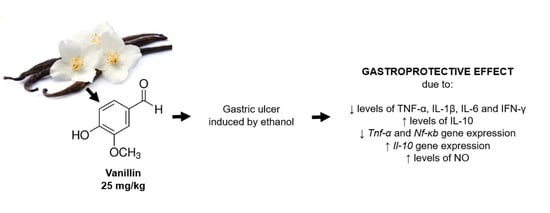
Anti-Inflammatory Effect of Vanillin Protects the Stomach against Ulcer Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethanol-Induced Gastric Ulcers
2.3. Histological Analysis
2.4. Measurement of Cytokine Levels
2.5. Gene Expression Analysis by Real-Time, Quantitative PCR (qPCR)
2.6. Levels of Nitrite/Nitrate (Total NOx) in the Stomach
2.7. Statistical Analysis
3. Results
3.1. Effect of Vanillin on Ethanol-Induced Gastric Ulcer
3.2. Histological Analysis
3.3. Effect of Vanillin in Gastric Inflammation
3.4. Effect of Vanillin in mRNA Expression
3.5. Effect of Vanillin in the Levels of Nitrite/Nitrate (Total NOx)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzokaky, A.A.; Abdelkader, E.M.; El-Dessouki, A.M.; Khaleel, S.A.; Raslan, N.A. C-phycocyanin protects against ethanol-induced gastric ulcers in rats: Role of HMGB1/NLRP3/NF-κB pathway. Basic Clin. Pharmacol. Toxicol. 2020, 127, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liao, H.; Liu, Y.; Zheng, Y.; Wu, X.; Su, Z.; Zhang, X.; Lai, Z.; Lai, X.; Lin, Z.-X.; et al. Protective effects of pogostone from Pogostemonis herba against ethanol-induced gastric ulcer in rats. Fitoterapia 2015, 100, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.; Nordin, N.; Hassandarvish, P.; Hajrezaie, M.; Azizan, A.H.S.; Fadaeinasab, M.; Majid, N.A.; Abdulla, M.A.; Hashim, N.M.; Ali, H.M. Methanol leaf extract of Actinodaphne sesquipedalis (Lauraceae) enhances gastric defense against ethanol-induced ulcer in rats. Drug Des. Devel. Ther. 2017, 11, 1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albaayit, S.F.A.; Abba, Y.; Abdullah, R.; Abdullah, N. Prophylactic effects of Clausena excavata Burum. f. leaf extract in ethanol-induced gastric ulcers. Drug Des. Devel. Ther. 2016, 10, 1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, G. Gastroenterology disease and lifestyle medicine. In Lifestyle Medicine; Springer: Cham, Switzerland, 2016; pp. 333–340. [Google Scholar]
- Boligon, A.A.; de Freitas, R.B.; de Brum, T.F.; Waczuk, E.P.; Klimaczewski, C.V.; de Ávila, D.S.; Athayde, M.L.; de Freitas Bauermann, L. Antiulcerogenic activity of Scutia buxifolia on gastric ulcers induced by ethanol in rats. Acta Pharm. Sin. B 2014, 4, 358–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, M.; Saluja, R.; Kanneganti, S.; Chinta, S.; Dikshit, M. Biochemical and molecular evaluation of neutrophil NOS in spontaneously hypertensive rats. Cell. Mol. Biol. 2007, 53, 84–93. [Google Scholar]
- Yoo, J.-H.; Park, E.-J.; Kim, S.H.; Lee, H.-J. Gastroprotective Effects of Fermented Lotus Root against Ethanol/HCl-Induced Gastric Mucosal Acute Toxicity in Rats. Nutrients 2020, 12, 808. [Google Scholar] [CrossRef] [Green Version]
- Hossen, M.J.; Hong, Y.D.; Baek, K.-S.; Yoo, S.; Hong, Y.H.; Kim, J.H.; Lee, J.-O.; Kim, D.; Park, J.; Cho, J.Y. In vitro antioxidative and anti-inflammatory effects of the compound K-rich fraction BIOGF1K, prepared from Panax ginseng. J. Ginseng Res. 2017, 41, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Paulrayer, A.; Adithan, A.; Lee, J.H.; Moon, K.H.; Kim, D.G.; Im, S.Y.; Kang, C.-W.; Kim, N.S.; Kim, J.-H. Aronia melanocarpa (black chokeberry) reduces ethanol-induced gastric damage via regulation of HSP-70, NF-κB, and MCP-1 signaling. Int. J. Mol. Sci. 2017, 18, 1195. [Google Scholar] [CrossRef]
- Golbabapour, S.; Hajrezaie, M.; Hassandarvish, P.; Abdul Majid, N.; Hadi, A.H.A.; Nordin, N.; Abdulla, M.A. Acute toxicity and gastroprotective role of M. pruriens in ethanol-induced gastric mucosal injuries in rats. Biomed Res Int. 2013, 2013, 974185. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Wong, I.C.K.; Leung, W.K. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: A population-based study. Gut 2017, 67, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Dou, Y.; Wu, X.; Li, H.; Wu, J.; Huang, Q.; Luo, D.; Yi, T.; Liu, Y.; Su, Z.; et al. Prophylactic efficacy of patchoulene epoxide against ethanol-induced gastric ulcer in rats: Influence on oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2018, 283, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Bezerra, C.F.; Camilo, C.J.; Do Nascimento Silva, M.K.; de Freitas, T.S.; Ribeiro-Filho, J.; Coutinho, H.D.M. Vanillin selectively modulates the action of antibiotics against resistant bacteria. Microb. Pathog. 2017, 113, 265–268. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, J.; Khang, G.; Lee, D. Reduction of inflammatory responses and enhancement of extracellular matrix formation by vanillin-incorporated poly(lactic-co-glycolic acid) scaffolds. Tissue Eng. Part A 2012, 18, 1967–1978. [Google Scholar] [CrossRef] [Green Version]
- Ho, K.; Yazan, L.S.; Ismail, N.; Ismail, M. Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol. 2009, 33, 155–160. [Google Scholar] [CrossRef]
- Pedroso, L.S.; Fávero, G.M.; de Camargo, L.E.A.; Mainardes, R.M.; Khalil, N.M. Effect of the o-methyl catechols apocynin, curcumin and vanillin on the cytotoxicity activity of tamoxifen. J. Enzym. Inhib. Med. Chem. 2013, 28, 734–740. [Google Scholar] [CrossRef]
- Al Asmari, A.; Al Shahrani, H.; Al Masri, N.; Al Faraidi, A.; Elfaki, I.; Arshaduddin, M. Vanillin abrogates ethanol induced gastric injury in rats via modulation of gastric secretion, oxidative stress and inflammation. Toxicol. Rep. 2016, 3, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Robert, A.; Nezamis, J.E.; Lancaster, C.; Hanchar, A.J. Cytoprotection by prostaglandins in rats: Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology 1979, 77, 433–443. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- El-Rady, A.; Nessren, M.; Dahpy, M.A.; Ahmed, A.; Elgamal, D.A.; Hadiya, S.; Ahmed, M.A.; Sayed, Z.E.-A.A.; Abdeltawab, D.; Abdelmohsen, A.S.; et al. Interplay of Biochemical, Genetic, and Immunohistochemical Factors in the Etio-Pathogenesis of Gastric Ulcer in Rats: A Comparative Study of the Effect of Pomegranate Loaded Nanoparticles Versus Pomegranate Peel Extract. Front. Physiol. 2021, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.C.; Vieira, A.J.; Beserra, F.P.; Pellizzon, C.H.; Nóbrega, R.H.; Rozza, A.L. Gastroprotective effect of limonene in rats: Influence on oxidative stress, inflammation and gene expression. Phytomedicine 2019, 53, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Sidahmed, H.M.A.; Vadivelu, J.; Loke, M.F.; Arbab, I.A.; Abdul, B.; Sukari, M.A.; Abdelwahab, S.I. Anti-ulcerogenic activity of dentatin from clausena excavata Burm. f. against ethanol-induced gastric ulcer in rats: Possible role of mucus and anti-oxidant effect. Phytomedicine 2019, 55, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Zhao, X.; Wang, W.; Zhang, Y.; Wang, H.; Liu, X.; Suo, H. Protective effect of silkworm pupa oil on hydrochloric acid/ethanol-induced gastric ulcers. J. Sci. Food Agric. 2019, 99, 2974–2986. [Google Scholar] [CrossRef]
- Mousa, A.M.; El-Sammad, N.M.; Hassan, S.K.; Abd El Nasser, A.M.; Hashim, A.N.; Moustafa, E.S.; Bakry, S.M.; Elsayed, E.A. Antiulcerogenic effect of Cuphea ignea extract against ethanol-induced gastric ulcer in rats. BMC Complement. Altern. Med. 2019, 19, 345. [Google Scholar] [CrossRef] [Green Version]
- Fahmy, N.M.; Al-Sayed, E.; Michel, H.E.; El-Shazly, M.; Singab, A.N.B. Gastroprotective effects of Erythrina speciosa (Fabaceae) leaves cultivated in Egypt against ethanol-induced gastric ulcer in rats. J. Ethnopharmacol. 2020, 248, 112297. [Google Scholar] [CrossRef]
- Luo, C.; Chen, H.; Wang, Y.; Lin, G.; Li, C.; Tan, L.; Su, Z.; Lai, X.; Xie, J.; Zeng, H. Protective effect of coptisine free base on indomethacin-induced gastric ulcers in rats: Characterization of potential molecular mechanisms. Life Sci. 2018, 193, 47–56. [Google Scholar] [CrossRef]
- Boutemine, I.-M.; Amri, M.; Amir, Z.-C.; Fitting, C.; Mecherara-Idjeri, S.; Layaida, K.; Sennoun, N.; Berkane, S.; Cavaillon, J.-M.; Touil-Boukoffa, C. Gastro-protective, therapeutic and anti-inflammatory activities of Pistacia lentiscus L. fatty oil against ethanol-induced gastric ulcers in rats. J. Ethnopharmacol. 2018, 224, 273–282. [Google Scholar] [CrossRef]
- Abdelfattah, M.S.; Elmallah, M.I.; Ebrahim, H.Y.; Almeer, R.S.; Eltanany, R.M.; Abdel Moneim, A.E. Prodigiosins from a marine sponge-associated actinomycete attenuate HCl/ethanol-induced gastric lesion via antioxidant and anti-inflammatory mechanisms. PLoS ONE 2019, 14, e0216737. [Google Scholar] [CrossRef]
- Antonisamy, P.; Subash-Babu, P.; Alshatwi, A.A.; Aravinthan, A.; Ignacimuthu, S.; Choi, K.C.; Kim, J.-H. Gastroprotective effect of nymphayol isolated from Nymphaea stellata (Willd.) flowers: Contribution of antioxidant, anti-inflammatory and anti-apoptotic activities. Chem. Biol. Interact. 2014, 224, 157–163. [Google Scholar] [CrossRef]
- Wang, R.; Sun, F.; Ren, C.; Zhai, L.; Xiong, R.; Yang, Y.; Yang, W.; Yi, R.; Li, C.; Zhao, X. Hunan insect tea polyphenols provide protection against gastric injury induced by HCl/ethanol through an antioxidant mechanism in mice. Food Funct. 2021, 12, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Salau, V.F.; Erukainure, O.L.; Olofinsan, K.A.; Ijomone, O.M.; Msomi, N.Z.; Islam, M. Vanillin modulates activities linked to dysmetabolism in psoas muscle of diabetic rats. Sci. Rep. 2021, 11, 18724. [Google Scholar] [CrossRef] [PubMed]
- Salau, V.F.; Erukainure, O.L.; Olofinsan, K.A.; Islam, M.S. Vanillin exerts therapeutic effects against hyperglycemia-altered glucose metabolism and purinergic activities in testicular tissues of diabetic rats. Reprod. Toxicol. 2021, 102, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.N.; Elsherbiny, N.M.; Shaheen, M.A.; Elseweidy, M.M. Modulation of NADPH oxidase and Nrf2/HO-1 pathway by vanillin in cisplatin-induced nephrotoxicity in rats. J. Pharm. Pharmacol. 2020, 72, 1546–1555. [Google Scholar] [CrossRef]





| Target Gene | Primer sequence 5′–3′ | Amplify Length (bp) | Annealing Temperature | NCBI Reference Sequence |
|---|---|---|---|---|
| TNFα | F: ATGGGCTCCCTCTCATCAGT | 60 °C | NM_012675.3 | |
| R: TGGTTTGCTACGACGTGGG | 100 | |||
| Nfκb | F: CCTCATCTTTCCCTCAGAGCC | 60 °C | NM_199267.2 | |
| R: CGCACTTGTAACGGAAACGC | 98 | |||
| Il10 | F: GACGCTGTCATCGATTTCTCC | 60 °C | NM_012854.2 | |
| R: GCTCCAAGACAAAGGTGTCTAC | 95 | |||
| βactin | F: CCCTGGCTCCTAGCACCAT | 60 °C | NM_031144.3 | |
| R: GATAGAGCCACCAATCCACACA | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciciliato, M.P.; de Souza, M.C.; Tarran, C.M.; de Castilho, A.L.T.; Vieira, A.J.; Rozza, A.L. Anti-Inflammatory Effect of Vanillin Protects the Stomach against Ulcer Formation. Pharmaceutics 2022, 14, 755. https://doi.org/10.3390/pharmaceutics14040755
Ciciliato MP, de Souza MC, Tarran CM, de Castilho ALT, Vieira AJ, Rozza AL. Anti-Inflammatory Effect of Vanillin Protects the Stomach against Ulcer Formation. Pharmaceutics. 2022; 14(4):755. https://doi.org/10.3390/pharmaceutics14040755
Chicago/Turabian StyleCiciliato, Murilo Piologo, Matheus Chiaradia de Souza, Carolina Mendes Tarran, Ana Laura Tironi de Castilho, Ana Júlia Vieira, and Ariane Leite Rozza. 2022. "Anti-Inflammatory Effect of Vanillin Protects the Stomach against Ulcer Formation" Pharmaceutics 14, no. 4: 755. https://doi.org/10.3390/pharmaceutics14040755