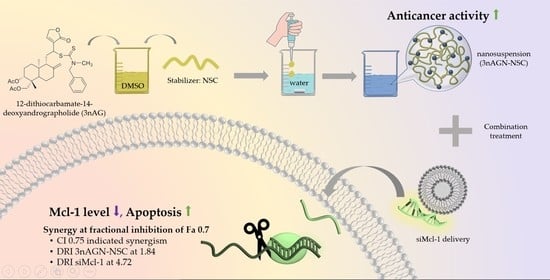
siRNA Targeting Mcl-1 Potentiates the Anticancer Activity of Andrographolide Nanosuspensions via Apoptosis in Breast Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. 3nAG Nanosuspension Preparation
2.2. Cytotoxicity of 3nAG, 3nAGN-NSC, and siMCl-1
2.3. Antitumor Interaction of Combination Treatment
2.4. Mcl-1 Expression of Andrographolide Treatment
2.5. Apoptosis Induction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. 3nAG Nanosuspension Preparation
4.3. Particle Size and Zeta Potential
4.4. Drug Content Analysis by High-Performance Liquid Chromatography (HPLC)
4.5. In Vitro Cytotoxicity
4.6. Antitumor Interaction of 3nAGN-NSC and siMcl-1 Delivery
4.7. Analysis by CompuSyn Software
4.8. mRNA Expression Level by Real-Time PCR
4.9. Apoptosis Evaluation
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, J.A.; Ahmed, S.; Jan, B.; Bender, O.; Al Hagbani, T.; Alqarni, A.; Anwar, S. Drugs repurposed: An advanced step towards the treatment of breast cancer and associated challenges. Biomed. Pharmacother. 2022, 145, 112375. [Google Scholar] [CrossRef]
- Lopes, C.M.; Dourado, A.; Oliveira, R. Phytotherapy and Nutritional Supplements on Breast Cancer. Biomed. Res. Int. 2017, 2017, 7207983. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wu, J.; Chen, Y.; Nie, J.; Chen, C. Activation of PI3K/AKT/mTOR Pathway Causes Drug Resistance in Breast Cancer. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Olopade, O.I.; Grushko, T.A.; Nanda, R.; Huo, D. Advances in Breast Cancer: Pathways to Personalized Medicine. Clin. Cancer Res. 2008, 14, 7988–7999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting MCL-1 in cancer: Current status and perspectives. J. Hematol. Oncol. 2021, 14, 67. [Google Scholar] [CrossRef]
- Mittal, P.; Singh, S.; Sinha, R.; Shrivastava, A.; Singh, A.; Singh, I.K. Myeloid cell leukemia 1 (MCL-1): Structural characteristics and application in cancer therapy. Inter. J. Biol. Macromol. 2021, 187, 999–1018. [Google Scholar] [CrossRef]
- Bolomsky, A.; Vogler, M.; Köse, M.C.; Heckman, C.A.; Ehx, G.; Ludwig, H.; Caers, J. MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents. J. Hematol. Oncol. 2020, 13, 173. [Google Scholar] [CrossRef]
- Li, A.; Keck, J.M.; Parmar, S.; Patterson, J.; Labrie, M.; Creason, A.L.; Johnson, B.E.; Downey, M.; Thomas, G.; Beadling, C.; et al. Characterizing advanced breast cancer heterogeneity and treatment resistance through serial biopsies and comprehensive analytics. NPJ Precis. Oncol. 2021, 5, 28. [Google Scholar] [CrossRef]
- Hernández-Lemus, E.; Martínez-García, M. Pathway-Based Drug-Repurposing Schemes in Cancer: The Role of Translational Bioinformatics. Front. Oncol. 2021, 10. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Tang, N.; Sun, D.; Lan, Y.; Yu, Z.; Zhao, X.; Feng, L.; Zhang, B.; Jin, L.; et al. Andrographolide inhibits breast cancer through suppressing COX-2 expression and angiogenesis via inactivation of p300 signaling and VEGF pathway. J. Exp. Clin. Cancer Res. 2018, 37, 248. [Google Scholar] [CrossRef] [Green Version]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pengnam, S.; Plianwong, S.; Yingyongnarongkul, B.-e.; Patrojanasophon, P.; Opanasopit, P. Delivery of small interfering RNAs by nanovesicles for cancer therapy. Drug Metab. Pharmacokinet. 2022, 42, 100425. [Google Scholar] [CrossRef] [PubMed]
- Leenders, F.; Möpert, K.; Schmiedeknecht, A.; Santel, A.; Czauderna, F.; Aleku, M.; Penschuck, S.; Dames, S.; Sternberger, M.; Röhl, T.; et al. PKN3 is required for malignant prostate cell growth downstream of activated PI 3-kinase. EMBO J. 2004, 23, 3303–3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Widden, H.; Placzek, W.J. The multiple mechanisms of MCL1 in the regulation of cell fate. Commun. Biol. 2021, 4, 1029. [Google Scholar] [CrossRef]
- Soo, H.L.; Quah, S.Y.; Sulaiman, I.; Sagineedu, S.R.; Lim, J.C.W.; Stanslas, J. Advances and challenges in developing andrographolide and its analogues as cancer therapeutic agents. Drug Discov. Today 2019, 24, 1890–1898. [Google Scholar] [CrossRef]
- Senichkin, V.V.; Streletskaia, A.Y.; Gorbunova, A.S.; Zhivotovsky, B.; Kopeina, G.S. Saga of Mcl-1: Regulation from transcription to degradation. Cell Death Differ. 2020, 27, 405–419. [Google Scholar] [CrossRef]
- Wen, Q.; Zhan, Y.; Zheng, H.; Zang, H.; Luo, J.; Zhang, Y.; Wang, W.; Feng, J.; Lu, J.; Chen, L.; et al. Elevated expression of mcl-1 inhibits apoptosis and predicts poor prognosis in patients with surgically resected non-small cell lung cancer. Diagn. Pathol. 2019, 14, 108. [Google Scholar] [CrossRef] [Green Version]
- Babu, A.; Munshi, A.; Ramesh, R. Combinatorial therapeutic approaches with RNAi and anticancer drugs using nanodrug delivery systems. Drug Dev. Ind. Pharm. 2017, 43, 1391–1401. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L. Biological properties of andrographolide, an active ingredient of Andrographis Paniculata: A narrative review. Ann. Transl. Med. 2021, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.; Parveen, R.; Parveen, B.; Zahiruddin, S.; Aasif Khan, M.; Khan, A.; Massey, S.; Ahmad, S.; Husain, S.A. Anticancer potential of andrographolide from Andrographis paniculata (Burm.f.) Nees and its mechanisms of action. J. Ethnopharmacol. 2021, 272, 113936. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Islam, M.A.; Shaw, S.; Khan, I.N.; Saravi, S.S.S.; Ahmad, S.; Rehman, S.; Gupta, V.K.; et al. Andrographolide, a diterpene lactone from Andrographis paniculata and its therapeutic promises in cancer. Cancer lett. 2018, 420, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Lim, S.H.; Sagineedu, S.R.; Lajis, N.H.; Stanslas, J. SRJ09, a promising anticancer drug lead: Elucidation of mechanisms of antiproliferative and apoptogenic effects and assessment of in vivo antitumor efficacy. Pharmacol. Res. 2016, 107, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.L.; Tan, H.K.; Oon, C.E.; Kuroyanagi, M.; Muhammad, T.S.T. Identification of genes involved in the regulation of 14-deoxy-11,12-didehydroandrographolide-induced toxicity in T-47D mammary cells. Food Chem. Toxicol. 2012, 50, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Arsakhant, P.; Sirion, U.; Chairoungdua, A.; Suksen, K.; Piyachaturawat, P.; Suksamrarn, A.; Saeeng, R. Design and synthesis of C-12 dithiocarbamate andrographolide analogues as an anticancer agent. Bioorg. Med. Chem. Lett. 2020, 30, 127263. [Google Scholar] [CrossRef]
- Agrawal, P.; Nair, M.S. An insight into the pharmacological and analytical potential of Andrographolide. Fundam. Clin. Pharmacol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Dumkliang, E.; Pamornpathomkul, B.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Yoksan, S.; Opanasopit, P. Feasibility of chitosan-based nanoparticles approach for intranasal immunisation of live attenuated Japanese encephalitis vaccine. Int. J. Biol. Macromol. 2021, 183, 1096–1105. [Google Scholar] [CrossRef]
- Khan, K.U.; Minhas, M.U.; Badshah, S.F.; Suhail, M.; Ahmad, A.; Ijaz, S. Overview of nanoparticulate strategies for solubility enhancement of poorly soluble drugs. Life Sci. 2022, 291, 120301. [Google Scholar] [CrossRef]
- Arora, D.; Khurana, B.; Rath, G.; Nanda, S.; Goyal, A.K. Recent Advances in Nanosuspension Technology for Drug Delivery. Curr. Pharm. Des. 2018, 24, 2403–2415. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of nanosuspensions in drug delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Liu, Y.; Xiao, Y.; Yang, X.; Su, W.; Zhang, M.; Liao, Y.; Kuang, H.; Wang, X. High drug payload curcumin nanosuspensions stabilized by mPEG-DSPE and SPC: In vitro and in vivo evaluation. Drug Deliv. 2017, 24, 109–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, M.; Chattopadhyay, S.; Choudhuri, T.; Bera, R.; Kumar, S.; Chakraborty, B.; Mukherjee, S.K. Cytotoxicity and cell cycle arrest induced by andrographolide lead to programmed cell death of MDA-MB-231 breast cancer cell line. J. Biomed. Sci. 2016, 23, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Zhang, N.; Chou, J.H.; Dong, H.-J.; Lin, S.-F.; Ulrich-Merzenich, G.S.; Chou, T.-C. Drug combination in vivo using combination index method: Taxotere and T607 against colon carcinoma HCT-116 xenograft tumor in nude mice. Synergy 2016, 3, 15–30. [Google Scholar] [CrossRef]
- Pengnam, S.; Plianwong, S.; Patrojanasophon, P.; Radchatawedchakoon, W.; Yingyongnarongkul, B.-e.; Opanasopit, P.; Charoensuksai, P. Synergistic Effect of Doxorubicin and siRNA-Mediated Silencing of Mcl-1 Using Cationic Niosomes against 3D MCF-7 Spheroids. Pharmaceutics 2021, 13, 550. [Google Scholar] [CrossRef]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Patrojanasophon, P. Catechol-modified chitosan/hyaluronic acid nanoparticles as a new avenue for local delivery of doxorubicin to oral cancer cells. Colloids Surf. B Biointerfaces 2020, 196, 111279. [Google Scholar] [CrossRef]
- Wang, L.; Du, J.; Zhou, Y.; Wang, Y. Safety of nanosuspensions in drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 455–469. [Google Scholar] [CrossRef]
- Shah, M.R.; Imran, M.; Ullah, S. Chapter 5—Nanosuspensions. In Lipid-Based Nanocarriers for Drug Delivery and Diagnosis; Shah, M.R., Imran, M., Ullah, S., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 139–172. [Google Scholar]
- Li, M.; Alvarez, P.; Orbe, P.; Bilgili, E. Multi-faceted Characterization of Wet-milled Griseofulvin Nanosuspensions for Elucidation of Aggregation State and Stabilization Mechanisms. AAPS PharmSciTech 2018, 19, 1789–1801. [Google Scholar] [CrossRef]
- Anstee, N.S.; Bilardi, R.A.; Ng, A.P.; Xu, Z.; Robati, M.; Vandenberg, C.J.; Cory, S. Impact of elevated anti-apoptotic MCL-1 and BCL-2 on the development and treatment of MLL-AF9 AML in mice. Cell Death Differ. 2019, 26, 1316–1331. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, R.; Lartigue, L.; Perkins, G. Targeting Mcl-1 and other Bcl-2 family member proteins in cancer therapy. Pharmacol. Ther. 2019, 195, 13–20. [Google Scholar] [CrossRef]
- Xiao, Y.; Nimmer, P.; Sheppard, G.S.; Bruncko, M.; Hessler, P.; Lu, X.; Roberts-Rapp, L.; Pappano, W.N.; Elmore, S.W.; Souers, A.J.; et al. MCL-1 Is a Key Determinant of Breast Cancer Cell Survival: Validation of MCL-1 Dependency Utilizing a Highly Selective Small Molecule Inhibitor. Mol. Cancer Ther. 2015, 14, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.-M.; Giltnane, J.M.; Balko, J.M.; Schwarz, L.J.; Guerrero-Zotano, A.L.; Hutchinson, K.E.; Nixon, M.J.; Estrada, M.V.; Sánchez, V.; Sanders, M.E.; et al. MYC and MCL1 Cooperatively Promote Chemotherapy-Resistant Breast Cancer Stem Cells via Regulation of Mitochondrial Oxidative Phosphorylation. Cell Metab. 2017, 26, 633–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.J.; Mason, S.M.; Winder, M.L.; Willemsen, R.B.E.; Cloix, C.; Lawson, H.; Rooney, N.; Dhayade, S.; Sims, A.H.; Blyth, K.; et al. Breast cancer dependence on MCL-1 is due to its canonical anti-apoptotic function. Cell Death Differ. 2021, 28, 2589–2600. [Google Scholar] [CrossRef]
- Su, M.; Mei, Y.; Sinha, S. Role of the Crosstalk between Autophagy and Apoptosis in Cancer. J. Oncol. 2013, 2013, 102735. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, J.R.; Bruno, P.M.; Gilbert, L.A.; Capron, K.L.; Lauffenburger, D.A.; Hemann, M.T. Defining principles of combination drug mechanisms of action. Proc. Natl. Acad. Sci. USA 2013, 110, E170. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440. [Google Scholar] [CrossRef] [Green Version]
- Parhi, P.; Mohanty, C.; Sahoo, S.K. Nanotechnology-based combinational drug delivery: An emerging approach for cancer therapy. Drug Discov. Today 2012, 17, 1044–1052. [Google Scholar] [CrossRef]
- Qin, S.-Y.; Cheng, Y.-J.; Lei, Q.; Zhang, A.-Q.; Zhang, X.-Z. Combinational strategy for high-performance cancer chemotherapy. Biomaterials 2018, 171, 178–197. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; He, Z.; Chen, M.; Ding, Y.; Yao, Y.; Duan, Y.; Zixuan, L.; Qi, C.; Zheng, L.; et al. Andrographolide Suppresses the Growth and Metastasis of Luminal-Like Breast Cancer by Inhibiting the NF-κB/miR-21-5p/PDCD4 Signaling Pathway. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Purwaningsih, E.; Susmiarsih, T.; Kusuma, I. Cytotoxicity of Pheophorbide and Andrographolide Combination on MCF-7 Cancer Cell Culture. J. Herbs Spices Med. Plants 2020, 26, 148–154. [Google Scholar] [CrossRef]
- Lim, S.-C.; Jeon, H.J.; Kee, K.H.; Lee, M.J.; Hong, R.; Han, S.I. Andrographolide induces apoptotic and non-apoptotic death and enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in gastric cancer cells. Oncol. Lett. 2017, 13, 3837–3844. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Das, B.; Sen, R.; Kundu, P.; Manna, A.; Sarkar, A.; Chowdhury, C.; Chatterjee, M.; Das, P. Andrographolide Analogue Induces Apoptosis and Autophagy Mediated Cell Death in U937 Cells by Inhibition of PI3K/Akt/mTOR Pathway. PLOS ONE 2015, 10, e0139657. [Google Scholar] [CrossRef] [Green Version]
- Jada, S.R.; Matthews, C.; Saad, M.S.; Hamzah, A.S.; Lajis, N.H.; Stevens, M.F.G.; Stanslas, J. Benzylidene derivatives of andrographolide inhibit growth of breast and colon cancer cells in vitro by inducing G(1) arrest and apoptosis. Br. J. Pharmacol. 2008, 155, 641–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, H.; Kapur, N.; Singh, R.; Sonpavde, G.; Lillard, J.W.; Singh, S. Andrographolide inhibits prostate cancer by targeting cell cycle regulators, CXCR3 and CXCR7 chemokine receptors. Cell Cycle 2016, 15, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Liu, T.; Liu, J.; Chen, Y.; Wang, Z. Andrographolide inhibits human hepatoma-derived Hep3B cell growth through the activation of c-Jun N-terminal kinase. Planta Med. 2007, 73, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, C.; Yacoub, A.; Hossein, H.; Martin, A.P.; Bareford, M.D.; Eulitt, P.; Yang, C.; Nephew, K.P.; Dent, P. Inhibition of MCL-1 in breast cancer cells promotes cell death in vitro and in vivo. Cancer Biol. Ther. 2010, 10, 903–917. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Yin, S.; Dong, Y.; Guo, X.; Fan, L.; Ye, M.; Hu, H. Autophagy-dependent EIF2AK3 activation compromises ursolic acid-induced apoptosis through upregulation of MCL1 in MCF-7 human breast cancer cells. Autophagy 2013, 9, 196–207. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.M.; Elion, D.L.; Rahman, B.; Hicks, D.J.; Sanchez, V.; Cook, R.S. Therapeutic inhibition of Mcl-1 blocks cell survival in estrogen receptor-positive breast cancers. Oncotarget 2019, 10, 5389–5402. [Google Scholar] [CrossRef] [Green Version]
- Kansom, T.; Sajomsang, W.; Saeeng, R.; Rojanarata, T.; Ngawhirunpat, T.; Patrojanasophon, P.; Opanasopit, P. Fabrication and characterization of andrographolide analogue (3A.1) nanosuspensions stabilized by amphiphilic chitosan derivatives for colorectal cancer therapy. J. Drug Deliv. Sci. Technol. 2019, 54, 101287. [Google Scholar] [CrossRef]







| Stabilizers | Size | PDI | Zeta Potential | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| NSC | 249.6 | 1.6 | 0.075 | 0.007 | −26.6 | 0.5 |
| BSC | 270.5 | 1.3 | 0.109 | 0.019 | −26.6 | 0.9 |
| OSC | 222.2 | 3.2 | 0.088 | 0.013 | −31.7 | 1.0 |
| Tween80 | 868.7 | 18.5 | 0.282 | 0.023 | −42.8 | 0.6 |
| SDS | 862.1 | 36.7 | 0.487 | 0.018 | −29.6 | 0.4 |
| 3nAG:NSC Ratios | Size | PDI | Zeta Potential | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| 0.5:1 | 239.20 | 3.82 | 0.209 | 0.009 | −28.93 | 1.01 |
| 1:1 | 213.53 | 2.25 | 0.062 | 0.007 | −19.17 | 1.25 |
| 1.5:1 | 222.50 | 1.49 | 0.085 | 0.027 | −25.63 | 0.23 |
| 2:1 | 235.50 | 0.79 | 0.052 | 0.030 | −27.30 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pengnam, S.; Charoensuksai, P.; Yingyongnarongkul, B.-e.; Saeeng, R.; Uludağ, H.; Patrojanasophon, P.; Opanasopit, P.; Plianwong, S. siRNA Targeting Mcl-1 Potentiates the Anticancer Activity of Andrographolide Nanosuspensions via Apoptosis in Breast Cancer Cells. Pharmaceutics 2022, 14, 1196. https://doi.org/10.3390/pharmaceutics14061196
Pengnam S, Charoensuksai P, Yingyongnarongkul B-e, Saeeng R, Uludağ H, Patrojanasophon P, Opanasopit P, Plianwong S. siRNA Targeting Mcl-1 Potentiates the Anticancer Activity of Andrographolide Nanosuspensions via Apoptosis in Breast Cancer Cells. Pharmaceutics. 2022; 14(6):1196. https://doi.org/10.3390/pharmaceutics14061196
Chicago/Turabian StylePengnam, Supusson, Purin Charoensuksai, Boon-ek Yingyongnarongkul, Rungnapha Saeeng, Hasan Uludağ, Prasopchai Patrojanasophon, Praneet Opanasopit, and Samarwadee Plianwong. 2022. "siRNA Targeting Mcl-1 Potentiates the Anticancer Activity of Andrographolide Nanosuspensions via Apoptosis in Breast Cancer Cells" Pharmaceutics 14, no. 6: 1196. https://doi.org/10.3390/pharmaceutics14061196