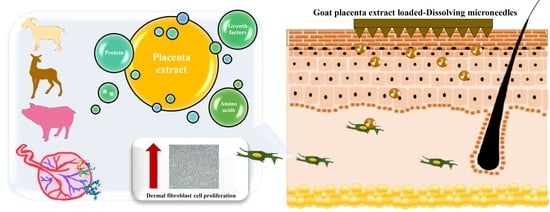
A Novel Approach for Skin Regeneration by a Potent Bioactive Placental-Loaded Microneedle Patch: Comparative Study of Deer, Goat, and Porcine Placentas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of DP, GP and PP
2.3. Determination of Protein, Growth Factors and Amino Acids
2.4. Cytotoxicity Study of Skin Cells
2.5. Determination of Cell Proliferation and Migration
2.6. Measurement of Skin Regeneration after UVB-Induced Skin Damage
2.7. Fabrication and Characterization of DMNs Loading Placenta Extract
2.8. In Vitro Skin Permeation Study
2.9. CLSM Study
2.10. Skin Histology
2.11. In Vivo Human Skin Study
2.12. Data Analysis
3. Results and Discussion
3.1. Physicochemical Properties of DP, GP and PP Extracts
3.2. Cytotoxicity of Placental Extracts on Skin Cells
3.3. Effect of Placental Extracts on Cell Proliferation and Migration
3.4. Skin Regeneration after UVB-Induced Skin Damage
3.5. DMN Loading of Bioactive Macromolecular Compounds
3.6. Skin Permeation and Deposition of Macromolecular Protein-Loaded DMNs
3.7. Skin Histology
3.8. Human Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.; Choi, W.; Yum, K.; Song, S.; Ock, S.; Park, S.; Kim, M. Efficacy and Safety of Human Placental Extract Solution on Fatigue: A Double-Blind, Randomized, Placebo-Controlled Study. Evid. Based Complement. Alternat. Med. 2012, 2012, 130875. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Chan, M.K.S.; Wong, M.B.F.; Klokol, D.; Chernykh, V. Placental therapy: An insight to their biological and therapeutic properties. J. Med. Ther. 2017, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Tebakari, M.; Daigo, Y.; Ishikawa, H.; Nakamura, M.; Kawashima, J.; Takano, F. Anti-inflammatory Effect of the Water-Soluble Portion of Porcine Placental Extract in Lipopolysaccharide-Stimulated RAW264.7 Murine Macrophage Cells. Biol. Pharm. Bull. 2018, 41, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.D.; Bhattacharyya, D. Isolation of fibronectin type III like peptide from human placental extract used as wound healer. J. Chromatogr. B 2005, 818, 67–73. [Google Scholar] [CrossRef]
- Park, S.Y.; Phark, S.; Lee, M.; Lim, J.Y.; Sul, D. Anti-oxidative and anti-inflammatory activities of placental extracts in benzo a pyrene-exposed rats. Placenta 2010, 31, 873–879. [Google Scholar] [CrossRef]
- Teng, D.; Fang, Y.; Song, X.; Gao, Y. Optimization of enzymatic hydrolysis parameters for antioxidant capacity of peptide from goat placenta. Food Bioprod. Process. 2011, 89, 202–208. [Google Scholar] [CrossRef]
- Bériot, M.; Tchimbou, A.F.; Barbato, O.; Beckers, J.F.; de Sousa, N.M. Identification of pregnancy-associated glycoproteins and alpha-fetoprotein in fallow deer (Dama dama) placenta. Acta Vet. Scand. 2014, 56, 4. [Google Scholar] [CrossRef] [Green Version]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair. Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [Green Version]
- El-Domyati, M.; El-Ammawi, T.S.; Moawad, O.; El-Fakahany, H.; Medhat, W.; Mahoney, M.G.; Uitto, J. Efficacy of mesotherapy in facial rejuvenation: A histological and immunohistochemical evaluation. Int. J. Dermatol. 2012, 51, 913–919. [Google Scholar] [CrossRef] [Green Version]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Tansathien, K.; Suriyaaumporn, P.; Charoenputtakhun, P.; Ngawhirunpat, T.; Opanasopit, P.; Rangsimawong, W. Development of Sponge Microspicule Cream as a Transdermal Delivery System for Protein and Growth Factors from Deer Antler Velvet Extract. Biol. Pharm. Bull. 2019, 42, 1207–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General Cytotoxicity Assessment by Means of the MTT Assay. In Protocols in In Vitro Hepatocyte Research; Vinken, M., Rogiers, V., Eds.; Springer: New York, NY, USA, 2015; pp. 333–348. [Google Scholar]
- Kansom, T.; Sajomsang, W.; Saeeng, R.; Charoensuksai, P.; Opanasopit, P.; Tonglairoum, P. Apoptosis Induction and Antimigratory Activity of Andrographolide Analog (3A.1)-Incorporated Self-Assembled Nanoparticles in Cancer Cells. AAPS PharmSciTech 2018, 19, 3123–3133. [Google Scholar] [CrossRef] [PubMed]
- Suriyaamporn, P.; Opanasopit, P.; Ngawhirunpat, T.; Rangsimawong, W. Computer-aided rational design for optimally Gantrez® S-97 and hyaluronic acid-based dissolving microneedles as a potential ocular delivery system. J. Drug Deliv. Sci. Technol. 2021, 61, 102319. [Google Scholar] [CrossRef]
- Tansathien, K.; Chareanputtakhun, P.; Ngawhirunpat, T.; Opanasopit, P.; Rangsimawong, W. Hair growth promoting effect of bioactive extract from deer antler velvet-loaded niosomes and microspicules serum. Int. J. Pharm. 2021, 597, 120352. [Google Scholar] [CrossRef]
- Mavon, A. Acetyl aspartic acid, a novel active ingredient, demonstrates potential to improve signs of skin ageing: From consumer need to clinical proof. Int. J. Cosmet. Sci. 2015, 37, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.W.; Song, S.H.; Kim, M. Synergistic effect of copper and amino acid mixtures on the production of extracellular matrix proteins in skin fibroblasts. Mol. Biol. Rep. 2021, 48, 3277–3284. [Google Scholar] [CrossRef]
- Russo, A.; Bonci, P.; Bonci, P. The effects of different preservation processes on the total protein and growth factor content in a new biological product developed from human amniotic membrane. Cell Tissue Bank. 2012, 13, 353–361. [Google Scholar] [CrossRef]
- Anitua, E.; Pino, A.; Orive, G. Plasma rich in growth factors promotes dermal fibroblast proliferation, migration and biosynthetic activity. J. Wound Care 2016, 25, 680–687. [Google Scholar] [CrossRef]
- De Araújo, R.; Lôbo, M.; Trindade, K.; Silva, D.F.; Pereira, N. Fibroblast Growth Factors: A Controlling Mechanism of Skin Aging. Skin Pharmacol. Physiol. 2019, 32, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.W.D.; Vickaryous, M.K.; Viloria-Petit, A.M. Signalling by Transforming Growth Factor Beta Isoforms in Wound Healing and Tissue Regeneration. Int. J. Dev. Biol. 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquirol Caussa, J.; Herrero Vila, E. Epidermal growth factor, innovation and safety. Med. Clin. 2015, 145, 305–312. [Google Scholar] [CrossRef]
- Aldag, C.; Nogueira Teixeira, D.; Leventhal, P.S. Skin rejuvenation using cosmetic products containing growth factors, cytokines, and matrikines: A review of the literature. Clin. Cosmet. Investig. Dermatol. 2016, 9, 411–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, F.; Huang, J.; Zhang, W.; Zhang, M.; Li, Z.; Si, L.; Long, X.; Wang, X. The Paracrine Effect of Adipose-Derived Stem Cells Orchestrates Competition between Different Damaged Dermal Fibroblasts to Repair UVB-Induced Skin Aging. Stem Cells Int. 2020, 2020, 8878370. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCrudden, M.T.C.; Zaid Alkilani, A.; Larrañeta, E.; McAlister, E.; Courtenay, A.J.; Kearney, M.-C.; Singh, T.R.R.; McCarthy, H.O.; Kett, V.L.; et al. Hydrogel-Forming Microneedles Prepared from “Super Swelling” Polymers Combined with Lyophilised Wafers for Transdermal Drug Delivery. PLoS ONE 2014, 9, e111547. [Google Scholar] [CrossRef]
- Kalluri, H.; Banga, A.K. Transdermal delivery of proteins. AAPS PharmSciTech 2011, 12, 431–441. [Google Scholar] [CrossRef]
- Andrews, S.N.; Jeong, E.; Prausnitz, M.R. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm. Res. 2013, 30, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.C.; Ling, M.H.; Kusuma, S.J. Poly-γ-glutamic Acid Microneedles with a Supporting Structure Design as a Potential Tool for Transdermal Delivery of Insulin. Acta Biomater. 2015, 24, 106–116. [Google Scholar] [CrossRef]
- Liu, S.; Wu, D.; Quan, Y.S.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Improvement of Transdermal Delivery of Exendin-4 Using Novel Tip-Loaded Microneedle Arrays Fabricated from Hyaluronic Acid. Mol. Pharm. 2016, 13, 272–279. [Google Scholar] [CrossRef]
- Chan, J.K. The wonderful colors of the hematoxylin-eosin stain in diagnostic surgical pathology. Int. J. Surg. Pathol. 2014, 22, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Layton, C. The hematoxylins and eosin. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Churchill Livingstone: London, UK, 2013; Volume 10, pp. 173–186. [Google Scholar]
- Kim, E.; Rebecca, V.W.; Fedorenko, I.V.; Messina, J.L.; Mathew, R.A.; Maria-Engler, S.S.; Basanta, D.; Smalley, K.S.; Anderson, A.R. Senescent fibroblasts can drive melanoma initiation and progression. arXiv 2013, arXiv:1304.1054. [Google Scholar]
- Liu, S.; Jin, M.N.; Quan, Y.S.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Transdermal delivery of relatively high molecular weight drugs using novel self-dissolving microneedle arrays fabricated from hyaluronic acid and their characteristics and safety after application to the skin. Eur. J. Pharm. Biopharm. 2014, 86, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Fan, L.M.; Dai, R.; Luan, M.; Xie, H.; Li, A.Q.; Li, L. Comparison of two series of non-invasive instruments used for the skin physiological properties measurements: The DermaLab® from cortex technology vs. the series of detectors from courage & khazaka. Skin Res. Technol. 2017, 23, 70–78. [Google Scholar] [CrossRef]
- Pires, L.R.; Vinayakumar, K.B.; Turos, M.; Miguel, V.; Gaspar, J. A Perspective on Microneedle-Based Drug Delivery and Diagnostics in Paediatrics. J. Pers. Med. 2019, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Vieira, D.; McEachern, F.; Filippelli, R.; Dimentberg, E.; Harvey, E.J.; Merle, G. Microelectrochemical Smart Needle for Real Time Minimally Invasive Oximetry. Biosensors 2020, 10, 157. [Google Scholar] [CrossRef]
- Pires, L.R.; Amado, I.R.; Gaspar, J. Dissolving microneedles for the delivery of peptides—Towards tolerance-inducing vaccines. Int. J. Pharm. 2020, 586, 119590. [Google Scholar] [CrossRef]

 ), DP extract (
), DP extract (  ), and PP extract (
), and PP extract (  ). Data are presented as the mean ± S.D. (n = 3). *, **, and *** indicate that the GP extract, DP extract, and PP extract were significantly different from the control group (untreated cells), respectively (p < 0.05).
). Data are presented as the mean ± S.D. (n = 3). *, **, and *** indicate that the GP extract, DP extract, and PP extract were significantly different from the control group (untreated cells), respectively (p < 0.05).
 ), DP extract (
), DP extract (  ), and PP extract (
), and PP extract (  ). Data are presented as the mean ± S.D. (n = 3). *, **, and *** indicate that the GP extract, DP extract, and PP extract were significantly different from the control group (untreated cells), respectively (p < 0.05).
). Data are presented as the mean ± S.D. (n = 3). *, **, and *** indicate that the GP extract, DP extract, and PP extract were significantly different from the control group (untreated cells), respectively (p < 0.05).
 ) and 72 h (
) and 72 h (  ) were significantly different from the untreated cells at 24 h (
) were significantly different from the untreated cells at 24 h (  ) (p < 0.05).
) (p < 0.05).
 ) and 72 h (
) and 72 h (  ) were significantly different from the untreated cells at 24 h (
) were significantly different from the untreated cells at 24 h (  ) (p < 0.05).
) (p < 0.05).







| Compounds | Contents (per 1 g of Extract) | ||
|---|---|---|---|
| GP Extract | DP Extract | PP Extract | |
| Protein | 705.51 ± 4.70 mg/g | 198.34 ± 3.26 mg/g | 151.44 ± 3.65 mg/g |
| Growth factors | |||
| EGF | 171.18 ± 8.65 pg/g | 39.02 ± 2.92 pg/g | 5.79 ± 2.47 pg/g |
| FGF | 401.79 ± 24.07 ng/g | 448.99 ± 4.29 ng/g | 65.34 ± 6.56 ng/g |
| IGF-1 | 155.58 ± 27.82 ng/g | 23.07 ± 3.19 ng/g | 40.38 ± 6.00 ng/g |
| TGF-β1 | 56.52 ± 15.61 pg/g | 14.21 ± 0.58 pg/g | 55.20 ± 1.21 pg/g |
| Compounds | Contents (per 1 g of Extract) | ||
|---|---|---|---|
| GP Extract | DP Extract | PP Extract | |
| Alanine | ND | 2841.08 µg/g | ND |
| Arginine | 0.23 µg/g | 34.94 µg/g | ND |
| Asparagine | ND | 1126.98 µg/g | ND |
| Aspartic acid | 23,842.50 µg/g | 1449.57 µg/g | ND |
| Cysteine | ND | ND | 124.72 µg/g |
| Glutamic acid | 15.45 µg/g | 1356.54 µg/g | 48.73 µg/g |
| Glycine | ND | 340.07 µg/g | ND |
| Histidine | 0.08 µg/g | 0.38 µg/g | 1.04 µg/g |
| Isoleucine | 6.16 µg/g | 1608.14 µg/g | 16.29 µg/g |
| Leucine | ND | 598.14 µg/g | 45.15 µg/g |
| Lysine | 47.69 µg/g | 3062.68 µg/g | 74.00 µg/g |
| Methionine | 40.14 µg/g | ND | 12.24 µg/g |
| Phenylalanine | 3.14 µg/g | ND | 26.63 µg/g |
| Serine | ND | 627.39 µg/g | 11.35 µg/g |
| Threonine | ND | 1757.01 µg/g | 38.64 µg/g |
| Tyrosine | 21.82 µg/g | 1860.82 µg/g | 16.96 µg/g |
| Valine | ND | 1027.41 µg/g | 51.43 µg/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tansathien, K.; Suriyaamporn, P.; Ngawhirunpat, T.; Opanasopit, P.; Rangsimawong, W. A Novel Approach for Skin Regeneration by a Potent Bioactive Placental-Loaded Microneedle Patch: Comparative Study of Deer, Goat, and Porcine Placentas. Pharmaceutics 2022, 14, 1221. https://doi.org/10.3390/pharmaceutics14061221
Tansathien K, Suriyaamporn P, Ngawhirunpat T, Opanasopit P, Rangsimawong W. A Novel Approach for Skin Regeneration by a Potent Bioactive Placental-Loaded Microneedle Patch: Comparative Study of Deer, Goat, and Porcine Placentas. Pharmaceutics. 2022; 14(6):1221. https://doi.org/10.3390/pharmaceutics14061221
Chicago/Turabian StyleTansathien, Kritsanaporn, Phuvamin Suriyaamporn, Tanasait Ngawhirunpat, Praneet Opanasopit, and Worranan Rangsimawong. 2022. "A Novel Approach for Skin Regeneration by a Potent Bioactive Placental-Loaded Microneedle Patch: Comparative Study of Deer, Goat, and Porcine Placentas" Pharmaceutics 14, no. 6: 1221. https://doi.org/10.3390/pharmaceutics14061221