Development of a Novel In Vitro Model to Study Lymphatic Uptake of Drugs via Artificial Chylomicrons
Abstract
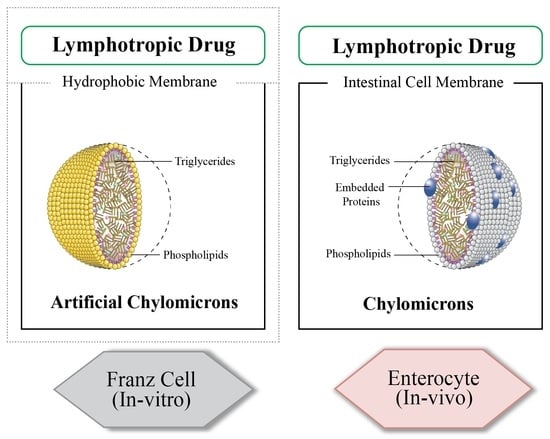
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Franz Cell for Investigating Intestinal Lymphatic Uptake
2.2.2. Entrapment Efficiency
2.2.3. Characterization of Intralipid®
Measurement of Size of Intralipid®
Morphological Characterization of Intralipid® via Transmission Electron Microscopy (TEM)
2.3. Statistical Analysis
3. Results and Discussion
3.1. Lymphatic Uptake via the In Vitro Model
3.2. Inhibition of the Lymphatic Uptake in the In Vitro Model
3.3. Enhancement of the Lymphatic Uptake in the In Vitro Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yáñez, J.A.; Wang, S.W.; Knemeyer, I.W.; Wirth, M.A.; Alton, K.B. Intestinal Lymphatic Transport for Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 923–942. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, N.L.; Charman, W.N.; Porter, C.J. Lipid-based Delivery Systems and Intestinal Lymphatic Drug Transport: A Mechanistic Update. Adv. Drug Deliv. Rev. 2008, 60, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Cifarelli, V.; Eichmann, A. The Intestinal Lymphatic System: Functions and Metabolic Implications. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 503–513. [Google Scholar] [CrossRef]
- Charman, W.N.; Stella, V.J. Estimating the Maximal Potential for Intestinal Lymphatic Transport of Lipophilic Drug Molecules. Int. J. Pharm. 1986, 34, 175–178. [Google Scholar] [CrossRef]
- Yousef, M.; Silva, D.; Chacra, N.B.; Davies, N.M.; Löbenberg, R. The Lymphatic System: A Sometimes-Forgotten Compartment in Pharmaceutical Sciences. J. Pharm. Pharm. Sci. 2021, 24, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, Y.; Qi, J.; Wu, W. An Update on Oral Drug Delivery via Intestinal Lymphatic Transport. Acta Pharm. Sin. B 2021, 11, 2449–2468. [Google Scholar] [CrossRef]
- Punjabi, M.S.; Naha, A.; Shetty, D.; Nayak, U.Y. Lymphatic Drug Transport and Associated Drug Delivery Technologies: A Comprehensive Review. Curr. Pharm. Des. 2021, 27, 1992–1998. [Google Scholar] [CrossRef]
- Khan, A.A.; Mudassir, J.; Mohtar, N.; Darwis, Y. Advanced Drug Delivery to the Lymphatic System: Lipid-Based Nanoformulations. Int. J. Nanomed. 2013, 8, 2733–2744. [Google Scholar] [CrossRef]
- Trevaskis, N.L.; Kaminskas, L.M.; Porter, C.J. From Sewer to Saviour-Targeting the Lymphatic System to Promote Drug Exposure and Activity. Nat. Rev. Drug Discov. 2015, 14, 781–803. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Garg, A.; Verma, A. Nano Lipid Based Carriers for Lymphatic Voyage of Anti-Cancer Drugs: An Insight into the In-Vitro, Ex-Vivo, In-Situ and In-Vivo Study Models. J. Drug Deliv. Sci. Technol. 2020, 59, 101899. [Google Scholar] [CrossRef]
- Yousef, M.; Park, C.; Le, T.S.; Chacra, N.B.; Davies, N.M.; Löbenberg, R. Simulated Lymphatic Fluid for In-Vitro Assessment in Pharmaceutical Development. Dissolution Technol. 2022, 29, 86–93. [Google Scholar] [CrossRef]
- Dahan, A.; Hoffman, A. Evaluation of a Chylomicron Flow Blocking Approach to Investigate the Intestinal Lymphatic Transport of Lipophilic Drugs. Eur. J. Pharm. Sci. 2005, 24, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Chalikwar, S.S.; Belgamwar, V.S.; Talele, V.R.; Surana, S.J.; Patil, M.U. Formulation and Evaluation of Nimodipine-Loaded Solid Lipid Nanoparticles Delivered Via Lymphatic Transport System. Colloids Surf. B Biointerfaces 2012, 97, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Charman, W.N.; Porter, C.J. Lipophilic Prodrugs Designed for Intestinal Lymphatic Transport. Adv. Drug Deliv. Rev. 1996, 19, 149–169. [Google Scholar] [CrossRef]
- Shackleford, D.M.; Faassen, W.F.; Houwing, N.; Lass, H.; Edwards, G.A.; Porter, C.J.; Charman, W.N. Contribution of Lymphatically Transported Testosterone Undecanoate to the Systemic Exposure of Testosterone after Oral Administration of Two Andriol Formulations in Conscious Lymph Duct-Cannulated Dogs. J. Pharmacol. Exp. Ther. 2003, 306, 925–933. [Google Scholar] [CrossRef]
- Muchow, M.; Maincent, P.; Müller, R.H.; Keck, C.M. Testosterone Undecanoate–Increase of Oral Bioavailability by Nanostructured Lipid Carriers (NLC). J. Adv. Pharm. Technol. Res. 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Noguchi, T.; Charman, W.N.; Stella, V.J. Lymphatic Appearance of DDT in Thoracic or Mesenteric Lymph Duct Cannulated Rats. Int. J. Pharm. 1985, 24, 185–192. [Google Scholar] [CrossRef]
- Trevaskis, N.L.; Hu, L.; Caliph, S.M.; Han, S.; Porter, C.J. The Mesenteric Lymph Duct Cannulated Rat Model: Application to the Assessment of Intestinal Lymphatic Drug Transport. J. Vis. Exp. 2015, 97, e52389. [Google Scholar] [CrossRef]
- Redgrave, T.G. Inhibition of Protein Synthesis and Absorption of Lipid into Thoracic Duct Lymph of Rats. Proc. Soc. Exp. Biol. Med. 1969, 130, 776–780. [Google Scholar] [CrossRef]
- Elz, A.S.; Trevaskis, N.L.; Porter, C.J.; Bowen, J.M.; Prestidge, C.A. Smart Design Approaches for Orally Administered Lipophilic Prodrugs to Promote Lymphatic Transport. J. Control. Release 2022, 341, 676–701. [Google Scholar] [CrossRef]
- Gibb, M.; Pradhan, S.H.; Mulenos, M.R.; Lujan, H.; Liu, J.; Ede, J.D.; Shatkin, J.A.; Sayes, C.M. Characterization of a Human In Vitro Intestinal Model for the Hazard Assessment of Nanomaterials Used in Cancer Immunotherapy. Appl. Sci. 2021, 11, 2113. [Google Scholar] [CrossRef]
- Dahan, A.; Hoffman, A. Rationalizing the Selection of Oral Lipid Based Drug Delivery Systems by an In-Vitro Dynamic Lipolysis Model for Improved Oral Bioavailability of Poorly Water Soluble Drugs. J. Control. Release 2008, 129, 1–10. [Google Scholar] [CrossRef]
- Gershkovich, P.; Hoffman, A. Uptake of Lipophilic Drugs by Plasma Derived Isolated Chylomicrons: Linear Correlation with Intestinal Lymphatic Bioavailability. Eur. J. Pharm. Pharm. Sci. 2005, 26, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Qiu, Y.; Qi, J.; Feng, M.; Ju, D.; Wu, W. Biomimetic Reassembled Chylomicrons as Novel Association Model for the Prediction of Lymphatic Transportation of Highly Lipophilic Drugs via the Oral Route. Int. J. Pharm. 2015, 483, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Lepore, M.; Delfino, I. Intralipid-Based Phantoms for the Development of New Optical Diagnostic Techniques. Open Biotechnol. J. 2019, 13, 163–172. [Google Scholar] [CrossRef]
- Khoo, S.M.; Edwards, G.A.; Porter, C.J.; Charman, W.N. A Conscious Dog Model for Assessing the Absorption, Enterocyte-Based Metabolism, and Intestinal Lymphatic Transport of Halofantrine. J. Pharm. Sci. 2001, 90, 1599–1607. [Google Scholar] [CrossRef]
- Khoo, S.M.; Shackleford, D.M.; Porter, C.J.; Edwards, G.A.; Charman, W.N. Intestinal Lymphatic Transport of Halofantrine Occurs After Oral Administration of a Unit-Dose Lipid-Based Formulation to Fasted Dogs. Pharm. Res. 2003, 20, 1460–1465. [Google Scholar] [CrossRef]
- Caliph, S.M.; Charman, W.N.; Porter, C.J. Effect of Short-, Medium-, and Long-Chain Fatty Acid-Based Vehicles on the Absolute Oral Bioavailability and Intestinal Lymphatic Transport of Halofantrine and Assessment of Mass Balance in Lymph-Cannulated and Non-Cannulated Rats. J. Pharm. Sci. 2000, 89, 1073–1084. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Lee, J. Liposomal Formulations for Enhanced Lymphatic Drug Delivery. Asian J. Pharm. Sci. 2013, 8, 96–103. [Google Scholar] [CrossRef]
- Murota, K.; Cermak, R.; Terao, J.; Wolffram, S. Influence of Fatty Acid Patterns on the Intestinal Absorption Pathway of Quercetin in Thoracic Lymph Duct-Cannulated Rats. Br. J. Nutr. 2013, 109, 2147–2153. [Google Scholar] [CrossRef]
- Jain, C.P.; Vyas, S.P.; Dixit, V.K. Niosomal System for Delivery of Rifampicin to Lymphatics. Indian J. Pharm. Sci. 2006, 68, 575–578. [Google Scholar] [CrossRef]
- Olivas-Aguirre, M.; Torres-López, L.; Pottosin, I.; Dobrovinskaya, O. Phenolic Compounds Cannabidiol, Curcumin and Quercetin Cause Mitochondrial Dysfunction and Suppress Acute Lymphoblastic Leukemia Cells. Int. J. Mol. Sci. 2020, 22, 204. [Google Scholar] [CrossRef] [PubMed]
- Jewell, A.; Brookes, A.; Feng, W.; Ashford, M.; Gellert, P.; Butler, J.; Fischer, P.M.; Scurr, D.J.; Stocks, M.J.; Gershkovich, P. Distribution of a Highly Lipophilic Drug Cannabidiol into Different Lymph Nodes Following Oral Administration in Lipidic Vehicle. Eur. J. Pharm. Biopharm. 2022, 174, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Holm, R.; Porter, C.J.; Müllertz, A.; Kristensen, H.G.; Charman, W.N. Structured Triglyceride Vehicles for Oral Delivery of Halofantrine: Examination of Intestinal Lymphatic Transport and Bioavailability in Conscious Rats. Pharm. Res. 2002, 19, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, S. Viral, Fungal, Protozoal and Helminthic Infections. In Clinical Pharmacology, 11th ed.; Bennett, P.N., Sharma, P., Brown, M.J., Eds.; Churchill Livingstone (Elsiever): London, UK, 2012; pp. 213–239. [Google Scholar] [CrossRef]
- Chen, I.L.; Tsai, Y.J.; Huang, C.M.; Tsai, T.H. Lymphatic Absorption of Quercetin and Rutin in Rat and Their Pharmacokinetics in Systemic Plasma. J. Agric. Food Chem. 2010, 58, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Lesser, S.; Cermak, R.; Wolffram, S. Bioavailability of Quercetin in Pigs is Influenced by the Dietary Fat Content. J. Nutr. 2004, 134, 1508–1511. [Google Scholar] [CrossRef] [PubMed]
- Zgair, A.; Wong, J.C.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; Barrett, D.A.; Constantinescu, C.S.; Fischer, P.M.; et al. Dietary Fats and Pharmaceutical Lipid Excipients Increase Systemic Exposure to Orally Administered Cannabis and Cannabis-Based Medicines. Am. J. Transl. Res. 2016, 8, 3448. [Google Scholar]
- Franco, V.; Gershkovich, P.; Perucca, E.; Bialer, M. The Interplay Between Liver First-Pass Effect and Lymphatic Absorption of Cannabidiol and Its Implications for Cannabidiol Oral Formulations. Clin. Pharmacokinet. 2020, 59, 1493–1500. [Google Scholar] [CrossRef]
- Zgair, A.; Lee, J.B.; Wong, J.; Taha, D.A.; Aram, J.; Di Virgilio, D.; McArthur, J.W.; Cheng, Y.K.; Hennig, I.M.; Barrett, D.A.; et al. Oral Administration of Cannabis with Lipids Leads to High Levels of Cannabinoids in the Intestinal Lymphatic System and Prominent Immunomodulation. Sci. Rep. 2017, 7, 14542. [Google Scholar] [CrossRef]
- Abu-Sawwa, R.; Scutt, B.; Park, Y. Emerging Use of Epidiolex (Cannabidiol) in Epilepsy. J. Pediatr. Pharmacol. Ther. 2020, 25, 485–499. [Google Scholar] [CrossRef]
- Singh, S.; Mariappan, T.T.; Shankar, R.; Sarda, N.; Singh, B. A critical Review of the Probable Reasons for the Poor Variable Bioavailability of Rifampicin from Anti-Tubercular Fixed-Dose Combination (FDC) Products, and the Likely Solutions to the Problem. Int. J. Pharm. 2001, 228, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Carcaboso, Á.M.; Glisoni, R.J.; Moretton, M.A.; Chiappetta, D.A. New Old Challenges in Tuberculosis: Potentially Effective Nanotechnologies in Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, N.V.; Khatri, H.N.; Patel, M.M. Formulation, Optimization, and Characterization of Rifampicin-Loaded Solid Lipid Nanoparticles for the Treatment of Tuberculosis. Drug Dev. Ind. Pharm. 2018, 44, 1975–1989. [Google Scholar] [CrossRef] [PubMed]
- Fatma, S.; Yakubov, R.; Anwar, K.; Hussain, M.M. Pluronic L81 Enhances Triacylglycerol Accumulation in the Cytosol and Inhibits Chylomicron Secretion. J. Lipid Res. 2006, 47, 2422–2432. [Google Scholar] [CrossRef] [PubMed]
- Glatzle, J.; Kalogeris, T.J.; Zittel, T.T.; Guerrini, S.; Tso, P.; Raybould, H.E. Chylomicron Components Mediate Intestinal Lipid-Induced Inhibition of Gastric Motor Function. Am. J. Physiol.-Gastrointest Liver Physiol. 2002, 282, G86–G91. [Google Scholar] [CrossRef]
- Tso, P.A.; Gollamudi, S.R. Pluronic L-81: A Potent Inhibitor of the Transport of Intestinal Chylomicrons. Am. J. Physiol.-Gastrointest Liver Physiol. 1984, 247, G32–G36. [Google Scholar] [CrossRef]
- Morita, S.Y.; Kawabe, M.; Nakano, M.; Handa, T. Pluronic L81 Affects the Lipid Particle Sizes and Apolipoprotein B Conformation. Chem. Phys. Lipids 2003, 126, 39–48. [Google Scholar] [CrossRef]
- Managuli, R.S.; Raut, S.Y.; Reddy, M.S.; Mutalik, S. Targeting the Intestinal Lymphatic System: A Versatile Path for Enhanced Oral Bioavailability of Drugs. Expert Opin. Drug Deliv. 2018, 15, 787–804. [Google Scholar] [CrossRef]
- Winstanley, P.A.; Orme, M.L. The Effects of Food on Drug Bioavailability. Br. J. Clin. Pharmacol. 1989, 28, 621. [Google Scholar] [CrossRef]
- Karmen, A.; Whyte, M.; Goodman, D.S. Fatty Acid Esterification and Chylomicron Formation During Fat Absorption: 1. Triglycerides and Cholesterol Esters. J. Lipid Res. 1963, 4, 312–321. [Google Scholar] [CrossRef]
- Gershkovich, P.; Qadri, B.; Yacovan, A.; Amselem, S.; Hoffman, A. Different Impacts of Intestinal Lymphatic Transport on the Oral Bioavailability of Structurally Similar Synthetic Lipophilic Cannabinoids: Dexanabinol and PRS-211,220. Eur. J. Pharm. Sci. 2007, 31, 298–305. [Google Scholar] [CrossRef]
- Porter, C.J.; Charman, W.N. Intestinal Lymphatic Drug Transport: An Update. Adv. Drug Deliv. Rev. 2001, 50, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kojima, H.; Sako, K.; Kondo, H. Drug Delivery to the Intestinal Lymph by Oral Formulations. Pharm. Dev. Technol. 2022, 27, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Shad, M.A.; Pervez, H.; Zafar, Z.I.; Nawaz, H.; Khan, H. Physicochemical Properties, Fatty Acid Profile and Antioxidant Activity of Peanut Oil. Pak. J. Bot. 2012, 44, 435–440. [Google Scholar]
- Clemente, T.E.; Cahoon, E.B. Soybean Oil: Genetic Approaches for Modification of Functionality and Total Content. Plant Physiol. 2009, 151, 1030–1040. [Google Scholar] [CrossRef]









| Model Drug | Mobile Phase | Flow Rate (mL/min) | Detection Wavelength (nm) |
|---|---|---|---|
| Rifampicin | Methanol, Acetate Buffer (pH = 5.8) (60:40) | 1.2 | 254 |
| Quercetin | Methanol, Acetate Buffer (pH = 5.8) (60:40) | 1.2 | 257, 370 |
| Cannabidiol | Acetonitrile, Phosphoric acid (0.2%) (72:28) | 1 | 210, 224 |
| Halofantrine | Methanol, Phosphate Buffer (pH = 7.5) (80:20) | 1 | 210, 259 |
| Drug | MW | HBA | PSA | LogP | MP (°C) | Density (g/cm3) | pKa | HBD | Structure |
|---|---|---|---|---|---|---|---|---|---|
| Rifampicin | 822.9 | 15 | 220.15 * | 4.9 | 183 | 1.178 ** | 1.7 7.9 | 6 |  |
| Cannabidiol | 314.469 * | 2 * | 40.46 * | 6.3 * | 66–67 # | 1.04 # | 9.13 * | 2 * |  |
| Quercetin | 302.23 | 7 | 127.45 * | 1.48 | 316–318 | 1.8 ## | 7.17 8.26 10.13 12.30 13.11 | 5 |  |
| Halofantrine | 500.4 | 5 | 23.5 * | 8.9 | 93–96 and 203–204 (for the hydrochloride salt) ** | 1.2 *** | 10.05 * 14.47 | 1 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousef, M.; Park, C.; Henostroza, M.; Bou Chacra, N.; Davies, N.M.; Löbenberg, R. Development of a Novel In Vitro Model to Study Lymphatic Uptake of Drugs via Artificial Chylomicrons. Pharmaceutics 2023, 15, 2532. https://doi.org/10.3390/pharmaceutics15112532
Yousef M, Park C, Henostroza M, Bou Chacra N, Davies NM, Löbenberg R. Development of a Novel In Vitro Model to Study Lymphatic Uptake of Drugs via Artificial Chylomicrons. Pharmaceutics. 2023; 15(11):2532. https://doi.org/10.3390/pharmaceutics15112532
Chicago/Turabian StyleYousef, Malaz, Chulhun Park, Mirla Henostroza, Nadia Bou Chacra, Neal M. Davies, and Raimar Löbenberg. 2023. "Development of a Novel In Vitro Model to Study Lymphatic Uptake of Drugs via Artificial Chylomicrons" Pharmaceutics 15, no. 11: 2532. https://doi.org/10.3390/pharmaceutics15112532