Enhanced Solubility and Antitumor Activity of Annona Squamosa Seed Oil via Nanoparticles Stabilized with TPGS: Preparation and In Vitro and In Vivo Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines and Animals
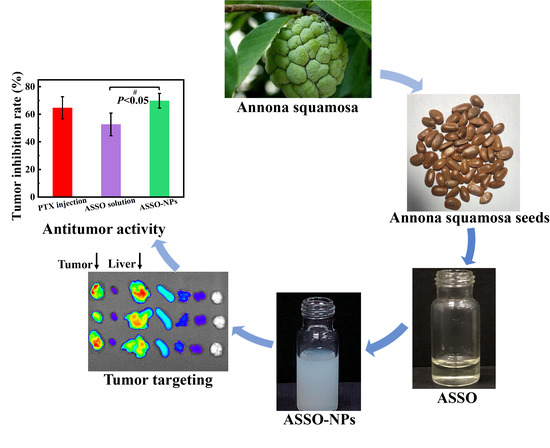
2.3. Extraction of ASSO
2.4. Preparation of ASSO-NPs
2.4.1. Screening Suitable Stabilizer of ASSO-NPs
2.4.2. Optimizing ASSO/Stabilizer Ratio and Preparation Concentration of ASSO-NPs
2.5. Characterization of ASSO-NPs
2.5.1. Dynamic Light Scattering Measurement
2.5.2. Morphology of ASSO-NPs
2.5.3. FT-IR Analysis
2.6. The Storage Stability and the Stability of ASSO-NPs in Physiological Media
2.7. The Pharmacological Evaluation of ASSO-NPs
2.7.1. In Vitro Cytotoxicity Assay
2.7.2. In Vivo Antitumor Activity in 4T1 Tumor-Bearing Mice
2.8. In Vivo Biodistribution Study Based ASSO-NPs
2.9. Statistical Analysis
3. Results and Discussion
3.1. Extraction of ASSO
3.2. Preparation of ASSO-NPs
3.2.1. Screening Suitable Stabilizer of ASSO-NPs
3.2.2. Optimizing Prescription of ASSO-NPs
3.3. Characterization of ASSO-NPs
3.4. The Storage Stability and the Stability of ASSO-NPs in Physiological Media
3.5. The Pharmacological Evaluation of ASSO-NPs
3.5.1. In Vitro Cytotoxicity Assay
3.5.2. In Vivo Antitumor Activity in 4T1 Tumor-Bearing Mice
3.6. In Vivo Biodistribution Study Based ASSO-NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, K.; Li, H.; Li, W.; Zhong, J.; Chen, Y.; Shen, C.; Yuan, C. Comparative transcriptomic analyses of normal and malformed flowers in sugar apple (Annona squamosa L.) to identify the differential expressed genes between normal and malformed flowers. BMC Plant Biol. 2017, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.A.; Millan-Hernandez, C.; Cline, A.R.; McElrath, T.C.; Irish, B.; Goenaga, R. Attraction of Pollinators to Atemoya (Annona squamosa × Annona cherimola) in Puerto Rico Using Commercial Lures and Food Attractants. J. Econ. Entomol. 2015, 108, 1923–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Chen, Y.; Chen, J.; Li, X.; Chen, Y. A Review on Annona squamosa L.: Phytochemicals and Biological Activities. Am. J. Chin. Med. 2017, 45, 933–964. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Hernández, G.; Vivar-Vera, M.D.L.; García-Magaña, M.D.L.; González-Silva, N.; Pérez-Larios, A.; Montalvo-González, E. Ultrasound-Assisted Extraction of Total Acetogenins from the Soursop Fruit by Response Surface Methodology. Molecules 2020, 25, 1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, D.K.; Singh, N.; Dev, K.; Sharma, R.; Sahai, M.; Palit, G.; Maurya, R. Anti-ulcer constituents of Annona squamosa twigs. Fitoterapia 2011, 82, 666–675. [Google Scholar] [CrossRef]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activities of 18-acetoxy-ent-kaur-16-ene from Annona squamosa L. bark. Inflammopharmacology 2011, 19, 111–115. [Google Scholar] [CrossRef]
- Kalidindi, N.; Thimmaiah, N.V.; Jagadeesh, N.V.; Nandeep, R.; Swetha, S.; Kalidindi, B. Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa Linn. leaves. J. Food Drug Anal. 2015, 23, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.S.; Rizwani, G.H.; Guo, H.; Ahmed, M.; Ahmed, M.; Hassan, S.Z.; Hassan, A.; Chen, Z.S.; Xu, R.H. Annona squamosa Linn: Cytotoxic activity found in leaf extract against human tumor cell lines. Pak. J. Pharm. Sci. 2014, 27, 1559–1563. [Google Scholar]
- Ruddaraju, L.K.; Pammi, S.V.; Pallela, P.V.; Padavala, V.S.; Kolapalli, V.R.M. Antibiotic potentiation and anti-cancer competence through bio-mediated ZnO nanoparticles. Mater. Sci. Eng. 2019, 103, 109756. [Google Scholar] [CrossRef]
- Vikas, B.; Anil, S.; Remani, P. Cytotoxicity Profiling of Annona Squamosa in Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2019, 20, 2831–2840. [Google Scholar] [CrossRef]
- Ma, C.Y.; Lu, J.H.; Li, X.; Liu, X.; Chen, J.W. Eight new cytotoxic annonaceous acetogenins from the seeds of Annona squamosa. Chin. J. Nat. Med. 2019, 17, 291–297. [Google Scholar] [CrossRef]
- Haykal, T.; Nasr, P.; Hodroj, M.H.; Taleb, R.I.; Sarkis, R.; Moujabber, M.N.E.; Rizk, S. Annona cherimola Seed Extract Activates Extrinsic and Intrinsic Apoptotic Pathways in Leukemic Cells. Toxins 2019, 11, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laguna-Hernández, G.; Brechú-Franco, A.E.; De la Cruz-Chacón, I.; González-Esquinca, A.R. A Histochemical Technique for the Detection of Annonaceous Acetogenins. In Histochemistry of Single Molecules; Humana Press: New York, NY, USA, 2017; Volume 1560, pp. 331–338. [Google Scholar] [CrossRef]
- Zahid, M.; Arif, M.; Rahman, M.A.; Singh, K.; Mujahid, M. Solvent Extraction and Gas Chromatography-Mass Spectrometry Analysis of Annona squamosa L. Seeds for Determination of Bioactives, Fatty Acid/Fatty Oil Composition, and Antioxidant Activity. J. Diet. Suppl. 2018, 15, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Bhoir, S.S.; Vishwapathi, V.; Singh, K.K. Antipsoriatic potential of Annona squamosa seed oil: An in vitro and in vivo evaluation. Phytomedicine 2019, 54, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N. Lipoxygenase inhibition by novel fatty acid ester from Annona squamosa seeds. J. Enzym. Inhib. Med. Chem. 2008, 23, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Shi, Y.; Ma, C.; Wang, X.; Li, Y.; Miao, Y.; Chen, J.; Li, X. Antitumor activity of Annona squamosa seed oil. J. Ethnopharmacol. 2016, 193, 362–367. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, Y.; Chen, J.; Wang, Y.; Xu, S.; Qiu, H.; Li, X. Chemical constituents and antitumor activity of fatty oil from Annona squamosa. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 109–111. [Google Scholar] [CrossRef]
- Setoguchi, S.; Hidaka, R.; Nagata-Akaho, N.; Watase, D.; Koga, M.; Matsunaga, K.; Karube, Y.; Takata, J. Novel Cationic Prodrug of Ubiquinol-10 Enhances Intestinal Absorption via Efficient Formation of Nanosized Mixed-Micelles with Bile Acid Anions. Molecules 2020, 25, 546. [Google Scholar] [CrossRef] [Green Version]
- Blanton, H.L.; Brelsfoard, J.; DeTurk, N.; Pruitt, K.; Narasimhan, M.; Morgan, D.J.; Guindon, J. Cannabinoids: Current and Future Options to Treat Chronic and Chemotherapy-Induced Neuropathic Pain. Drugs 2019, 79, 969–995. [Google Scholar] [CrossRef]
- Pires, F.Q.; da Silva, J.K.R.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Lipid nanoparticles as carriers of cyclodextrin inclusion complexes: A promising approach for cutaneous delivery of a volatile essential oil. Colloids Surf. B Biointerfaces 2019, 182, 110382. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Iacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amado, J.R.R.; Prada, A.L.; Diaz, J.G.; Souto, R.N.P.; Arranz, J.C.E.; de Souza, T.P. Development, larvicide activity, and toxicity in nontarget species of the Croton linearis Jacq essential oil nanoemulsion. Environ. Sci. Pollut. Res. 2020, 27, 9410–9423. [Google Scholar] [CrossRef] [PubMed]
- Sieniawska, E.; Świątek, Ł.; Wota, M.; Rajtar, B.; Polz-Dacewicz, M. Microemulsions of essentials oils—Increase of solubility and antioxidant activity or cytotoxicity? Food Chem. Toxicol. 2019, 129, 115–124. [Google Scholar] [CrossRef]
- Deda, D.K.; Iglesias, B.A.; Alves, E.; Araki, K.; Garcia, C.R.S. Porphyrin Derivative Nanoformulations for Therapy and Antiparasitic Agents. Molecules 2020, 25, E2080. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fu, X. Spheroidization on Fructus Mori polysaccharides to enhance bioavailability and bioactivity by anti-solvent precipitation method. Food Chem. 2019, 300, 125245. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Li, Y.; Li, Y.; Xiao, Y.; Kuang, H.; Wang, X. Annonaceous acetogenins nanosuspensions stabilized by PCL-PEG block polymer: Significantly improved antitumor efficacy. Int. J. Nanomed. 2016, 11, 3239–3253. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Fan, X.; Li, J.; Bi, F.; Fu, X.; Zhai, L. Experimental and theoretical studies on stability of new stabilizers for N-methyl-P-nitroaniline derivative in CMDB propellants. J. Hazard. Mater. 2017, 327, 187–196. [Google Scholar] [CrossRef]
- Sokol, M.B.; Nikolskaya, E.D.; Yabbarov, N.G.; Zenin, V.A.; Faustova, M.R.; Belov, A.V.; Zhunina, O.A.; Mollaev, M.D.; Zabolotsky, A.I.; Tereshchenko, O.G.; et al. Development of novel PLGA nanoparticles with co-encapsulation of docetaxel and abiraterone acetate for a highly efficient delivery into tumor cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1150–1158. [Google Scholar] [CrossRef]
- Kirimlioğlu, G.Y.; Öztürk, A.A. Levocetirizine Dihydrochloride-Loaded Chitosan Nanoparticles: Formulation and In Vitro Evaluation. Turk. J. Pharm. Sci. 2020, 17, 27–35. [Google Scholar] [CrossRef]
- Li, H.; Jin, K.; Luo, M.; Wang, X.; Zhu, X.; Liu, X.; Jiang, T.; Zhang, Q.; Wang, S.; Pang, Z. Size Dependency of Circulation and Biodistribution of Biomimetic Nanoparticles: Red Blood Cell Membrane-Coated Nanoparticles. Cells 2019, 8, 881. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, Y.; Chen, G.; Wei, P.; Ping, Q. PLGA nanoparticles for the oral delivery of 5-Fluorouracil using high pressure homogenization-emulsification as the preparation method and in vitro/in vivo studies. Drug Dev. Ind. Pharm. 2008, 34, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.S.; Sawant, K.K. Formulation optimization of etoposide loaded PLGA nanoparticles by double factorial design and their evaluation. Curr. Drug Deliv. 2010, 7, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, T.; Li, H.; Fu, J.; Ao, H.; Lu, L.; Han, M.; Guo, Y.; Yue, F.; Wang, X. Hydrous icaritin nanorods with excellent stability improves the in vitro and in vivo activity against breast cancer. Drug Deliv. 2020, 27, 228–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, B.B.; Dantas, F.G.D.S.; Galvão, F.; Cupozak-Pinheiro, W.J.; Wender, H.; Pizzuti, L.; Rosa, P.P.; Tenório, K.V.; Gatto, C.C.; Negri, M.; et al. Synthesis, structural characterization, and prospects for new cobalt (II) complexes with thiocarbamoyl-pyrazoline ligands as promising antifungal agents. J. Inorg. Biochem. 2020, 213, 111277. [Google Scholar] [CrossRef]
- Kamata, S.; Hashiyama, R.; Hana-Ika, H.; Ohkubo, I.; Saito, R.; Honda, A.; Anan, Y.; Akahoshi, N.; Noguchi, K.; Kanda, Y.; et al. Cytotoxicity comparison of 35 developmental neurotoxicants in human induced pluripotent stem cells (iPSC), iPSC-derived neural progenitor cells, and transformed cell lines. Toxicol. Vitr. 2020, 69, 104999. [Google Scholar] [CrossRef]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef]
- Li, Y.; Zhi, X.; Lin, J.; You, X.; Yuan, J. Preparation and characterization of DOX loaded keratin nanoparticles for pH/GSH dual responsive release. Mater. Sci. Eng. C 2017, 73, 189–197. [Google Scholar] [CrossRef]
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist. Updates 2020, 50, 100682. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Wang, J.; Cui, M.; Yeung, B.Z.; Cole, D.J.; Wientjes, M.G.; Au, J.L. Paclitaxel tumor priming promotes delivery and transfection of intravenous lipid-siRNA in pancreatic tumors. J. Control. Release 2015, 216, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Kisu, I.; Sakamoto, Y. Safety Evaluation of Paclitaxel Injection NK in Tri-Weekly Administration of Paclitaxel plus Carboplatin(TC Therapy) for Gynecological Cancers. Gan Kagaku Ryoho. Cancer Chemother. 2017, 44, 1007–1010. [Google Scholar]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, Q.; Wang, J.; Zhang, S.; Ding, B.; Wei, Y.; Dong, M.; Ryu, J.Y.; Yoon, T.Y.; Shi, X.; et al. Microfluidic Synthesis of Hybrid Nanoparticles with Controlled Lipid Layers: Understanding Flexibility-Regulated Cell-Nanoparticle Interaction. ACS Nano 2015, 9, 9912–9921. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Stabilizer | ASSO/Stabilizer Ratio | Concentration (mg/mL) | DLS Results | ||
|---|---|---|---|---|---|
| Size (nm) | PDI | Zeta Potential (mV) | |||
| Sodium oleate | 2:1 | 4 | 321.6 ± 6.3 | 0.49 ± 0.01 | 0.3 ± 0.1 |
| Solutol® HS 15 | 343.0 ± 23.0 | 0.58 ± 0.03 | −33.1 ± 0.9 | ||
| SDS | 311.8 ± 3.8 | 0.40 ± 0.08 | −89.8 ± 2.2 | ||
| TPGS | 253.1 ± 6.7 | 0.28 ± 0.03 | −0.6 ± 0.7 | ||
| Stabilizer | ASSO/Stabilizer Ratio | Concentration (mg/mL) | DLS Results | ||
|---|---|---|---|---|---|
| Size (nm) | PDI | Zeta Potential (mV) | |||
| TPGS | 2:1 | 4 | 253.1 ± 6.7 | 0.28 ± 0.03 | −0.6 ± 0.7 |
| 1:1 | 4 | 259.3 ± 3.3 | 0.20 ± 0.01 | −2.4 ± 0.8 | |
| 1:2 | 4 | 209.8 ± 2.1 | 0.21 ± 0.01 | −0.6 ± 0.3 | |
| 1:2 | 2 | 193.7 ± 4.1 | 0.10 ± 0.09 | −0.5 ± 0.3 | |
| 1:2 | 6 | 258.8 ± 2.5 | 0.27 ± 0.02 | −1.6 ± 0.2 | |
| Group | Liver Index | Spleen Index |
|---|---|---|
| Normal saline | 0.06149 ± 0.00573 | 0.03423 ± 0.00458 |
| PTX group | 0.06484 ± 0.00270 | 0.02370 ± 0.00304 ** |
| ASSO solution | 0.06105 ± 0.00604 | 0.02452 ± 0.00354 ** |
| ASSO-NPs | 0.06648 ± 0.00326 | 0.03150 ± 0.00490 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ao, H.; Lu, L.; Li, M.; Han, M.; Guo, Y.; Wang, X. Enhanced Solubility and Antitumor Activity of Annona Squamosa Seed Oil via Nanoparticles Stabilized with TPGS: Preparation and In Vitro and In Vivo Evaluation. Pharmaceutics 2022, 14, 1232. https://doi.org/10.3390/pharmaceutics14061232
Ao H, Lu L, Li M, Han M, Guo Y, Wang X. Enhanced Solubility and Antitumor Activity of Annona Squamosa Seed Oil via Nanoparticles Stabilized with TPGS: Preparation and In Vitro and In Vivo Evaluation. Pharmaceutics. 2022; 14(6):1232. https://doi.org/10.3390/pharmaceutics14061232
Chicago/Turabian StyleAo, Hui, Likang Lu, Manzhen Li, Meihua Han, Yifei Guo, and Xiangtao Wang. 2022. "Enhanced Solubility and Antitumor Activity of Annona Squamosa Seed Oil via Nanoparticles Stabilized with TPGS: Preparation and In Vitro and In Vivo Evaluation" Pharmaceutics 14, no. 6: 1232. https://doi.org/10.3390/pharmaceutics14061232