Novel Liposomal Formulation of Baicalein for the Treatment of Pancreatic Ductal Adenocarcinoma: Design, Characterization, and Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
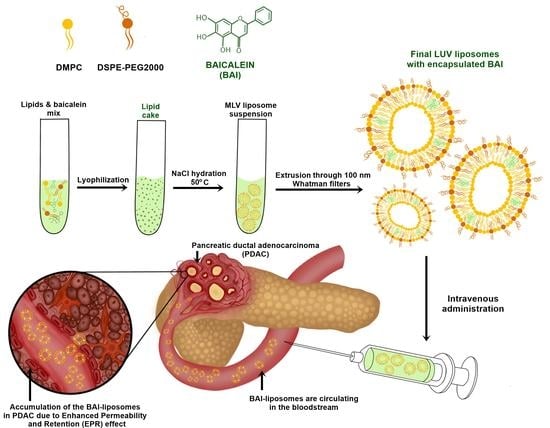
2.2. Liposome Preparation
2.3. Liposome Determination of Mean Diameter Size, Polydispersity (PDI), and Zeta Potential of BAI-Loaded and Empty Liposomes
2.4. BAI Encapsulation Efficiency Determination and Process Optimization
2.5. Long-Term Stability of BAI-Loaded Liposomes: Size, PDI, and Retention Evaluation
2.6. In Vitro Release of Baicalein from Liposomes in the Presence of Serum Albumins
2.7. Cryogenic Transmission Electron Microscopy (Cryo-EM) Visualization
2.8. Cell Culture
2.9. Cell Viability Assay Using MTT Colorimetric Test
2.10. Cellular Uptake
2.11. DNA Fragmentation Assay
2.12. RealTime-Glo Apoptosis and Necrosis Assay
2.13. Preparation of Three-Dimensional Spheroids
2.14. ATP Cell Viability Assay for 3D Spheroid Model
2.15. Determination of Hemolysis of Human Erythrocytes
2.16. Statistical Analysis
3. Results
3.1. Preparation and Analysis of BAI-Loaded PEGylated Liposomes
3.2. Characterization of Obtained BAI-Loaded Liposomal Suspension by Dynamic Light Scattering (DLS) System
3.3. Cryogenic Transmission Electron Microscopy (Cryo-TEM) Visualization of Nanoparticles
3.4. Long-Term Stability Studies of BAI Liposomes
3.5. BAI-Liposome Stability in Presence of Serum Albumins and Hemolytic Activity
3.6. Cytotoxic Activity of BAI Liposomes towards PDAC Cell Lines and Normal NHDF Cell Line
3.7. Baicalein Formulation Induces Apoptosis in Pancreatic Cancer BxPC-3 Cell Line
3.8. Cellular Uptake of BAI-Loaded Liposomes
3.9. Three-Dimensional Cultures
3.10. DNA Fragmentation Induction by BAI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The International Agency for Research on Cancer (IARC). Global Cancer Observatory. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/ (accessed on 18 October 2022).
- Hu, J.-X.; Zhao, C.-F.; Chen, W.-B.; Liu, Q.-C.; Li, Q.-W.; Lin, Y.-Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.; Metzger, P.; Gerum, S.; Mayerle, J.; Schneider, G.; Belka, C.; Schnurr, M.; Lauber, K. Pancreatic ductal adenocarcinoma: Biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019, 14, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengtsson, A.; Andersson, R.; Ansari, D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci. Rep. 2020, 10, 16425. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aquila, E.; Fulgenzi, C.A.M.; Minelli, A.; Citarella, F.; Stellato, M.; Pantano, F.; Russano, M.; Cursano, M.C.; Napolitano, A.; Zeppola, T.; et al. Prognostic and predictive factors in pancreatic cancer. Oncotarget 2020, 11, 924–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Principe, D.R.; Underwood, P.W.; Korc, M.; Trevino, J.G.; Munshi, H.G.; Rana, A. The Current Treatment Paradigm for Pancreatic Ductal Adenocarcinoma and Barriers to Therapeutic Efficacy. Front. Oncol. 2021, 11, 688377. [Google Scholar] [CrossRef]
- Zeng, S.; Pöttler, M.; Lan, B.; Grützmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 4504. [Google Scholar] [CrossRef] [Green Version]
- Stott, M.C.; Oldfield, L.; Hale, J.; Costello, E.; Halloran, C.M. Recent advances in understanding pancreatic cancer. Fac. Rev. 2022, 11, 9. [Google Scholar] [CrossRef]
- Edwards, P.; Kang, B.W.; Chau, I. Targeting the Stroma in the Management of Pancreatic Cancer. Front. Oncol. 2021, 11, 691185. [Google Scholar] [CrossRef]
- Narayanan, S.; Vicent, S.; Ponz-Sarvisé, M. PDAC as an Immune Evasive Disease: Can 3D Model Systems Aid to Tackle This Clinical Problem? Front. Cell Dev. Biol. 2021, 9, 787249. [Google Scholar] [CrossRef]
- Javadrashid, D.; Baghbanzadeh, A.; Derakhshani, A.; Leone, P.; Silvestris, N.; Racanelli, V.; Solimando, A.; Baradaran, B. Pancreatic Cancer Signaling Pathways, Genetic Alterations, and Tumor Microenvironment: The Barriers Affecting the Method of Treatment. Biomedicines 2021, 9, 373. [Google Scholar] [CrossRef]
- Miquel, M.; Zhang, S.; Pilarsky, C. Pre-clinical Models of Metastasis in Pancreatic Cancer. Front. Cell Dev. Biol. 2021, 9, 748631. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Meng, H. Transcytosis—An effective targeting strategy that is complementary to “EPR effect” for pancreatic cancer nano drug delivery. Theranostics 2019, 9, 8018–8025. [Google Scholar] [CrossRef]
- Inagaki, F.F.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Enhanced nanodrug delivery in tumors after near-infrared photoimmunotherapy. Nanophotonics 2019, 8, 1673–1688. [Google Scholar] [CrossRef]
- Gabizon, A.; Tzemach, D.; Mak, L.; Bronstein, M.; Horowitz, A.T. Dose Dependency of Pharmacokinetics and Therapeutic Efficacy of Pegylated Liposomal Doxorubicin (DOXIL) in Murine Models. J. Drug Target. 2002, 10, 539–548. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Lamichhane, N.; Udayakumar, T.; D’Souza, W.; Simone, C., II; Raghavan, S.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef] [Green Version]
- Green, A.E.; Rose, P.G. Pegylated liposomal doxorubicin in ovarian cancer. Int. J. Nanomed. 2006, 1, 229–239. [Google Scholar]
- Salehi, B.; Selamoglu, Z.; Mileski, K.; Pezzani, R.; Redaelli, M.; Cho, W.; Kobarfard, F.; Rajabi, S.; Martorell, M.; Kumar, P.; et al. Liposomal Cytarabine as Cancer Therapy: From Chemistry to Medicine. Biomolecules 2019, 9, 773. [Google Scholar] [CrossRef] [Green Version]
- Gang, W.; Jie, W.J.; Ping, Z.L.; Ming, D.S.; Ying, L.J.; Lei, W.; Fang, Y. Liposomal quercetin: Evaluating drug delivery in vitro and biodistribution in vivo. Expert Opin. Drug Deliv. 2012, 9, 599–613. [Google Scholar] [CrossRef]
- Lipka, D.; Gubernator, J.; Filipczak, N.; Barnert, S.; Süss, R.; Kozubek, A.; Legut, M. Vitamin C-driven epirubicin loading into liposomes. Int. J. Nanomed. 2013, 8, 3573–3585. [Google Scholar] [CrossRef]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome®): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koudelka, T.; Turánek, J. Liposomal paclitaxel formulations. J. Control. Release 2012, 163, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Nowak, M.; Lazebna, A.; Więcek, K.; Jabłońska, I.; Szpadel, K.; Grzeszczak, A.; Gubernator, J.; Ziółkowski, P. The Comparison of In Vitro Photosensitizing Efficacy of Curcumin-Loaded Liposomes Following Photody-namic Therapy on Melanoma MUG-Mel2, Squamous Cell Carcinoma SCC-25, and Normal Keratinocyte HaCaT Cells. Pharmaceuticals 2021, 1, 374. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.; Moreno, E.; Larrea, E.; Sanmartín, C.; Irache, J.M.; Espuelas, S. Berberine-Loaded Liposomes for the Treatment of Leishmania infantum-Infected BALB/c Mice. Pharmaceutics 2020, 12, 858. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, K.; Zhang, Y.; Li, H.; Li, A.; Xu, Y.; Wei, B. Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci. Rep. 2020, 10, 2440. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-K. Liposomes for enhanced bioavailability of water-insoluble drugs: In vivo evidence and recent approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Ding, Y.; Zhang, Y.; Ho, R.J.; Zhao, Y.; Zhang, T.; Guo, C. Antibody-modified liposomes for tumor-targeting delivery of timosaponin AIII. Int. J. Nanomed. 2018, 13, 1927–1944. [Google Scholar] [CrossRef] [Green Version]
- Morelli, G.; Accardo, A.; Tesauro, D.; Cicala, C.; Salzano, G.; De Rosa, G.; Morisco, A.; Aloj, L.; Aurilio, M.; Maione, F.; et al. Peptide-modified liposomes for selective targeting of bombesin receptors overexpressed by cancer cells: A potential theranostic agent. Int. J. Nanomed. 2012, 7, 2007–2017. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Yamashita, K.; Itoh, Y.; Yoshino, K.; Nozawa, S.; Kasukawa, H. Comparative studies of polyethylene glycol-modified liposomes prepared using different PEG-modification methods. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2801–2807. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Dehelean, C.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Sandoval, V.; Sanz-Lamora, H.; Arias, G.; Marrero, P.F.; Haro, D.; Relat, J. Metabolic Impact of Flavonoids Consumption in Obesity: From Central to Peripheral. Nutrients 2020, 12, 2393. [Google Scholar] [CrossRef]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Asgharian, P.; Tazehkand, A.P.; Soofiyani, S.R.; Hosseini, K.; Martorell, M.; Tarhriz, V.; Ahangari, H.; Cruz-Martins, N.; Sharifi-Rad, J.; Almarhoon, Z.M.; et al. Quercetin Impact in Pancreatic Cancer: An Overview on Its Therapeutic Effects. Oxidative Med. Cell. Longev. 2021, 2021, 4393266. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Qu, C.; Chen, L.; Geng, Y.; Cheng, C.; Yu, S.; Wang, D.; Yang, L.; Meng, Z.; et al. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer 2021, 21, 396. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Bakhoda, M.R.; Bahmanpour, Z.; Ilkhani, K.; Zarrabi, A.; Makvandi, P.; Khan, H.; Mazaheri, S.; Darvish, M.; Mirzaei, H. Apigenin as Tumor Suppressor in Cancers: Biotherapeutic Activity, Nanodelivery, and Mechanisms with Emphasis on Pancreatic Cancer. Front. Chem. 2020, 8, 829. [Google Scholar] [CrossRef]
- Kato, H.; Naiki-Ito, A.; Suzuki, S.; Inaguma, S.; Komura, M.; Nakao, K.; Naiki, T.; Kachi, K.; Kato, A.; Matsuo, Y.; et al. DPYD, down-regulated by the potentially chemopreventive agent luteolin, interacts with STAT3 in pancreatic cancer. Carcinogenesis 2021, 42, 940–950. [Google Scholar] [CrossRef]
- Moreau, M.; Ibeh, U.; Decosmo, K.; Bih, N.; Yasmin-Karim, S.; Toyang, N.; Lowe, H.; Ngwa, W. Flavonoid Derivative of Cannabis Demonstrates Therapeutic Potential in Preclinical Models of Metastatic Pancreatic Cancer. Front. Oncol. 2019, 9, 660. [Google Scholar] [CrossRef] [Green Version]
- Hertzer, K.; Eibl, G. Baicalein—An Intriguing Therapeutic Phytochemical in Pancreatic Cancer. Curr. Drug Targets 2012, 13, 1772–1776. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Salazar, L.A.; Shaheen, S.; Ayatollahi, S.A.; Kobarfard, F.; Imran, M.; Imran, A.; Custódio, L.; López, M.D.; et al. The Therapeutic Potential of Wogonin Observed in Preclinical Studies. Evid.-Based Complement. Altern. Med. 2021, 2021, 9935451. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Li, M.; Zhang, C.-L.; Liu, D. Pharmacological properties of baicalin on liver diseases: A narrative review. Pharmacol. Rep. 2021, 73, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, Y.; Gao, Y.; Du, Z.; Wang, Y.; Cheng, P.; Chen, A.; Huang, H. The Fascinating Effects of Baicalein on Cancer: A Review. Int. J. Mol. Sci. 2016, 17, 1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, H.; Chen, M.C.; Pham, H.; Angst, E.; King, J.C.; Park, J.; Brovman, E.Y.; Ishiguro, H.; Harris, D.M.; Reber, H.A.; et al. Baicalein, a component of Scutellaria baicalensis, in-duces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1813, 1465–1474. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Pi, J.; Zhang, Y.; Ma, X.; Wang, S.; Qi, D.; Li, N.; Guo, P.; Liu, Z. A strategy to improve the oral availability of baicalein: The baicalein-theophylline cocrystal. Fitoterapia 2018, 129, 85–93. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Q.; Wang, J.-R.; Mei, X. Cocrystals of Baicalein with Higher Solubility and Enhanced Bioavailability. Cryst. Growth Des. 2017, 17, 1893–1901. [Google Scholar] [CrossRef]
- Kolluru, L.; Atre, P.; Rizvi, S. Characterization and Applications of Colloidal Systems as Versatile Drug Delivery Carriers for Parenteral Formulations. Pharmaceuticals 2021, 14, 108. [Google Scholar] [CrossRef]
- Stewart, J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Jaromin, A.; Korycińska, M.; Piętka-Ottlik, M.; Musiał, W.; Peczyńska-Czoch, W.; Kaczmarek, Ł.; Kozubek, A. Membrane Perturbations Induced by New Analogs of Neocryptolepine. Biol. Pharm. Bull. 2012, 35, 1432–1439. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, S.; Zhang, X.; Li, C.; Ji, S.; Mao, H.; Jiang, X. Mitochondrion-specific dendritic lipopeptide liposomes for targeted sub-cellular delivery. Nat. Commun. 2021, 12, 2390. [Google Scholar] [CrossRef]
- Pan, D.; Sayanagi, J.; Acevedo-Cintrón, J.A.; Schellhardt, L.; Snyder-Warwick, A.K.; Mackinnon, S.E.; Wood, M.D. Liposomes embedded within fibrin gels facilitate localized macrophage manipulations within nerve. J. Neurosci. Methods 2020, 348, 108981. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, L.; Lin, Y.; Gerhard, E.M.; Jiang, X.; Li, L.; Yang, J.; Gu, Z. Enhanced anticancer efficacy of paclitaxel through multistage tumor-targeting liposomes modified with RGD and KLA peptides. Int. J. Nanomed. 2017, 12, 1517–1537. [Google Scholar] [CrossRef] [Green Version]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Mo, F.; Ma, J.; Zhang, P.; Zhang, D.; Fan, H.; Yang, X.; Zhi, L.; Zhang, J. Solubility and thermodynamic properties of baicalein in water and ethanol mixtures from 283.15 to 328.15 K. Chem. Eng. Commun. 2019, 208, 183–196. [Google Scholar] [CrossRef]
- Wang, S.X.; Wen, X.; Bell, C.; Appiah, S. Liposome-delivered baicalein induction of myeloid leukemia K562 cell death via reactive oxygen species generation. Mol. Med. Rep. 2018, 17, 4524–4530. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.-L.; Wang, Y.; Tsai, K.H.-Y.; Chang, H.-I. Liposome-Encapsulated Baicalein Suppressed Lipogenesis and Extracellular Matrix Formation in Hs68 Human Dermal Fibroblasts. Front. Pharmacol. 2018, 9, 155. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Wu, W.; Liu, Q.; Chen, S. Long-circulating nanoliposomes (LCNs) sustained delivery of baicalein (BAI) with desired oral bioavailability in vivo. Drug Deliv. 2013, 20, 319–323. [Google Scholar] [CrossRef]
- Di Sotto, A.; Paolicelli, P.; Nardoni, M.; Abete, L.; Garzoli, S.; Di Giacomo, S.; Mazzanti, G.; Casadei, M.A.; Petralito, S. SPC Liposomes as Possible Delivery Systems for Improving Bioavailability of the Natural Sesquiterpene β-Caryophyllene: Lamellarity and Drug-Loading as Key Features for a Rational Drug Delivery Design. Pharmaceutics 2018, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondria, cholesterol and cancer cell metabolism. Clin. Transl. Med. 2016, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Belfiore, L.; Aghaei, B.; Law, A.M.; Dobrowolski, J.C.; Raftery, L.J.; Tjandra, A.D.; Yee, C.; Piloni, A.; Volkerling, A.; Ferris, C.J.; et al. Generation and analysis of 3D cell culture models for drug discovery. Eur. J. Pharm. Sci. 2021, 163, 105876. [Google Scholar] [CrossRef] [PubMed]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures—Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef] [Green Version]
- Nowacka, M.; Sterzynska, K.; Andrzejewska, M.; Nowicki, M.; Januchowski, R. Drug resistance evaluation in novel 3D in vitro model. Biomed. Pharmacother. 2021, 138, 111536. [Google Scholar] [CrossRef]
- Olive, P.L.; Durand, R.E. Drug and radiation resistance in spheroids: Cell contact and kinetics. Cancer Metastasis Rev. 1994, 13, 121–138. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Khater, M.; Ravishankar, D.; Greco, F.; Osborn, H.M. Metal complexes of flavonoids: Their synthesis, characterization and enhanced antioxidant and anticancer activities. Futur. Med. Chem. 2019, 11, 2845–2867. [Google Scholar] [CrossRef]
- Rodríguez-Arce, E.; Saldías, M. Antioxidant properties of flavonoid metal complexes and their potential inclusion in the development of novel strategies for the treatment against neurodegenerative diseases. Biomed. Pharmacother. 2021, 143, 112236. [Google Scholar] [CrossRef]
- Wang, F.; Jiao, P.; Qi, M.; Frezza, M.; Dou, Q.; Yan, B. Turning Tumor-Promoting Copper into an Anti-Cancer Weapon via High-Throughput Chemistry. Curr. Med. Chem. 2010, 17, 2685–2698. [Google Scholar] [CrossRef] [Green Version]
- Kucharzewski, M.; Braziewicz, J.; Majewska, U.; Gozdz, S. Selenium, Copper, and Zinc Concentrations in Intestinal Cancer Tissue and in Colon and Rectum Polyps. Biol. Trace Element Res. 2003, 92, 1–10. [Google Scholar] [CrossRef]
- Fabris, C.; Farini, R.; Del Favero, G.; Gurrieri, G.; Piccoli, A.; Sturniolo, G.C.; Panucci, A.; Naccarato, R. Copper, zinc and copper/zinc ratio in chronic pancreatitis and pancreatic cancer. Clin. Biochem. 1985, 18, 373–375. [Google Scholar] [CrossRef]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like copper redox chemistry revisited: Hydrogen per-oxide and superoxide mediation of copper-catalyzed oxidant production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Goldstein, S.; Meyerstein, D.; Czapski, G. The Fenton reagents. Free. Radic. Biol. Med. 1993, 15, 435–445. [Google Scholar] [CrossRef]
- Przybylo, M.; Glogocka, D.; Dobrucki, J.W.; Fraczkowska, K.; Podbielska, H.; Kopaczynska, M.; Borowik, T.; Langner, M. The cellular internalization of liposome encapsulated protoporphyrin IX by HeLa cells. Eur. J. Pharm. Sci. 2016, 85, 39–46. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, W.Y.; Ko, Y.T. The Effect of Surface Charges on the Cellular Uptake of Liposomes Investigated by Live Cell Imaging. Pharm. Res. 2017, 34, 704–717. [Google Scholar] [CrossRef]
- Kim, M.W.; Niidome, T.; Lee, R. Glycol Chitosan-Docosahexaenoic Acid Liposomes for Drug Delivery: Synergistic Effect of Doxorubicin-Rapamycin in Drug-Resistant Breast Cancer. Mar. Drugs 2019, 17, 581. [Google Scholar] [CrossRef] [Green Version]
- Skupin-Mrugalska, P.; Minko, T. Development of Liposomal Vesicles for Osimertinib Delivery to EGFR Mutation—Positive Lung Cancer Cells. Pharmaceutics 2020, 12, 939. [Google Scholar] [CrossRef]















| Sample | BAI-L | Empty Liposomes |
|---|---|---|
| Size (nm) | 100.9 ± 2.7 | 99.6 ± 1.1 |
| PDI | 0.12 ± 0.02 | 0.14 ± 0.01 |
| Zeta potential (mV) | 9.8 ± 0.4 | 7.8 ± 0.3 |
| Time (h) | 0 | 24 | 48 |
|---|---|---|---|
| Size (nm) | 94.1 ± 1.3 | 93.3 ± 0.3 | 103.9 ± 2.9 |
| PDI | 0.13 ± 0.01 | 0.07 ± 0.01 | 0.15 ± 0.02 |
| Cell Line | ||||||
|---|---|---|---|---|---|---|
| AsPC-1 | BxPC-3 | NHDF | ||||
| Sample | IC50 48 h | IC50 72 h | IC50 48 h | IC50 72 h | IC50 48 h | IC50 72 h |
| BAI-L | 24.9 ± 4.2 | 21 ± 3.6 | 27.6 ± 4.1 | 23.1 ± 4.3 | Non-toxic | Non-toxic |
| Empty liposomes | 41.7 ± 1 | 51.9 ± 7 | 59.4 ± 34 | 112.3 ± 7.9 | Non-toxic | 140 ± 4.6 |
| BAI-DMSO | 30.4 ± 1.6 | 36 ± 3.6 | 34 ± 6.7 | 25.1 ± 7 | Non-toxic | 49.3 ± 12 |
| DMSO | Non-toxic | Non-toxic | Non-toxic | Non-toxic | Non-toxic | Non-toxic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowski, A.; Zaremba-Czogalla, M.; Jaromin, A.; Olczak, E.; Zygmunt, A.; Etezadi, H.; Boyd, B.J.; Gubernator, J. Novel Liposomal Formulation of Baicalein for the Treatment of Pancreatic Ductal Adenocarcinoma: Design, Characterization, and Evaluation. Pharmaceutics 2023, 15, 179. https://doi.org/10.3390/pharmaceutics15010179
Markowski A, Zaremba-Czogalla M, Jaromin A, Olczak E, Zygmunt A, Etezadi H, Boyd BJ, Gubernator J. Novel Liposomal Formulation of Baicalein for the Treatment of Pancreatic Ductal Adenocarcinoma: Design, Characterization, and Evaluation. Pharmaceutics. 2023; 15(1):179. https://doi.org/10.3390/pharmaceutics15010179
Chicago/Turabian StyleMarkowski, Adam, Magdalena Zaremba-Czogalla, Anna Jaromin, Ewa Olczak, Adrianna Zygmunt, Haniyeh Etezadi, Ben J. Boyd, and Jerzy Gubernator. 2023. "Novel Liposomal Formulation of Baicalein for the Treatment of Pancreatic Ductal Adenocarcinoma: Design, Characterization, and Evaluation" Pharmaceutics 15, no. 1: 179. https://doi.org/10.3390/pharmaceutics15010179