therapeutic molecules loaded into SBA-15 ordered mesoporous silica carriers: A case study with Itraconazole and Ibuprofen. Langmuir 2008, 24, 8651–8659. [Google Scholar] [CrossRef] [PubMed]
- Ainhoa, R.; Vallet-Regi, M. Solid state NMR characterisation of encapsulated molecules in mesoporous silica. JSST 2004, 31, 219–223. [Google Scholar] [CrossRef]
- Chen, Z.; Xia, Y.; Liao, S.; Huang, Y.; Li, Y.; He, Y.; Tong, Z.; Li, B. Thermal degradation kinetics study of curcumin with nonlinear methods. Food Chem. 2014, 155, 81–86. [Google Scholar] [CrossRef] [PubMed]







| X_M | ||
|---|---|---|
| X—STRUCTURE | M—MODIFICATION | DESCRIPTION |
| MFI | Commercial zeolite | 1_ZSM-5_c |
| MFI | Unmodified hierarchical zeolite | 1_ZSM-5 |
| MFI | Inulin modified hierarchical zeolite | 1_ZSM-5_i |
| MFI | Hyaluronic acid modified hierarchical zeolite | 1_ZSM-5_kh |
| MFI | Heparin modified hierarchical zeolite | 1_ZSM-5_h |
| BEA | Commercial zeolite | 2_B_c |
| BEA | Unmodified hierarchical zeolite | 2_B |
| BEA | Inulin modified hierarchical zeolite | 2_B_i |
| BEA | Hyaluronic acid modified hierarchical zeolite | 2_B_kh |
| BEA | Heparin modified hierarchical zeolite | 2_B_h |
| FAU | Commercial zeolite | 3_Y_c |
| FAU | Unmodified hierarchical zeolite | 3_Y |
| FAU | Inulin modified hierarchical zeolite | 3_Y_i |
| FAU | Hyaluronic acid modified hierarchical zeolite | 3_Y_kh |
| FAU | Heparin modified hierarchical zeolite | 3_Y_h |
| Material | %N | %C | %H | %S |
|---|---|---|---|---|
| 1_ZSM-5_c | 0.123 | 0.742 | 0.349 | 0.073 |
| 1_ZSM-5 | 0.041 | 0.023 | 0.908 | 0.004 |
| 1_ZSM-5_i | 0.120 | 3.095 | 4.088 | 0.425 |
| 1_ZSM-5_i (leaching) | 0.117 | 3.086 | 4.075 | 0.418 |
| 1_ZSM-5_kh | 0.088 | 3.772 | 1.125 | 0.335 |
| 1_ZSM-5_kh (leaching) | 0.086 | 3.705 | 1.105 | 0.320 |
| 1_ZSM-5_h | 0.207 | 4.633 | 1.348 | 0.494 |
| 1_ZSM-5_h (leaching) | 0.205 | 4.601 | 1.302 | 0.487 |
| 2_B_c | 0.095 | 0.080 | 1.333 | 0.045 |
| 2_B | 0.014 | 0.059 | 0.655 | 0.261 |
| 2_B_i | 0.278 | 6.589 | 2.124 | 0.368 |
| 2_B_i (leaching) | 0.208 | 6.498 | 2.012 | 0.302 |
| 2_B_kh | 0.271 | 6.781 | 2.176 | 0.340 |
| 2_B_kh (leaching) | 0.206 | 6.691 | 2.087 | 0.328 |
| 2_B_h | 0.341 | 7.535 | 2.298 | 0.140 |
| 2_B_h (leaching) | 0.332 | 7.451 | 2.176 | 0.132 |
| 3_Y_c | 0.011 | 0.024 | 2.836 | 0.273 |
| 3_Y | 0.014 | 0.011 | 2.582 | 0.303 |
| 3_Y_i | 0.018 | 2.652 | 2.709 | 0.140 |
| 3_Y_i (leaching) | 0.017 | 2.543 | 2.602 | 0.131 |
| 3_Y_kh | 0.022 | 3.066 | 2.957 | 0.027 |
| 3_Y_kh (leaching) | 0.018 | 2.990 | 2.876 | 0.022 |
| 3_Y_h | 0.144 | 3.280 | 3.526 | 0.108 |
| 3_Y_h (leaching) | 0.140 | 3.214 | 3.455 | 0.104 |
| Material | Specific Surface Area, BET (m2/g) | Pore Volume, (cm3/g) | Mesopore Size, (nm) | ||
|---|---|---|---|---|---|
| Total Pore Volume | Micropores Volume | Mesoporous Volume | |||
| 1_ZSM-5_c | 347 | 0.26 | 0.13 | 0.13 | 3.0 |
| 1_ZSM-5 | 829 | 0.57 | 0.23 | 0.34 | 2.7 |
| 1_ZSM-5_i | 422 | 0.56 | 0.16 | 0.40 | 3.1 |
| 1_ZSM-5_kh | 405 | 0.35 | 0.15 | 0.20 | 3.5 |
| 1_ZSM-5_h | 445 | 0.40 | 0.13 | 0.27 | 3.3 |
| 2_B_c | 533 | 0.29 | 0.25 | 0.04 | - |
| 2_B | 704 | 0.42 | 0.18 | 0.24 | 3.2 |
| 2_B_i | 440 | 0.54 | 0.12 | 0.42 | 3.1 |
| 2_B_kh | 529 | 0.36 | 0.17 | 0.19 | 3.1 |
| 2_B_h | 611 | 0.46 | 0.17 | 0.29 | 3.0 |
| 3_Y_c | 718 | 0.37 | 0.34 | 0.03 | - |
| 3_Y | 792 | 0.49 | 0.19 | 0.30 | 3.4 |
| 3_Y_i | 610 | 0.66 | 0.22 | 0.44 | 3.1 |
| 3_Y_kh | 604 | 0.47 | 0.23 | 0.24 | 3.0 |
| 3_Y_h | 641 | 0.61 | 0.18 | 0.43 | 3.3 |
| Materials | % LOAD | ||
|---|---|---|---|
IBUPROFEN | CURCUMIN | FERULIC ACID | |
| 1_ZSM-5 | 49 | 39 | 70 |
| 1_ZSM-5_i | 68 | 39 | 49 |
| 1_ZSM-5_kh | 50 | 39 | 54 |
| 1_ZSM-5_h | 43 | 39 | 44 |
| 2_B | 54 | 38 | 64 |
| 2_B_i | 46 | 40 | 58 |
| 2_B_kh | 49 | 40 | 47 |
| 2_B_h | 53 | 39 | 44 |
| 3_Y | 54 | 44 | 62 |
| 3_Y_i | 43 | 41 | 48 |
| 3_Y_kh | 79 | 42 | 50 |
| 3_Y_h | 53 | 40 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyńczak, A.; Nowak, I.; Woźniak, N.; Chudzińska, J.; Feliczak-Guzik, A. Synthesis and Characterization of Hierarchical Zeolites Modified with Polysaccharides and Its Potential Role as a Platform for Drug Delivery. Pharmaceutics 2023, 15, 535. https://doi.org/10.3390/pharmaceutics15020535
Wawrzyńczak A, Nowak I, Woźniak N, Chudzińska J, Feliczak-Guzik A. Synthesis and Characterization of Hierarchical Zeolites Modified with Polysaccharides and Its Potential Role as a Platform for Drug Delivery. Pharmaceutics. 2023; 15(2):535. https://doi.org/10.3390/pharmaceutics15020535
Chicago/Turabian StyleWawrzyńczak, Agata, Izabela Nowak, Natalia Woźniak, Jagoda Chudzińska, and Agnieszka Feliczak-Guzik. 2023. "Synthesis and Characterization of Hierarchical Zeolites Modified with Polysaccharides and Its Potential Role as a Platform for Drug Delivery" Pharmaceutics 15, no. 2: 535. https://doi.org/10.3390/pharmaceutics15020535