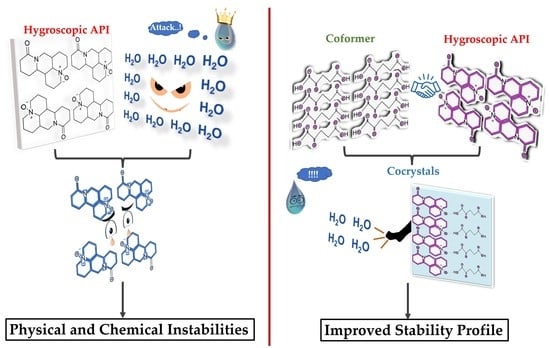
Co-Crystallization Approach to Enhance the Stability of Moisture-Sensitive Drugs
Abstract
:1. Introduction
2. Moisture Interaction with Solid
2.1. Interaction Mechanism
2.2. Hygroscopicity of Hydrochloride Salts: Case of Berberine Chloride, Palmatine Chloride, and Adiphenine Hydrochloride
3. Impact of Moisture-Sensitive Materials on a Process and the Final Product
3.1. Chemical Instability
3.2. Physical Instability
3.2.1. Flow Property
3.2.2. Compaction
3.2.3. Tablet Hardness/Friability
3.2.4. Dissolution
Excipient Form Change
Gelatin Capsule Shell Changes
Polymorphic Transformations of API
- Hydrate/Anhydrate: Water molecules may be present in APIs, either in a bound or unbound form. Generally, the water of hydration in crystals is reversible at a particular temperature and RH [4]. It is generally known that anhydrous forms and hydrates of the same API exhibit different dissolution profiles. Hence, any changes in the API’s hydration state during storage results in product quality failure [62].
- Polymorph conversion: Hydrate and anhydrous form interconversion is often reversible, but polymorphic form interconversions are usually irreversible. The primary concern for polymorph conversions is whether the polymorphic API form is the thermodynamically stable form during the whole storage temperature and humidity range [63]. Moisture can also induce polymorphic changes in some compounds, such as theophylline, and cause major batch inconsistencies [34].
- Amorphous to crystalline: Amorphous and metastable forms generally have high volumes, high surface free energy, and weak intermolecular interaction. Hence, solvent association in such forms of solids is comparatively more than that of crystalline forms due to the easy breaking down of weaker intramolecular bonding by water. This is followed by the supersaturation of drugs, nucleation, and crystal growth of drugs. Hence, the amorphous form generally adsorbs significantly more moisture and converts into a crystalline form, leading to alterations in the dissolution profile [4,64].
4. Manufacturing Process-Related Issues of Moisture-Sensitive Drugs
5. Co-Crystals Approach to Improve Hygroscopic Stability
5.1. Molecular Orientation and Aromatic Interactions
5.2. Correlation with Lattice Energy and Its Measurement
5.3. Hygroscopic API and Hydrophobic Cofomer
5.4. Recent Works
| Drug | Coformer | Synthon | Observation |
|---|---|---|---|
| Indomethacin | Saccharin | The acid and imide dimers of API and co-former are interconnected by an N–H-O hydrogen bond | Moisture uptake by co-crystal was almost negligible (<0.05%) at 98% RH than the stable indomethacin [97] |
| Theophylline | Flufenamic acid | API and co-former connected by an acid-imide heterosynthon involving O−H···O and N−H···O hydrogen bonding. The C−H···F interactions lead to the generation of ladder networks. | Hygroscopicity of theophylline was decreased by co-crystal formation [91] |
| S -oxiracetam | Gallic acid (GA) | Tetramer formation is caused by the intermolecular hydrogen bonding between O−H···O (at 4 places) and N−H···O (at 2 places). Construction of R22(9) synthon is by amide and two hydroxy groups of phenol. | Significant improvement in the hygroscopic stability of S-oxiracetam-GA co-crystal at 98 % RH [98] |
| Caffeine | Oxalic acid | Caffeine and oxlic acid connected through a heteromeric synthon of O-H-N C-H-O hydrogen bonding | The co-crystal of caffeine with oxalic acid was completely stable at 98% RH for several weeks compared to pure caffeine [92] |
| Temozolomide (TMZ) |
| The carboxamide group of TMZ and carboxylic acid of the co-former is connected through hydrogen bonding via amide–acid hetero synthon | Both the co-crystals of TMZ show longer half-lives (2 times greater) compared to the TMZ alone [95] |
| Flucytosine (FLC) |
| API and co-formers are interconnected through hydrogen bonding between O-H∙∙∙O, N-H∙∙∙O, and N-H∙∙∙N homosynthons | FLC-2,3HBA, FLCGAA, and FLC-GLA exhibited higher -% RH and stability at both 70–75% RH and 90–95% RH conditions [99] |
| Tramadol | Paracetamol | Interconnected through hydrogen bond between Cl of tramadol to H of paracetamol | Hygroscopicity of co-crystal was decreased [100,101] |
| Oxymatrine (OMT) |
| The API and co-formers are interconnected through either of O-H∙∙∙O, C-O∙∙∙H, N-H∙∙∙O−-N+, N-H∙∙∙O=C hydrogen bonding. In the case of urea co-crystals, the API and co-former are interconnected through the water molecule in between. | Hygroscopicity of the co-crystals was in the following order: Urea > THP > KTA > HNA > SUA [36] |
| Phenazopyridine hydrochloride | Phthalimide | Drug and co-former forms a dimer with O-H∙∙∙N, N-H∙∙∙N hydrogen bonds. Furthermore, the dimers are linked together, forming scissor-like chains. | Hygroscopic stability of phenazopyridine greatly improved after co-crystal formation [96] |
6. Methods and Tools for Moisture Content Determination
| Method | Principle | Advantages | Limitations |
|---|---|---|---|
| Loss on drying [110] | Works on the thermogravimetry principle. Material is heated until no more weight is lost and the final loss of weight is calculated. |
|
|
| Karl Fischer titration [103,110] | Water reacts with iodine and reaches the endpoint when all the water is consumed |
|
|
| Near Infrared spectroscopy [111] | Absorption of electromagnetic radiation by the sample, and its transmittance and absorbance are measured by the detector |
|
|
| Raman spectroscopy [108] | The Raman shift is unique to each molecule and water being a weak Raman scatterer can be identified and quantified. It is also possible to quantify the water content from the Raman intensity. |
|
|
| Microwave technology [109] | When microwave interacts with water within the material they slow down and weaken attenuate as the energy is transferred to the water. The signal is received by the receiver antenna and is compared to the transmitted signals. |
|
|
| Dynamic vapor sorption [112] | The gravimetric technique measures the amount of solvent absorbed with respect to its mass by varying vapor concentration surrounding the sample |
|
|
| Dielectric capacitance [110] | Measures the difference between the dielectric constant of water and the material located between the sensor’s capacitor electrodes |
|
|
7. Future Perspectives
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reutzel-Edens, S.M.; Braun, D.E.; Newman, A.W. Hygroscopicity and Hydrates in Pharmaceutical Solids; Wiley-VCH: Weinheim, Germany, 2018; Volume 2. [Google Scholar]
- Roy, S.; Siddique, S.; Majumder, S.; Abdul, M.; Rahman, S.; Lateef, D.; Dan, S.; Bose, A. A systemic approach on understanding the role of moisture in pharmaceutical product degradation and its prevention: Challenges and perspectives. Biomed. Res. 2018, 29, 3336–3343. [Google Scholar] [CrossRef]
- Newman, A.W.; Reutzel-Edens, S.M.; Zografi, G. Characterization of the “hygroscopic” properties of active pharmaceutical ingredients. J. Pharm. Sci. 2008, 97, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Waterman, K.C.; MacDonald, B.C. Package selection for moisture protection for solid, oral drug products. J. Pharm. Sci. 2010, 99, 4437–4452. [Google Scholar] [CrossRef]
- Mauer, L.J.; Taylor, L.S. Water-solids interactions: Deliquescence. Annu. Rev. Food Sci. Technol. 2010, 1, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Mauer, L.J.; Taylor, L.S. Deliquescence of pharmaceutical systems. Pharm. Dev. Technol. 2010, 15, 582–594. [Google Scholar] [CrossRef]
- Kumar, D.; Thipparaboina, R.; Sreedhar, B.; Shastri, N.R. The role of surface chemistry in crystal morphology and its associated properties. CrystEngComm 2015, 17, 6646–6650. [Google Scholar] [CrossRef]
- Watanabe, T.; Ito, M.; Suzuki, H.; Terada, K.; Noguchi, S. Reduced deliquescency of isosorbide by cocrystallization and mechanisms for hygroscopicity. Int. J. Pharm. 2021, 607, 120959. [Google Scholar] [CrossRef]
- Zografi, G. States of water associated with solids. Drug Dev. Ind. Pharm. 1988, 14, 1905–1926. [Google Scholar] [CrossRef]
- Chaurasia, G. A review on pharmaceutical preformulation studies in formulation and development of new drug molecules. Int. J. Pharm. Sci. Res. 2016, 7, 2313–2320. [Google Scholar]
- Paoli, A.D., Jr. The HVAC process. J. Valid. Technol. 2011, 17, 20. [Google Scholar]
- Kerry, J. 9—Aluminium foil packaging. In Packaging Technology; Emblem, A., Emblem, H., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 163–177. [Google Scholar]
- Zier, K.-I.; Schultze, W.; Leopold, C.S. Combination of a hot-melt subcoating and an enteric coating for moisture protection of hygroscopic Sennae fructus tablets. Pharm. Dev. Technol. 2019, 24, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Hauss, D.J. Oral lipid-based formulations. Adv. Drug Deliv. Rev. 2007, 59, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Moraes e Silva, A.; Honorato, J.o.; Machado Viana, A.L.; Faria, H.; Ruela, A.; Doriguetto, A.C.; Alves Oliveira, C.M.; Martins, F.T. New Multicomponent Crystal Forms of Adiphenine with Low Hygroscopicity. Cryst. Growth Des. 2022, 22, 3688–3697. [Google Scholar] [CrossRef]
- Lu, Q.; Dun, J.; Chen, J.-M.; Liu, S.; Sun, C.C. Improving solid-state properties of berberine chloride through forming a salt cocrystal with citric acid. Int. J. Pharm. 2019, 554, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, S.; Gao, Z. Crystal structure, dissolution and hygroscopicity of a novel cocrystal hydrate of berberine hydrochloride with L (+)-lactic acid. Die Pharm.-Int. J. Pharm. Sci. 2020, 75, 483–487. [Google Scholar]
- Perumalla, S.R.; Sun, C.C. Improved solid-state stability of salts by cocrystallization between conjugate acid–base pairs. CrystEngComm 2013, 15, 5756–5759. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Duan, Y.; Liu, L.-X.; Chang, L.; Feng, Y.-R.; Wu, L.-L.; Zhang, L.; Zhang, Y.-J.; Zou, D.-Y.; Liu, Y.-L. On Improving The Hygroscopic Stability Of Palmatine Chloride With Crystalline Palmatine Sulfosalicyate Pharmaceutical Salt. J. Struct. Chem. 2022, 63, 52–61. [Google Scholar] [CrossRef]
- Behrooz, F.; Mariun, N.; Marhaban, M.; Mohd Radzi, M.; Ramli, A. Review of Control Techniques for HVAC Systems—Nonlinearity Approaches Based on Fuzzy Cognitive Maps. Energies 2018, 11, 495. [Google Scholar] [CrossRef] [Green Version]
- Salawi, A. Pharmaceutical Coating and Its Different Approaches, a Review. Polymers 2022, 14, 3318. [Google Scholar] [CrossRef]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Physical stability enhancement of theophylline via cocrystallization. Int. J. Pharm. 2006, 320, 114–123. [Google Scholar] [CrossRef]
- Zhang, Y.-n.; Li, Y.-j.; Chang, L.; Liu, L.-x.; Feng, Y.-r.; Wu, L.-l.; Zhang, L.; Zhang, Y.-j. Improving hygroscopic stability of palmatine chloride by forming a pharmaceutical salt cocrystal of palmatine chloride-gallic acid with neutral molecule. J. Drug Deliv. Sci. Technol. 2021, 66, 102839. [Google Scholar] [CrossRef]
- Nugrahani, I.; Parwati, R.D. Challenges and Progress in Nonsteroidal Anti-Inflammatory Drugs Co-Crystal Development. Molecules 2021, 26, 4185. [Google Scholar] [CrossRef]
- Mirza, S.; Miroshnyk, I.; Heinämäki, J.; Yliruusi, J. Co-crystals: An emerging approach for enhancing properties of pharmaceutical solids. Dosis 2008, 24, 90–96. [Google Scholar]
- Liu, L.; Wang, J.-R.; Mei, X. Enhancing the stability of active pharmaceutical ingredients by cocrystal strategy. CrystEngComm 2022. [Google Scholar] [CrossRef]
- Karangutkar, A.V.; Ananthanarayan, L. Co-crystallization of Basella rubra extract with sucrose: Characterization of co-crystals and evaluating the storage stability of betacyanin pigments. J. Food Eng. 2020, 271, 109776. [Google Scholar] [CrossRef]
- Stanton, S.A.; Du, J.J.; Lai, F.; Stanton, G.; Hawkins, B.A.; Ong, J.A.; Groundwater, P.W.; Platts, J.A.; Hibbs, D.E. Understanding hygroscopicity of theophylline via a novel cocrystal polymorph: A charge density study. J. Phys. Chem. A 2021, 125, 9736–9756. [Google Scholar] [CrossRef]
- Thakuria, R.; Sarma, B. Drug-drug and drug-nutraceutical cocrystal/salt as alternative medicine for combination therapy: A crystal engineering approach. Crystals 2018, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- CDER; USFDA. Regulatory Classification of Pharmaceutical Co-Crystals: Guidance for Industry. 2018. Available online: https://www.fda.gov/files/drugs/published/Regulatory-Classification-of-Pharmaceutical-Co-Crystals.pdf (accessed on 8 December 2022).
- European Medicines Agency. Reflection Paper on the Use of Cocrystals of Active Substances in Medicinal Products; European Medicines Agency: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Sandler, N.; Reiche, K.; Heinämäki, J.; Yliruusi, J. Effect of moisture on powder flow properties of theophylline. Pharmaceutics 2010, 2, 275–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, H.; Miyata, T.; Mohri, K.; Sugimoto, I.; Manabe, H. Water of Crystallization of Berberine Chloride. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1978, 98, 981–985. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, K.; Matsuoka, M. Solid-state polymorphic transition of theophylline anhydrate and humidity effect. Cryst. Growth Des. 2007, 7, 411–415. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Chang, L.; Ji, Y.; Liu, L.; Feng, Y.; Wu, L.; Zhang, L.; Zhang, Y.; Zou, D. Crystalline palmatine saccharinate pharmaceutical salt without reducing solubility and improving its hygroscopic stability with regard to palmatine chloride. J. Mol. Struct. 2021, 1230, 129631. [Google Scholar] [CrossRef]
- Qi, M.-H.; Li, H.; Zhu, B.; Hong, M.; Ren, G.-B. Cocrystals of Oxymatrine: Reducing Hygroscopicity and Affecting the Dissolution Rate. Cryst. Growth Des. 2021, 21, 3874–3888. [Google Scholar] [CrossRef]
- Kumar, D.; Thipparaboina, R.; Modi, S.R.; Bansal, A.K.; Shastri, N.R. Effect of HPMC concentration on crystal habit of nifedipine. CrystEngComm 2015, 17, 1615–1624. [Google Scholar] [CrossRef]
- Kumar, D.; Thipparaboina, R.; Modi, S.R.; Bansal, A.K.; Shastri, N.R. Effect of surfactant concentration on nifedipine crystal habit and its related pharmaceutical properties. J. Cryst. Growth 2015, 422, 44–51. [Google Scholar] [CrossRef]
- Elder, D.P.; Delaney, E.; Teasdale, A.; Eyley, S.; Reif, V.D.; Jacq, K.; Facchine, K.L.; Oestrich, R.S.; Sandra, P.; David, F. The Utility of Sulfonate Salts in Drug Development. J. Pharm. Sci. 2010, 99, 2948–2961. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Huang, Y.; Zhang, M.; Lou, B. Preparation, crystal structures, and oral bioavailability of two cocrystals of emodin with berberine chloride. Cryst. Growth Des. 2018, 18, 7481–7488. [Google Scholar] [CrossRef]
- Shalaev, E.Y.; Zografi, G. How does residual water affect the solid-state degradation of drugs in the amorphous state? J. Pharm. Sci. 1996, 85, 1137–1141. [Google Scholar] [CrossRef]
- Szakonyi, G.; Zelkó, R. The effect of water on the solid state characteristics of pharmaceutical excipients: Molecular mechanisms, measurement techniques, and quality aspects of final dosage form. Int. J. Pharm. Investig. 2012, 2, 18. [Google Scholar]
- Yoshioka, S.; Aso, Y. Correlations between molecular mobility and chemical stability during storage of amorphous pharmaceuticals. J. Pharm. Sci. 2007, 96, 960–981. [Google Scholar] [CrossRef]
- Steendam, R.; Frijlink, H.W.; Lerk, C.F. Plasticisation of amylodextrin by moisture. Consequences for compaction behaviour and tablet properties. Eur. J. Pharm. Sci. 2001, 14, 245–254. [Google Scholar] [CrossRef]
- Ruotsalainen, M.; Heinämäki, J.; Taipale, K.; Yliruusi, J. Influence of the aqueous film coating process on the properties and stability of tablets containing a moisture-labile drug. Pharm. Dev. Technol. 2003, 8, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Guo, D.; Shen, Y.; Guo, S.; Ruan, K. Effects of highly hygroscopic excipients on the hydrolysis of simvastatin in tablet at high relative humidity. Indian J. Pharm. Sci. 2012, 74, 527. [Google Scholar] [PubMed] [Green Version]
- Mihranyan, A.; Strömme, M.; Ek, R. Influence of cellulose powder structure on moisture-induced degradation of acetylsalicylic acid. Eur. J. Pharm. Sci. 2006, 27, 220–225. [Google Scholar] [CrossRef]
- Worku, Z.A.; Kumar, D.; Gomes, J.V.; He, Y.; Glennon, B.; Ramisetty, K.A.; Rasmuson, Å.C.; O’Connell, P.; Gallagher, K.H.; Woods, T.; et al. Modelling and understanding powder flow properties and compactability of selected active pharmaceutical ingredients, excipients and physical mixtures from critical material properties. Int. J. Pharm. 2017, 531, 191–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo-Osuna, I.; Ferrero, C.; Jiménez-Castellanos, M. Influence of moisture content on the mechanical properties of methyl methacrylate–starch copolymers. Eur. J. Pharm. Biopharm. 2007, 66, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.; Oliver, J.; Pugsley, T.; Sharma, J.; Zhou, J. Flowability of moist pharmaceutical powders. Powder Technol. 2009, 189, 409–415. [Google Scholar] [CrossRef]
- Coelho, M.; Harnby, N. The effect of humidity on the form of water retention in a powder. Powder Technol. 1978, 20, 197–200. [Google Scholar] [CrossRef]
- Nokhodchi, A. An overview of the effect of moisture on compaction and compression. Pharm. Technol. (2003) 2005, 29, 46–66. [Google Scholar]
- Khan, K.A.; Musikabhumma, P.; Warr, J.P. The Effect of Moisture Content of Microcrystalline Cellulose on the Compressional Properties of Some Formulations. Drug Dev. Ind. Pharm. 1981, 7, 525–538. [Google Scholar] [CrossRef]
- Zografi, G.; Kontny, M.J. The interactions of water with cellulose-and starch-derived pharmaceutical excipients. Pharm. Res. 1986, 3, 187–194. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Rubinstein, M.; Larhrib, H.; Guyot, J. The effect of moisture on the properties of ibuprofen tablets. Int. J. Pharm. 1995, 118, 191–197. [Google Scholar] [CrossRef]
- Akbuga, J.; Gürsoy, A. The effect of moisture sorption and desorption on furosemide tablet properties. Drug Dev. Ind. Pharm. 1987, 13, 1827–1845. [Google Scholar] [CrossRef]
- Viljoen, J.M.; Steenekamp, J.H.; Marais, A.F.; Kotzé, A.F. Effect of moisture content, temperature and exposure time on the physical stability of chitosan powder and tablets. Drug Dev. Ind. Pharm. 2014, 40, 730–742. [Google Scholar] [CrossRef]
- Collier, J.W.; Shah, R.B.; Gupta, A.; Sayeed, V.; Habib, M.J.; Khan, M.A. Influence of formulation and processing factors on stability of levothyroxine sodium pentahydrate. Aaps Pharmscitech 2010, 11, 818–825. [Google Scholar] [CrossRef]
- Bele, M.H.; Derle, D.V. Effect of sorbed water on disintegrant performance of four brands of polacrilin potassium NF. Aaps Pharmscitech 2012, 13, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Rao, K.R.; Venugopal, K.; Manikandan, R. Alteration in dissolution characteristics of gelatin-containing formulations. Pharm. Technol. 2002, 26, 36–54. [Google Scholar]
- Ofner III, C.M.; Zhang, Y.E.; Jobeck, V.C.; Bowman, B.J. Crosslinking studies in gelatin capsules treated with formaldehyde and in capsules exposed to elevated temperature and humidity. J. Pharm. Sci. 2001, 90, 79–88. [Google Scholar] [CrossRef]
- Healy, A.M.; Worku, Z.A.; Kumar, D.; Madi, A.M. Pharmaceutical solvates, hydrates and amorphous forms: A special emphasis on cocrystals. Adv. Drug Deliv. Rev. 2017, 117, 25–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Rohani, S. Polymorphism and crystallization of active pharmaceutical ingredients (APIs). Curr. Med. Chem. 2009, 16, 884–905. [Google Scholar] [CrossRef]
- Skrdla, P.J.; Floyd, P.D.; Dell’Orco, P.C. Predicted amorphous solubility and dissolution rate advantages following moisture sorption: Case studies of indomethacin and felodipine. Int. J. Pharm. 2019, 555, 100–108. [Google Scholar] [CrossRef]
- Bharate, S.S.; Bharate, S.B.; Bajaj, A.N. Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: A comprehensive review. J. Excip. Food Chem. 2016, 1, 1131. [Google Scholar]
- Airaksinen, S.; Karjalainen, M.; Kivikero, N.; Westermarck, S.; Shevchenko, A.; Rantanen, J.; Yliruusi, J. Excipient selection can significantly affect solid-state phase transformation in formulation during wet granulation. Aaps Pharmscitech 2005, 6, E311–E322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, R.; Bloomfield, S.F. Microbial Quality Assurance in Cosmetics, Toiletries, and Non-Sterile Pharmaceuticals, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Li, Y.; Sanzgiri, Y.D.; Chen, Y. A study on moisture isotherms of formulations: The use of polynomial equations to predict the moisture isotherms of tablet products. AAPS PharmSciTech 2003, 4, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S. Granulation techniques and technologies: Recent progresses. BioImpacts BI 2015, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Koranne, S.; Sahoo, A.; Krzyzaniak, J.F.; Luthra, S.; Arora, K.K.; Suryanarayanan, R. Challenges in transitioning cocrystals from bench to bedside: Dissociation in prototype drug product environment. Mol. Pharm. 2018, 15, 3297–3307. [Google Scholar] [CrossRef]
- Veith, H.; Zaeh, M.; Luebbert, C.; Rodríguez-Hornedo, N.; Sadowski, G. Stability of Pharmaceutical Co-Crystals at Humid Conditions Can Be Predicted. Pharmaceutics 2021, 13, 433. [Google Scholar] [CrossRef]
- McNamara, D.P.; Childs, S.L.; Giordano, J.; Iarriccio, A.; Cassidy, J.; Shet, M.S.; Mannion, R.; O’Donnell, E.; Park, A. Use of a glutaric acid cocrystal to improve oral bioavailability of a low solubility API. Pharm. Res. 2006, 23, 1888–1897. [Google Scholar] [CrossRef]
- Stanton, M.K.; Bak, A. Physicochemical properties of pharmaceutical co-crystals: A case study of ten AMG 517 co-crystals. Cryst. Growth Des. 2008, 8, 3856–3862. [Google Scholar] [CrossRef]
- Hickey, M.B.; Peterson, M.L.; Scoppettuolo, L.A.; Morrisette, S.L.; Vetter, A.; Guzmán, H.; Remenar, J.F.; Zhang, Z.; Tawa, M.D.; Haley, S. Performance comparison of a co-crystal of carbamazepine with marketed product. Eur. J. Pharm. Biopharm. 2007, 67, 112–119. [Google Scholar] [CrossRef]
- Huang, S.; Xue, Q.; Xu, J.; Ruan, S.; Cai, T. Simultaneously improving the physicochemical properties, dissolution performance, and bioavailability of apigenin and daidzein by co-crystallization with theophylline. J. Pharm. Sci. 2019, 108, 2982–2993. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Zhang, M.; Zhang, Y.; Lou, B. A Drug–Drug Cocrystal of Dihydromyricetin and Pentoxifylline. J. Pharm. Sci. 2022, 111, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; An, Q.; Zhang, Y.; Sun, W.; Li, J.; Feng, Y.; Geng, Y.; Cheng, G. Improving the solubility, hygroscopicity and permeability of enrofloxacin by forming 1: 2 pharmaceutical salt cocrystal with neutral and anionic co-existing p-nitrobenzoic acid. J. Drug Deliv. Sci. Technol. 2022, 76, 103732. [Google Scholar] [CrossRef]
- Lai, F.; Du, J.J.; Williams, P.A.; Váradi, L.; Baker, D.; Groundwater, P.W.; Overgaard, J.; Platts, J.A.; Hibbs, D.E. A comparison of the experimental and theoretical charge density distributions in two polymorphic modifications of piroxicam. Phys. Chem. Chem. Phys. 2016, 18, 28802–28818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, F.; Xiong, Y.; Peng, R.; Zhang, C. Molecular Packing Density Coefficient Contradiction of High-Density Energetic Compounds and a Strategy to Achieve High Packing Density. Cryst. Growth Des. 2022, 22, 3252–3263. [Google Scholar] [CrossRef]
- Zieliński, W.; Katrusiak, A. Hydrate smaller than the anhydrate. CrystEngComm 2015, 17, 5468–5473. [Google Scholar] [CrossRef]
- Braun, D.E.; Karamertzanis, P.G.; Price, S.L. Which, if any, hydrates will crystallise? Predicting hydrate formation of two dihydroxybenzoic acids. Chem. Commun. 2011, 47, 5443–5445. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Nie, J.; Stephen Chan, H.C.; Zhang, H.; Li, L.; Lin, H.; Tong, H.H.Y.; Ma, A.; Zhou, Z. Phase solubility diagrams and energy surface calculations support the solubility enhancement with low hygroscopicity of Bergenin: 4-Aminobenzamide (1: 1) cocrystal. Int. J. Pharm. 2021, 601, 120537. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Du, J.J.; Lai, F.; Stanton, S.A.; Williams, P.A.; Groundwater, P.W.; Platts, J.A.; Overgaard, J.; Hibbs, D.E. An experimental and theoretical charge density study of theophylline and malonic acid cocrystallization. RSC Adv. 2022, 12, 15670–15684. [Google Scholar] [CrossRef]
- Thomas, S.P.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Accurate Lattice Energies for Molecular Crystals from Experimental Crystal Structures. J. Chem. Theory Comput. 2018, 14, 1614–1623. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Chow, S.F.; Chen, M.; Shi, L.; Chow, A.H.L.; Sun, C.C. Simultaneously Improving the Mechanical Properties, Dissolution Performance, and Hygroscopicity of Ibuprofen and Flurbiprofen by Cocrystallization with Nicotinamide. Pharm. Res. 2012, 29, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Weng, X.; Wei, Y.; Gao, Y.; Zhang, J.; Qian, S. Modification of hygroscopicity and tabletability of l-carnitine by a cocrystallization technique. CrystEngComm 2021, 23, 2138–2149. [Google Scholar] [CrossRef]
- de Almeida, A.C.; Torquetti, C.; Ferreira, P.O.; Fernandes, R.P.; dos Santos, E.C.; Kogawa, A.C.; Caires, F.J. Cocrystals of ciprofloxacin with nicotinic and isonicotinic acids: Mechanochemical synthesis, characterization, thermal and solubility study. Thermochim. Acta 2020, 685, 178346. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Q.; Zhu, B.; Zhang, Z.; Bao, J.; Ding, Q.; Ren, G.; Mei, X. Pharmaceutical Cocrystals of Nicorandil with Enhanced Chemical Stability and Sustained Release. Cryst. Growth Des. 2020, 20, 6995–7005. [Google Scholar] [CrossRef]
- Han, S.; Hong, J.; Luo, Q.; Xu, H.; Tan, H.; Wang, Q.; Tao, J.; Zhou, Y.; Peng, L.; He, Y. Hygroscopicity of organic compounds as a function of organic functionality, water solubility, molecular weight and oxidation level. Atmos. Chem. Phys. 2022, 22, 3985–4004. [Google Scholar] [CrossRef]
- Aitipamula, S.; Wong, A.B.; Chow, P.S.; Tan, R.B. Cocrystallization with flufenamic acid: Comparison of physicochemical properties of two pharmaceutical cocrystals. CrystEngComm 2014, 16, 5793–5801. [Google Scholar] [CrossRef]
- Aher, S.; Dhumal, R.; Mahadik, K.; Ketolainen, J.; Paradkar, A. Effect of cocrystallization techniques on compressional properties of caffeine/oxalic acid 2: 1 cocrystal. Pharm. Dev. Technol. 2013, 18, 55–60. [Google Scholar] [CrossRef]
- Shinozaki, T.; Ono, M.; Higashi, K.; Moribe, K. A novel drug-drug cocrystal of levofloxacin and metacetamol: Reduced hygroscopicity and improved photostability of levofloxacin. J. Pharm. Sci. 2019, 108, 2383–2390. [Google Scholar] [CrossRef]
- Wu, D.; Li, J.; Xiao, Y.; Ji, X.; Li, C.; Zhang, B.; Hou, B.; Zhou, L.; Xie, C.; Gong, J.; et al. New Salts and Cocrystals of Pymetrozine with Improvements on Solubility and Humidity Stability: Experimental and Theoretical Study. Cryst. Growth Des. 2021, 21, 2371–2388. [Google Scholar] [CrossRef]
- Babu, N.J.; Sanphui, P.; Nangia, A. Crystal Engineering of Stable Temozolomide Cocrystals. Chem.–Asian J. 2012, 7, 2274–2285. [Google Scholar] [CrossRef]
- Tao, Q.; Chen, J.-M.; Ma, L.; Lu, T.-B. Phenazopyridine cocrystal and salts that exhibit enhanced solubility and stability. Cryst. Growth Des. 2012, 12, 3144–3152. [Google Scholar] [CrossRef]
- Basavoju, S.; Boström, D.; Velaga, S.P. Indomethacin–saccharin cocrystal: Design, synthesis and preliminary pharmaceutical characterization. Pharm. Res. 2008, 25, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Z.; Chen, J.-M.; Lu, T.-B. Enhancing the hygroscopic stability of S-oxiracetam via pharmaceutical cocrystals. Cryst. Growth Des. 2012, 12, 4562–4566. [Google Scholar] [CrossRef]
- Nechipadappu, S.K.; Ramachandran, J.; Shivalingegowda, N.; Lokanath, N.K.; Trivedi, D.R. Synthesis of cocrystals/salts of flucytosine: Structure and stability. New J. Chem. 2018, 42, 5433–5446. [Google Scholar] [CrossRef]
- Gadade, D.D.; Pekamwar, S.S. Pharmaceutical cocrystals: Regulatory and strategic aspects, design and development. Adv. Pharm. Bull. 2016, 6, 479. [Google Scholar] [CrossRef]
- Almansa, C.; Merce, R.; Tesson, N.; Farran, J.; Tomas, J.; Plata-Salaman, C.R. Co-Crystal of Tramadol hydrochloride–Celecoxib (CTC): A novel API–API co-crystal for the treatment of pain. Cryst. Growth Des. 2017, 17, 1884–1892. [Google Scholar] [CrossRef]
- Padivitage, N.L.; Smuts, J.P.; Armstrong, D.W. Water determination. In Specification of Drug Substances and Products; Elsevier: Amsterdam, The Netherlands, 2014; pp. 223–241. [Google Scholar]
- Tavčar, E.; Turk, E.; Kreft, S. Simple Modification of Karl-Fischer Titration Method for Determination of Water Content in Colored Samples. J. Anal. Methods Chem. 2012, 2012, 379724. [Google Scholar] [CrossRef] [Green Version]
- Hansuld, E.M.; Briens, L. A review of monitoring methods for pharmaceutical wet granulation. Int. J. Pharm. 2014, 472, 192–201. [Google Scholar] [CrossRef]
- Gradinarsky, L.; Brage, H.; Lagerholm, B.; Björn, I.N.; Folestad, S. In situ monitoring and control of moisture content in pharmaceutical powder processes using an open-ended coaxial probe. Meas. Sci. Technol. 2006, 17, 1847. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. 2004. Available online: http://www.fda.gov/cder/guidance/published.html (accessed on 8 December 2022).
- Nambiar, A.G.; Singh, M.; Mali, A.R.; Serrano, D.R.; Kumar, R.; Healy, A.M.; Agrawal, A.K.; Kumar, D. Continuous Manufacturing and Molecular Modeling of Pharmaceutical Amorphous Solid Dispersions. AAPS PharmSciTech 2022, 23, 249. [Google Scholar] [CrossRef]
- Jørgensen, A.; Rantanen, J.; Karjalainen, M.; Khriachtchev, L.; Räsänen, E.; Yliruusi, J. Hydrate formation during wet granulation studied by spectroscopic methods and multivariate analysis. Pharm. Res. 2002, 19, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Okamura, S. Microwave technology for moisture measurement. Subsurf. Sens. Technol. Appl. 2000, 1, 205–227. [Google Scholar] [CrossRef]
- Spitzlei, M. Choosing a method for measuring your material’s moisture content. Powder Bulk Eng. 2000, 14, 39–52. [Google Scholar]
- Rosas, J.G.; Blanco, M.; González, J.M.; Alcalà, M. Real-time determination of critical quality attributes using near-infrared spectroscopy: A contribution for Process Analytical Technology (PAT). Talanta 2012, 97, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Murikipudi, V.; Gupta, P.; Sihorkar, V. Efficient throughput method for hygroscopicity classification of active and inactive pharmaceutical ingredients by water vapor sorption analysis. Pharm. Dev. Technol. 2013, 18, 348–358. [Google Scholar] [CrossRef] [PubMed]











| Control Strategy | Advantages | Disadvantages | References |
|---|---|---|---|
| Manufacturing Controls (dehumidifiers and HVAC) |
|
| [11,20] |
| Protective Packaging (aluminum foil pack and use of desiccants) |
|
| [12] |
| Polymer/Film Coating |
|
| [21] |
| Lipid-based Technologies |
|
| [14] |
| Changing Salt forms of API |
|
| [15,19] |
| Co-crystals |
|
| [22,23] |
| Substance | Critical Relative Humidity (%RH) | Water Adsorbed at 30% RH/Unit Surface Area (mg/m2) |
|---|---|---|
| ISO | 48 | 1.636 |
| ISO-PZ | 56 | 0.572 |
| ISO-HCT | 85 | 4.963 |
| ISO-DHBA | 69 | 10.113 |
| ISO-GA | 67 | 1.172 |
| Compound | Coformer | % Weight Gain (w/w) | ||
|---|---|---|---|---|
| 70% RH | 95% RH | Changes in Solubility | ||
| Berberine chloride (BCl) [16,17] | -- | 8.80% | 16.30% | -- |
| Citric acid-berberine | Citric acid [16] | < 2.0% | 8.00% | Co-crystal had similar solubility as that of BCl |
| Emodin-berberine chloride (EM-BCl) | Emodin [40] | ~ 0.50% | 1.30% | Solubility in the order: BCl > EM-BCl > 2EM-BCl-Et |
| 2 Emodin-berberine chloride-ethanol (2EM-BCl-Et) | ~ 0.75% | 0.90% | ||
| L(+)-Lactic acid-berberine chloride | L(+)-Lactic acid [17] | ~ 4.00% | 10.00% | Co-crystal had similar solubility as that of BCl |
| Palmatine chloride [35] | -- | 8.14% | 19.00% | -- |
| Palmatine-saccharinate (Salt) [35] | -- | 3.72% | 2.30% | Solubility of new salt reduced |
| Palmatine-sulfosalicyate (Salt) [19] | -- | 5.90% | 13.43% # | Solubility of new salt reduced |
| Gallic acid-palmatine chloride [23] | Gallic acid | 2.83% | 5.78% # | Solubility of co-crystal reduced |
| Adiphenine hydrochloride [15] | -- | ~ 2.5% | 22% @ | Retained 5% water during desorption even at 0% RH |
| Adiphenine citrate (Salt) [15] | -- | ~ 0.75% | 3.2% @ | Significant reduction in aqueous solubility |
| Adiphenine oxalate [15] | -- | ~ 0.25% | 2.6% @ | Significant reduction in aqueous solubility |
| Co-Crystal | IDR (mg cm−2 min−1) | Solubility (mg ml−1) | Hygroscopicity |
|---|---|---|---|
| OMT-Urea-2 H2O | 30.1 | >48 | 87.69% |
| OMT-SUA | 11.4 | >52 | 0.97% |
| OMT-THP | 2.8 | >54 | 45–50% |
| OMT-KTA-H2O | 21.7 | >50 | 6.06% |
| OMT-HNA | 0.1 | 2.92 | 2.45% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhondale, M.R.; Thakor, P.; Nambiar, A.G.; Singh, M.; Agrawal, A.K.; Shastri, N.R.; Kumar, D. Co-Crystallization Approach to Enhance the Stability of Moisture-Sensitive Drugs. Pharmaceutics 2023, 15, 189. https://doi.org/10.3390/pharmaceutics15010189
Dhondale MR, Thakor P, Nambiar AG, Singh M, Agrawal AK, Shastri NR, Kumar D. Co-Crystallization Approach to Enhance the Stability of Moisture-Sensitive Drugs. Pharmaceutics. 2023; 15(1):189. https://doi.org/10.3390/pharmaceutics15010189
Chicago/Turabian StyleDhondale, Madhukiran R., Pradip Thakor, Amritha G. Nambiar, Maan Singh, Ashish K. Agrawal, Nalini R. Shastri, and Dinesh Kumar. 2023. "Co-Crystallization Approach to Enhance the Stability of Moisture-Sensitive Drugs" Pharmaceutics 15, no. 1: 189. https://doi.org/10.3390/pharmaceutics15010189