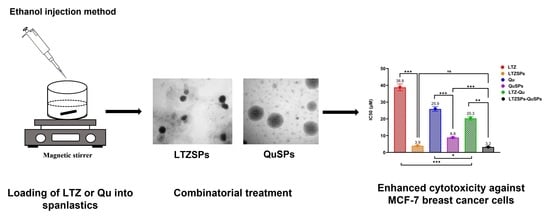
Combinatorial Therapy of Letrozole- and Quercetin-Loaded Spanlastics for Enhanced Cytotoxicity against MCF-7 Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LTZSPs and QuSPs
2.3. In Vitro Characterization of LTZSPs and QuSPs
2.3.1. Particle Size, Polydispersity Index and Zeta-Potential Measurements
2.3.2. Estimation of the Encapsulation Efficiencies of LTZSPs and QuSPs
drug concentration) × 100
2.3.3. Evaluation of LTZSPs and QuSPs Morphology
2.3.4. Fourier Transform Infrared Spectroscopy (FTIR) Studies
2.3.5. In Vitro Release Study of the Selected LTZSPs (L8) and QuSPs (Q5)
2.4. Ex Vivo Skin Permeation and Deposition Studies of LTZSPs and QuSPs
2.5. Cell Culture and Cell Viability Assay
2.6. Cell Cycle Analysis by Flow Cytometry
2.7. Assessment of Intracellular Reactive Oxygen Species (ROS) by ELISA
2.8. Statistical Analyses
3. Results and Discussion
3.1. Preparation and Characterization of Drug-Loaded Spanlastics
3.1.1. Particle Size of the Prepared LTZSPs and QuSPs
3.1.2. Encapsulation Efficiencies Percent (EE%) of the Prepared LTZSPs and QuSPs
3.1.3. Zeta Potential of the Prepared LTZSPs and QuSPs
3.1.4. TEM Measurements
3.1.5. Fourier-Transform Infrared (FTIR) Spectroscopy Studies
3.2. In Vitro Drug Release Study
3.3. Ex Vivo Permeation Study
3.4. Cell Viability Assay of LTZSPs, QuSPs, and Their Combinatorial Treatment
3.5. Cell Cycle Analysis by Flow Cytometry
3.6. Assessment of Reactive Oxygen Species (ROS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Houghton, S.C.; Hankinson, S.E. Cancer progress and priorities: Breast cancer. Cancer Epidemiol. Biomark. Prev. 2021, 30, 822–844. [Google Scholar] [CrossRef]
- Society, A.C. Breast Cancer Facts & Figures 2019–2020. 2019. Available online: https://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html (accessed on 15 January 2022).
- Ahmad, A. (Ed.) Breast Cancer Metastasis and Drug Resistance: Challenges and Progress, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Hart, C.D.; Migliaccio, I.; Malorni, L.; Guarducci, C.; Biganzoli, L.; Di Leo, A. Challenges in the management of advanced, ER-positive, HER2-negative breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 541–552. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Fang, L.; Liu, Y.; Sun, Y.; Wang, M.; Zhao, N.; He, Z. The transdermal patches for site-specific delivery of letrozole: A new option for breast cancer therapy. AAPS PharmSciTech 2010, 11, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.J. The what, why and how of aromatase inhibitors: Hormonal agents for treatment and prevention of breast cancer. Int. J. Clin. Pract. 2007, 61, 2051–2063. [Google Scholar] [CrossRef]
- Maniyar, M.; Chakraborty, A.; Kokare, C. Formulation and evaluation of letrozole-loaded spray dried liposomes with PEs for topical application. J. Liposome Res. 2020, 30, 274–284. [Google Scholar] [CrossRef]
- Cavadias, I.; Rouzier, R.; Lerebours, F.; Héquet, D. Hot flushes and breast cancer with positive hormone receptors: Mechanisms and management. Bull. Cancer 2020, 107, 1171–1185. [Google Scholar] [CrossRef]
- Nunes, F.A.P.; de Farias, M.L.F.; Oliveira, F.P.; Vieira, L.N.; Lima, L.F.C.; de Paula Paranhos, F.N.; de Mendonça, L.M.C.; Madeira, M. Use of aromatase inhibitors in patients with breast cancer is associated with deterioration of bone microarchitecture and density. Arch. Endocrinol. Metab. 2021, 65, 505–511. [Google Scholar] [CrossRef]
- Xi, H.; Yang, Y.; Zhao, D.; Fang, L.; Sun, L.; Mu, L.; Liu, J.; Zhao, N.; Zhao, Y.; Zheng, N.; et al. Transdermal patches for site-specific delivery of anastrozole: In vitro and local tissue disposition evaluation. Int. J. Pharm. 2010, 391, 73–78. [Google Scholar] [CrossRef]
- Regenthal, R.; Voskanian, M.; Baumann, F.; Teichert, J.; Brätter, C.; Aigner, A.; Abraham, G. Pharmacokinetic evaluation of a transdermal anastrozole-in-adhesive formulation. Drug Des. Devel. Ther. 2018, 12, 3653–3664. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Mostafa, S.K.; Helmy, M.W.; El Demellawy, M.A.; Sheweita, S.A. Multi-Reservoir phospholipid shell encapsulating protamine nanocapsules for co-delivery of letrozole and celecoxib in breast cancer therapy. Pharm. Res. 2017, 34, 1956–1969. [Google Scholar] [CrossRef]
- Jelovac, D.; Macedo, L.; Goloubeva, O.G.; Handratta, V.; Brodie, A.M. Additive antitumor effect of aromatase inhibitor letrozole and antiestrogen fulvestrant in a postmenopausal breast cancer model. Cancer Res. 2005, 65, 5439–5444. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, M.A.; Fumarola, C.; Alfieri, R.R.; La Monica, S.; Cavazzoni, A.; Galetti, M.; Gatti, R.; Belletti, S.; Harris, A.L.; Fox, S.B.; et al. Synergistic activity of letrozole and sorafenib on breast cancer cells. Breast Cancer Res. Treat. 2010, 124, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Yeter, Ç.; Özge, Ç. Anticancer effect of the letrozole-quercetin combination mediated by FOXOs and estrogen receptors in breast cancer cells. J. Res. Pharm. 2021, 25, 479–489. [Google Scholar] [CrossRef]
- Lamb, H.M.; Adkins, J.C. Letrozole. Drugs 1998, 56, 1125–1140. [Google Scholar] [CrossRef]
- Wang, R.; Yang, L.; Li, S.; Ye, D.; Yang, L.; Liu, Q.; Zhao, Z.; Cai, Q.; Tan, J.; Li, X. Quercetin inhibits breast cancer stem cells via downregulation of aldehyde dehydrogenase 1A1 (ALDH1A1), chemokine receptor type 4 (CXCR4), mucin 1 (MUC1), and epithelial cell adhesion molecule (EpCAM). Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 412. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.; Yang, L.; Li, P. Quercetin inhibits the proliferation and metastasis of human non-small cell lung cancer cell line: The key role of Src-mediated fibroblast growth factor-inducible 14 (Fn14)/nuclear factor kappa B (NF-κB) pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e920537-1–e920537-11. [Google Scholar] [CrossRef]
- Ong, C.S.; Tran, E.; Nguyen, T.T.; Ong, C.K.; Lee, S.K.; Lee, J.J.; Ng, C.P.; Leong, C.; Huynh, H. Quercetin-induced growth inhibition and cell death in nasopharyngeal carcinoma cells are associated with increase in Bad and hypophosphorylated retinoblastoma expressions. Oncol. Rep. 2004, 11, 727–733. [Google Scholar] [CrossRef]
- Han, C.; Gao, H.; Zhang, X. Quercetin anti-cancer effect in renal cancer through regulating survivin expression and caspase 3 activity. Med. One 2016, 1, 3. [Google Scholar]
- Zhang, X.-A.; Zhang, S.; Yin, Q.; Zhang, J. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharmacogn. Mag. 2015, 11, 404. [Google Scholar] [CrossRef]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018, 16, 108. [Google Scholar] [CrossRef]
- Angst, E.; Park, J.L.; Moro, A.; Lu, Q.-Y.; Lu, X.; Li, G.; King, J.; Chen, M.; Reber, H.A.; Go, V.L.W. The flavonoid quercetin inhibits pancreatic cancer growth in vitro and in vivo. Pancreas 2013, 42, 223–229. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019, 12, 55. [Google Scholar] [CrossRef]
- Ranganathan, S.; Halagowder, D.; Sivasithambaram, N.D. Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PLoS ONE 2015, 10, e0141370. [Google Scholar] [CrossRef]
- Schmitt-Ney, M.; Camussi, G. The PAX3-FOXO1 fusion protein present in rhabdomyosarcoma interferes with normal FOXO activity and the TGF-β pathway. PLoS ONE 2015, 10, e0121474. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133. [Google Scholar] [CrossRef]
- Tomoda, K.; Makino, K. Nanoparticles for transdermal drug delivery system (TDDS). In Colloid and Interface Science in Pharmaceutical Research and Development; Elsevier: Amsterdam, The Netherlands, 2014; pp. 131–147. [Google Scholar]
- Ansari, M.D.; Saifi, Z.; Pandit, J.; Khan, I.; Solanki, P.; Sultana, Y.; Aqil, M. Spanlastics a novel nanovesicular carrier: Its potential application and emerging trends in therapeutic delivery. AAPS PharmSciTech 2022, 23, 112. [Google Scholar] [CrossRef]
- Badria, F.; Mazyed, E. Formulation of nanospanlastics as a promising approach for improving the topical delivery of a natural leukotriene inhibitor (3- Acetyl-11-Keto-β-Boswellic Acid): Statistical optimization, in vitro characterization, and ex vivo permeation study. Drug Des. Dev. Ther. 2020, 14, 3697. [Google Scholar] [CrossRef]
- Sharma, A.; Pahwa, S.; Bhati, S.; Kudeshia, P. Spanlastics: A modern approach for nanovesicular drug delivery system. Int. J. Pharm. Sci. Res. 2020, 11, 1057–1065. [Google Scholar]
- Mazyed, E.A.; Helal, D.A.; Elkhoudary, M.M.; Abd Elhameed, A.G.; Yasser, M. Formulation and optimization of nanospanlastics for improving the bioavailability of green tea epigallocatechin gallate. Pharmaceuticals 2021, 14, 68. [Google Scholar] [CrossRef]
- Mohamed, H.B.; Attia Shafie, M.A.; Mekkawy, A.I. Chitosan nanoparticles for meloxicam ocular delivery: Development, in vitro characterization, and in vivo evaluation in a rabbit eye model. Pharmaceutics 2022, 14, 893. [Google Scholar] [CrossRef]
- Abdelbari, M.A.; El-Mancy, S.S.; Elshafeey, A.H.; Abdelbary, A.A. Implementing spanlastics for improving the ocular delivery of clotrimazole: In vitro characterization, ex vivo permeability, microbiological assessment and in vivo safety study. Int. J. Nanomed. 2021, 16, 6249–6261. [Google Scholar] [CrossRef]
- Allam, A.; Elsabahy, M.; El Badry, M.; Eleraky, N.E. Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma. Int. J. Pharm. 2021, 598, 120380. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.M.; El-Setouhy, D.A.; Ibrahim, A.B.; Habib, B.A.; Tayel, S.A.; Bayoumi, N.A. Penetration enhancer-containing spanlastics (PECSs) for transdermal delivery of haloperidol: In vitro characterization, ex vivo permeation and in vivo biodistribution studies. Drug Deliv. 2018, 25, 12–22. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Ahmed, A.A.; Osman, M.A. Penetration enhancers in proniosomes as a new strategy for enhanced transdermal drug delivery. Saudi Pharm. J. 2015, 23, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, D.A.; Aboelwafa, A.A.; Hamza, M.Y.; Mohamed, M.I. Topical delivery of fenoprofen calcium via elastic nano-vesicular spanlastics: Optimization using experimental design and in vivo evaluation. AAPS PharmSciTech 2017, 18, 2898–2909. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, H.; Lin, Z.; Chen, D.; Zhu, Y.; Hou, S.; Shi, X. Quercetin deformable liposome: Preparation and efficacy against ultraviolet B induced skin damages in vitro and in vivo. J. Photochem. Photobiol. B 2013, 127, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Enhanced non invasive trans-tympanic delivery of ciprofloxacin through encapsulation into nano-spanlastic vesicles: Fabrication, in-vitro characterization, and comparative ex-vivo permeation studies. Int. J. Pharm. 2017, 522, 157–164. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y. Effects of length and unsaturation of the alkyl chain on the hydrophobic binding of curcumin with Tween micelles. Food Chem. 2018, 246, 242–248. [Google Scholar] [CrossRef]
- Elhabak, M.; Ibrahim, S.; Abouelatta, S.M. Topical delivery of l-ascorbic acid spanlastics for stability enhancement and treatment of UVB induced damaged skin. Drug Deliv. 2021, 28, 445–453. [Google Scholar] [CrossRef]
- Kheradmandnia, S.; Vasheghani-Farahani, E.; Nosrati, M.; Atyabi, F. The effect of process variables on the properties of ketoprofen loaded solid lipid nanoparticles of beeswax and carnauba wax. Iran. J. Chem. Chem. Eng. 2010, 29, 181–187. [Google Scholar] [CrossRef]
- Ali, A.S.M.; Sarhan, H.A.; Magdy, T. Preparation and characterization of phenytoin sodium niosomes for enhanced closure of skin injuries. Int. J. Pharm. Pharm. Sci. 2014, 6, 542–546. [Google Scholar]
- Aburahma, M.H.; Abdelbary, G.A. Novel diphenyl dimethyl bicarboxylate provesicular powders with enhanced hepatocurative activity: Preparation, optimization, in vitro/in vivo evaluation. Int. J. Pharm. 2012, 422, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G.; El-Gendy, N. Niosome-encapsulated gentamicin for ophthalmic controlled delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Ferrara, F.; Hallan, S.S.; Baldisserotto, A.; Drechsler, M.; Malatesta, M.; Costanzo, M.; Cortesi, R.; Puglia, C.; Valacchi, G. Ethosomes and transethosomes for mangiferin transdermal delivery. Antioxidants 2021, 10, 768. [Google Scholar] [CrossRef]
- Dey, S.K.; Mandal, B.; Bhowmik, M.; Ghosh, L.K. Development and in vitro evaluation of Letrozole loaded biodegradable nanoparticles for breast cancer therapy. Braz. J. Pharm. Sci. 2009, 45, 585–591. [Google Scholar] [CrossRef]
- Albash, R.; Abdelbary, A.A.; Refai, H.; El-Nabarawi, M.A. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: In vitro, ex vivo, and in vivo evaluation. Int. J. Nanomed. 2019, 14, 1953–1968. [Google Scholar] [CrossRef]
- Badria, F.A.; Fayed, H.A.; Ibraheem, A.K.; State, A.F.; Mazyed, E.A. Formulation of sodium valproate nanospanlastics as a promising approach for drug repurposing in the treatment of androgenic alopecia. Pharmaceutics 2020, 12, 866. [Google Scholar] [CrossRef]
- Alaaeldin, E.; Mostafa, M.; Mansour, H.F.; Soliman, G.M. Spanlastics as an efficient delivery system for the enhancement of thymoquinone anticancer efficacy: Fabrication and cytotoxic studies against breast cancer cell lines. J. Drug Deliv. Sci. Technol. 2021, 65, 102725. [Google Scholar] [CrossRef]
- Mekkawy, A.I.; El-Mokhtar, M.A.; Nafady, N.A.; Yousef, N.; Hamad, M.A.; El-Shanawany, S.M.; Ibrahim, E.H.; Elsabahy, M. In vitro and in vivo evaluation of biologically synthesized silver nanoparticles for topical applications: Effect of surface coating and loading into hydrogels. Int. J. Nanomed. 2017, 12, 759–777. [Google Scholar] [CrossRef]
- Siddiqa, A.J.; Shrivastava, N.K.; Ali Mohsin, M.; Abidi, M.H.; Sharaf, M.A.F.; Shaikh, T.A. In vitro release and degradation study of letrozole-loaded poly (lactic-co-glycolic acid) microparticles. JOM 2021, 73, 450–459. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F.; Piccolella, S.; Marciano, S.; Nocera, P.; Pacifico, S. Silica/quercetin sol–gel hybrids as antioxidant dental implant materials. Sci. Technol. Adv. Mater. 2015, 16, 035001. [Google Scholar] [CrossRef] [PubMed]
- Mokale, V.J.; Patil, H.I.; Patil, A.P.; Shirude, P.R.; Naik, J.B. Formulation and optimisation of famotidine proniosomes: An in vitro and ex vivo study. J. Exp. Nanosci. 2016, 11, 97–110. [Google Scholar] [CrossRef]
- Maghsoodi, M.; Montazam, S.h.; Rezvantalab, H.; Jelvehgari, M. Response surface methodology for optimization of process variables of atorvastatin suspension preparation by microprecipitation method using desirability function. Pharm. Sci. 2020, 26, 61–74. [Google Scholar] [CrossRef]
- El-Sayed, M.M.; Hussein, A.K.; Sarhan, H.A.; Mansour, H.F. Flurbiprofen-loaded niosomes-in-gel system improves the ocular bioavailability of flurbiprofen in the aqueous humor. Drug Dev. Ind. Pharm. 2017, 43, 902–910. [Google Scholar] [CrossRef]
- Fathalla, D.; Youssef, E.M.; Soliman, G.M. Liposomal and ethosomal gels for the topical delivery of anthralin: Preparation, comparative evaluation and clinical assessment in psoriatic patients. Pharmaceutics 2020, 12, 446. [Google Scholar] [CrossRef]
- Mazyed, E.A.; Zakaria, S. Enhancement of dissolution characteristics of clopidogrel bisulphate by proniosomes. Int. J. Appl. Pharm. 2019, 11, 77–85. [Google Scholar] [CrossRef]
- ElMeshad, A.N.; Mohsen, A.M. Enhanced corneal permeation and antimycotic activity of itraconazole against Candida albicans via a novel nanosystem vesicle. Drug Deliv. 2016, 23, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, F.E.; Elsayed, I.; Gad, M.K.; Elshafeey, A.H.; Mohamed, M.I. Response surface optimization, Ex vivo and In vivo investigation of nasal spanlastics for bioavailability enhancement and brain targeting of risperidone. Int. J. Pharm. 2017, 530, 1–11. [Google Scholar] [CrossRef]
- Gupta, M.; Vaidya, B.; Mishra, N.; Vyas, S.P. Effect of surfactants on the characteristics of fluconazole niosomes for enhanced cutaneous delivery. Artif. Cells Blood Substit. Immobil. Biotechnol. 2011, 39, 376–384. [Google Scholar] [CrossRef]
- Tabbakhian, M.; Tavakoli, N.; Jaafari, M.R.; Daneshamouz, S. Enhancement of follicular delivery of finasteride by liposomes and niosomes 1. In vitro permeation and in vivo deposition studies using hamster flank and ear models. Int. J. Pharm. 2006, 323, 1–10. [Google Scholar] [CrossRef]
- Alaaeldin, E.; Abou-Taleb, H.A.; Mohamad, S.A.; Elrehany, M.; Gaber, S.S.; Mansour, H.F. Topical Nano-Vesicular Spanlastics of Celecoxib: Enhanced Anti-Inflammatory Effect and Down-Regulation of TNF-α, NF-κB and COX-2 in Complete Freund’s Adjuvant-Induced Arthritis Model in Rats. Int. J. Nanomed. 2021, 16, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Kanaani, L.; Javadi, I.; Ebrahimifar, M.; Ebrahimi Shahmabadi, H.; Akbarzadeh Khiyav, A.; Mehrdiba, T. Effects of cisplatin-loaded niosomal nanoparticleson BT-20 human breast carcinoma cells. Asian Pac. J. Cancer Prev. 2017, 18, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Shaker, D.S.; Shaker, M.A.; Hanafy, M.S. Cellular uptake, cytotoxicity and in-vivo evaluation of Tamoxifen citrate loaded niosomes. Int. J. Pharm. 2015, 493, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Fatemizadeh, M.; Tafvizi, F.; Shamsi, F.; Amiri, S.; Farajzadeh, A.; Akbarzadeh, I. Apoptosis Induction, Cell Cycle Arrest and Anti-Cancer Potential of Tamoxifen-Curcumin Loaded Niosomes Against MCF-7 Cancer Cells. Iran. J. Pathol. 2022, 17, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Sarieddine, R.; Alwattar, J.K.; Chouaib, R.; Gali-Muhtasib, H. Anticancer activity of thymoquinone cubic phase nanoparticles against human breast cancer: Formulation, cytotoxicity and subcellular localization. Int. J. Nanomed. 2020, 15, 9557–9570. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Bae, S.M.; Ahn, W.S. Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Arch. Pharm. Res. 2008, 31, 1281–1285. [Google Scholar] [CrossRef]
- Li, X.; Zhou, N.; Wang, J.; Liu, Z.; Wang, X.; Zhang, Q.; Liu, Q.; Gao, L.; Wang, R. Quercetin suppresses breast cancer stem cells (CD44+/CD24−) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018, 196, 56–62. [Google Scholar] [CrossRef]
- Niazvand, F.; Orazizadeh, M.; Khorsandi, L.; Abbaspour, M.; Mansouri, E.; Khodadadi, A. Effects of quercetin-loaded nanoparticles on MCF-7 human breast cancer cells. Medicina 2019, 55, 114. [Google Scholar] [CrossRef]
- Azria, D.; Larbouret, C.; Cunat, S.; Ozsahin, M.; Gourgou, S.; Martineau, P.; Evans, D.B.; Romieu, G.; Pujol, P.; Pèlegrin, A. Letrozole sensitizes breast cancer cells to ionizing radiation. Breast Cancer Res. 2005, 7, R156–R163. [Google Scholar] [CrossRef]
- Gibellini, L.; Pinti, M.; Nasi, M.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS metabolism in cancer cells: The potential role of quercetin. Cancers 2010, 2, 1288–1311. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Al-Rawas, A.M.; Al-Maqbali, M.; Al-Saleh, M.; Enriquez, M.B.; Al-Siyabi, S.; Al-Hashmi, K.; Al-Lawati, I.; Bulthuis, M.L. Systemic oxidative stress is increased in postmenopausal women and independently associates with homocysteine levels. Int. J. Mol. Sci. 2020, 21, 314. [Google Scholar] [CrossRef] [PubMed]
- Doshi, S.; Agarwal, A. The role of oxidative stress in menopause. J. Midlife Health 2013, 4, 140–146. [Google Scholar] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]







| No. | Formulation Code | Drug (mg/mL) | EA | EA (mg/mL) | Span 60 (mg/mL) | EA/Span 60 Ratio | |
|---|---|---|---|---|---|---|---|
| LTZ | Qu | ||||||
| 1 | L1 | 1.0 | Tween 80 | 2.0 | 8.0 | 20/80 | |
| 2 | L2 | 1.0 | Brij 35 | 2.0 | 8.0 | 20/80 | |
| 3 | L3 | 1.0 | Cremophor RH40 | 2.0 | 8.0 | 20/80 | |
| 4 | L4 | 1.0 | Tween 80 | 4.0 | 6.0 | 40/60 | |
| 5 | L5 | 1.0 | Brij 35 | 4.0 | 6.0 | 40/60 | |
| 6 | L6 | 1.0 | Cremophor RH40 | 4.0 | 6.0 | 40/60 | |
| 7 | L7 | 1.5 | Tween 80 | 4.0 | 6.0 | 40/60 | |
| 8 | L8 | 1.5 | Brij 35 | 4.0 | 6.0 | 40/60 | |
| 9 | L9 | 1.5 | Cremophor RH40 | 4.0 | 6.0 | 40/60 | |
| 10 | Q1 | 0.5 | Tween 80 | 2.0 | 8.0 | 20/80 | |
| 11 | Q2 | 0.5 | Cremophor RH40 | 2.0 | 8.0 | 20/80 | |
| 12 | Q3 | 0.5 | Tween 80 | 4.0 | 6.0 | 40/60 | |
| 13 | Q4 | 0.5 | Cremophor RH40 | 4.0 | 6.0 | 40/60 | |
| 14 | Q5 | 1.0 | Tween 80 | 2.0 | 8.0 | 20/80 | |
| 15 | Q6 | 1.0 | Cremophor RH40 | 2.0 | 8.0 | 20/80 | |
| 16 | Q7 | 1.0 | Tween 80 | 4.0 | 6.0 | 40/60 | |
| 17 | Q8 | 1.0 | Cremophor RH40 | 4.0 | 6.0 | 40/60 | |
| Formulations | * Average Particle Size (nm) ± SD | PDI ± SD | Zeta Potential (mV) ± SD | EE% ± SD |
|---|---|---|---|---|
| L1 | 242.1 ± 8.2 | 0.5 ± 0.06 | −19.9 ± 0.6 | 95.8 ± 0.5 |
| L2 | 240.3 ± 10.1 | 0.5 ± 0.03 | −25.4 ± 1.6 | 94.3 ± 0.5 |
| L3 | 310.3 ± 8.4 | 0.4 ± 0.02 | −9.9 ± 1.7 | 95.4 ± 0.8 |
| L4 | 192.0 ± 3.5 | 0.4 ± 0.04 | −43.8 ± 0.7 | 96.8 ± 1.2 |
| L5 | 129.0 ± 2.6 | 0.4 ± 0.01 | −39.6 ± 1.8 | 95.3 ± 0.5 |
| L6 | 257.0 ± 1.7 | 0.4 ± 0.02 | −21.6 ± 0.4 | 94.5 ± 0.6 |
| L7 | 188.5 ± 3.7 | 0.4 ± 0.01 | −28.8 ± 0.6 | 97.2 ± 0.8 |
| L8 | 164.9 ± 4.8 | 0.4 ± 0.01 | −35.5 ± 2.1 | 96.3 ± 0.3 |
| L9 | 289.7 ± 3.9 | 0.4 ± 0.04 | −7.8 ± 1.3 | 97.1 ± 0.5 |
| Q1 | 367.2 ± 40.1 | 0.5 ± 0.06 | −26.5 ± 1.4 | 99.4 ± 0.1 |
| Q2 | 406.4 ± 51.7 | 0.6 ± 0.08 | −19.2 ± 0.5 | 99.6 ± 0.1 |
| Q3 | 240.0 ± 79.6 | 0.6 ± 0.11 | −22.5 ± 0.6 | 97.9 ± 0.2 |
| Q4 | 560.3 ± 76.0 | 0.5 ± 0.06 | −17.6 ± 1.6 | 99.5 ± 0.2 |
| Q5 | 450.1 ± 45.4 | 0.2 ± 0.06 | −33.23 ± 2.0 | 99.1 ± 0.1 |
| Q6 | 480.6 ± 3.9 | 0.4 ± 0.04 | −23.6 ± 1.4 | 99.5 ± 0.1 |
| Q7 | 434.9 ± 91.3 | 0.6 ± 0.05 | −23.2 ± 0.2 | 99.0 ± 0.2 |
| Q8 | 498.4 ± 30.0 | 0.7 ± 0.01 | −18.6 ± 1.6 | 99.4 ± 0.2 |
| Formulation | Papp (×10−6) ± SD (×10−6) cm/s | Jss (mg cm−2 h−1) |
|---|---|---|
| LTZSPs | 2.25 ± 1.08 | 0.006 ± 0.003 |
| LTZ dispersion | 7.95 ± 1.67 ** | 0.021 ± 0.005 ** |
| QuSPs | 1.45 ± 0.28 ** | 0.005 ± 0.001 ** |
| Qu dispersion | 0.39 ± 0.02 | 0.0014 ± 0.00007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekkawy, A.I.; Eleraky, N.E.; Soliman, G.M.; Elnaggar, M.G.; Elnaggar, M.G. Combinatorial Therapy of Letrozole- and Quercetin-Loaded Spanlastics for Enhanced Cytotoxicity against MCF-7 Breast Cancer Cells. Pharmaceutics 2022, 14, 1727. https://doi.org/10.3390/pharmaceutics14081727
Mekkawy AI, Eleraky NE, Soliman GM, Elnaggar MG, Elnaggar MG. Combinatorial Therapy of Letrozole- and Quercetin-Loaded Spanlastics for Enhanced Cytotoxicity against MCF-7 Breast Cancer Cells. Pharmaceutics. 2022; 14(8):1727. https://doi.org/10.3390/pharmaceutics14081727
Chicago/Turabian StyleMekkawy, Aml I., Nermin E. Eleraky, Ghareb M. Soliman, Mohamed G. Elnaggar, and Marwa G. Elnaggar. 2022. "Combinatorial Therapy of Letrozole- and Quercetin-Loaded Spanlastics for Enhanced Cytotoxicity against MCF-7 Breast Cancer Cells" Pharmaceutics 14, no. 8: 1727. https://doi.org/10.3390/pharmaceutics14081727