Development of Peptide Biopharmaceuticals in Russia
Abstract
:1. Introduction
2. Nomenclature, Classification, and the Roles of Endogenous Peptides
3. Therapeutic Applications of Peptides
4. Problems Associated with Manufacturing Synthetic Peptides
5. The Main Strategies for Developing the Next Generation of Peptide Drugs
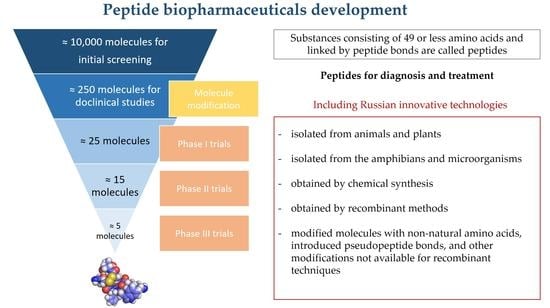
6. Primary Strategies for Developing the Next Generation of Peptide Drugs in Russia
7. Ongoing Clinical Trials for Peptide Drug Applications in Russia
7.1. Drugs Targeting AD
7.2. Inflammation
7.3. Cerebral Ischemia
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fischer, E. Untersuchungen über Aminosäuren, Polypeptide und Proteïne (1899–1906): Manuldruck 1925; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Góngora-Benítez, M.; Tulla-Puche, J.; Albericio, F. Multifaceted roles of disulfide bonds. peptides as therapeutics. Chem. Rev. 2014, 114, 901–926. [Google Scholar] [CrossRef] [PubMed]
- Sun, L. Peptide-based drug development. Mod. Chem. Appl. 2013, 1, e103. [Google Scholar] [CrossRef]
- Moore, A. The big and small of drug discovery. Biotech versus pharma: Advantages and drawbacks in drug development. EMBO Rep. 2003, 4, 114–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.H.; Ahmad, K.; Saeed, M.; Alharbi, A.M.; Barreto, G.E.; Ashraf, G.M.; Choi, I. Peptide based therapeutics and their use for the treatment of neurodegenerative and other diseases. Biomed. Pharmacother. 2018, 103, 574–581. [Google Scholar] [CrossRef] [PubMed]
- La Manna, S.; Di Natale, C.; Florio, D.; Marasco, D. Peptides as Therapeutic Agents for Inflammatory-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thundimadathil, J. Cancer Treatment Using Peptides: Current Therapies and Future Prospects. J. Amino Acids 2012, 2012, 967347. [Google Scholar] [CrossRef] [Green Version]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2019, 120, 3210–3229. [Google Scholar] [CrossRef] [Green Version]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem. 2018, 61, 1382–1414. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Zaykov, A.N.; Mayer, J.P.; Dimarchi, R.D. Pursuit of a perfect insulin. Nat. Rev. Drug Discov. 2016, 15, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Millington, G.W.M. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr. Metab. 2007, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, M. Are Neuropeptides Brain Hormones? J. Neuroendocrinol. 2011, 23, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Catt, K.J.; Dufau, M.L. Basic concepts of the mechanism of action of peptide hormones. Biol. Reprod. 1976, 14, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, R.M.; Dijkstra, P.D.; Hofmann, H.A. Complex structural and regulatory evolution of the pro-opiomelanocortin gene family. Gen. Comp. Endocrinol. 2014, 195, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Schulte, I.; Tammen, H.; Selle, H.; Schulz-Knappe, P. Peptides in body fluids and tissues as markers of disease. Exp. Rev. Mol. Diagn. 2005, 5, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Deigin, V.I.; Semenets, T.N.; Zamulaeva, I.A.; Maliutina, Y.V.; Selivanova, E.I.; Saenko, A.S.; Semina, O.V. The effects of the EW dipeptide optical and chemical isomers on the CFU-S population in intact and irradiated mice. Int. Immunopharmacol. 2007, 7, 375–382. [Google Scholar] [CrossRef]
- Pagel-Langenickel, I. Evolving role of natriuretic peptides from diagnostic tool to therapeutic modality. Adv. Exp. Med. Biol. 2018, 1067, 109–131. [Google Scholar] [CrossRef]
- Henry, M.S.; Gendron, L.; Tremblay, M.E.; Drolet, G. Enkephalins: Endogenous analgesics with an emerging role in stress resilience. Neural Plast. 2017, 2017, 1546125. [Google Scholar] [CrossRef]
- Shende, P.; Desai, D. Physiological and Therapeutic Roles of Neuropeptide Y on Biological Functions. Adv. Exp. Med. Biol. 2020, 1237, 37–47. [Google Scholar] [CrossRef]
- Mazziotti, G.; Mosca, A.; Frara, S.; Vitale, G.; Giustina, A. Somatostatin analogs in the treatment of neuroendocrine tumors: Current and emerging aspects. Exp. Opin. Pharmacother. 2017, 18, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Ou, L.; Wang, W.; Guo, D.Y. Gastrin, Cholecystokinin, Signaling, and Biological Activities in Cellular Processes. Front. Endocrinol. 2020, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, depression, and the microbiome: A role for gut peptides. Neurotherapeutic 2017, 15, 36–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barykin, E.P.; Garifulina, A.I.; Tolstova, A.P.; Anashkina, A.A.; Adzhubei, A.A.; Mezentsev, Y.V.; Shelukhina, I.V.; Kozin, S.A.; Tsetlin, V.I.; Makarov, A.A. Tetrapeptide Ac-HAEE-NH2 Protects α4β2 nAChR from Inhibition by Aβ. Int. J. Mol. Sci. 2020, 21, 6272. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Ben-Said, L.; Gaudreau, H.; Bedart, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2020, 45, fuaa039. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Kim, J.I.; Lee, I.; Park, S.; Bae, J.-Y.; Park, M.-S. Towards the application of human defensins as antivirals. Biomol. Ther. 2018, 26, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Knappe, P.; Schrader, M.; Zucht, H.-D. The peptidomics concept. Comb. Chem. 2005, 8, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Moiola, M.; Memeo, M.G.; Quadrelli, P. Stapled peptides—A useful improvement for peptide-based drugs. Molecules 2019, 24, 3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. 2010, 15, 40–56. [Google Scholar] [CrossRef]
- Peptide Therapeutics Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2021–2026. Available online: https://www.imarcgroup.com/peptide-therapeutics-market (accessed on 16 December 2021).
- Kaspar, A.A.; Reichert, J.M. Future directions for peptide therapeutics development. Drug Discov. 2013, 18, 807–817. [Google Scholar] [CrossRef]
- Agyei, D.; Ahmed, I.; Akram, Z.; Iqbal, M.N. Protein and peptide biopharmaceuticals: An overview. Protein Pept. Biopharm. Overv. 2017, 24, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Agyei, D.; Danquah, M. Industrial-scale manufacturing of pharmaceutical-grade bioactive peptides. Biotechnol. Adv. 2011, 39, 272–277. [Google Scholar] [CrossRef]
- Muheem, A.; Shakeel, F.; Jahangir, M.A.; Anwar, M.; Mallick, N.; Jain, G.K.; Warsi, M.H.; Ahmad, F.J. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm. J. 2016, 24, 413–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brayden, D.J.; Mrsny, R.J. Oral peptide delivery: Prioritizing the leading technologies. Ther. Deliv. 2011, 2, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Di, L. Strategic Approaches to Optimizing Peptide ADME Properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Ratnaparkhi, M.P.; Pandya, C.S.P. Peptides and proteins in pharmaceuticals. Int. J. Curr. Pharm. Res. 2011, 3, 1–9. [Google Scholar]
- Park, K.; Kwon, I.C.; Park, K. Oral protein delivery: Current status and future prospect. React. Funct. Polym. 2011, 71, 280–287. [Google Scholar] [CrossRef]
- Antunes, F.; Andrade, F.; Ferreira, D.; Morck Nielsen, H.; Sarmento, B. Models to Predict Intestinal Absorption of Therapeutic Peptides and Proteins. Curr. Drug Metab. 2012, 14, 4–20. [Google Scholar] [CrossRef]
- Wöhr, T.; Wahl, F.; Nefzi, A.; Rohwedder, B.; Sato, T.; Sun, X.; Mutter, M. Pseudo-prolines as a solubilizing, structure-disrupting protection technique in peptide synthesis. J. Am. Chem. Soc. 1996, 118, 9218–9227. [Google Scholar] [CrossRef]
- Harris, P.W.R.; Kowalczyk, R.; Hay, D.L.; Brimble, M.A. A single pseudoproline and microwave solid phase peptide synthesis facilitates an efficient synthesis of human amylin 1–37. Int. J. Pept. Res. Ther. 2013, 19, 147–155. [Google Scholar] [CrossRef]
- Räder, A.F.B.; Reichart, F.; Weinmuller, M.; Kessler, H. Improving oral bioavailability of cyclic peptides by N-methylation. Bioorg. Med. Chem. 2018, 26, 2766–2773. [Google Scholar] [CrossRef] [PubMed]
- Munegumi, T. Hydrophobicity of peptides containing D-amino acids. Chem. Biodivers. 2010, 7, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Ano, R.; Kimura, Y.; Shima, M.; Matsuno, R.; Ueno, T.; Akamatsu, M. Relationships between structure and high-throughput screening permeability of peptide derivatives and related compounds with artificial membranes: Application to prediction of Caco-2 cell permeability. Bioorg. Med. Chem. 2004, 12, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Viswanathan, M.; Kent, R.B.; Wood, C.R. Therapeutic peptides: Technological advances driving peptides into development. Curr. Opin. Biotechnol. 2006, 17, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.; Meibohm, B. Pharmacokinetics and pharmacokinetic–pharmacodynamic correlations of therapeutic peptides. Clin. Pharmacokinet. 2013, 52, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Rand, A.C.; Leung, S.S.F.; Eng, H.; Rotter, C.J.; Sharma, R.; Kalgutkar, A.; Zhang, Y.; Varma, M.V.; Farley, K.A.; Khunte, B.; et al. Optimizing PK properties of cyclic peptides: The effect of side chain substitutions on permeability and clearance. Medchemcomm 2012, 3, 1282–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdine, G.; Hilinski, G.J. Stapled peptides for intracellular drug targets. Methods Enzymol. 2012, 503, 3–33. [Google Scholar] [CrossRef]
- Jevševar, S.; Kunstelj, M.; Porekar, V.G. PEGylation of therapeutic proteins. Wiley Online Libr. 2010, 5, 113–128. [Google Scholar] [CrossRef] [Green Version]
- Werle, M.; Bernkop-Schnürch, A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids 2006, 30, 351–367. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [Green Version]
- Antosova, Z.; Mackova, M.; Kral, V.; Macek, T. Therapeutic application of peptides and proteins: Parenteral forever? Trends Biotechnol. 2009, 27, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.P.; Ottaway, N.L.; Perez-Tilve, D.; Ma, D.; Gelfanov, V.M.; Tschop, M.H.; DiMarchi, R.D. Peptide lipidation stabilizes structure to enhance biological function. Mol. Metab. 2013, 2, 468–479. [Google Scholar] [CrossRef]
- Tugyi, R.; Uray, K.; Iván, D.; Fellinger, E.; Perkins, A.; Hubecz, F. Partial D-amino acid substitution: Improved enzymatic stability and preserved Ab recognition of a MUC2 epitope peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rink, R.; Arkema-Meter, A.; Baudoin, I.; Post, E.; Kuipers, A.; Nelemans, S.A.; Akanbi, H.J.; Moll, G.N. To protect peptide pharmaceuticals against peptidases. J. Pharmacol. Toxicol. Methods 2010, 61, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Sharman, A.; Low, J. Vasopressin and its role in critical care. Contin. Educ. Anaesth. Crit. Care 2008, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Agersø, H.; Larsen, L.S.; Riis, A.; Lövgren, U.; Karlsson, M.O.; Senderovitz, T. Pharmacokinetics and renal excretion of desmopressin after intravenous administration to healthy subjects and renally impaired patients. Wiley Online Libr. 2004, 58, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Harris, A.G. Somatostatin and somatostatin analogues: Pharmacokinetics and pharmacodynamic effects. Gut 1994, 35, S1–S4. [Google Scholar] [CrossRef] [Green Version]
- Kilian, G.; Jamie, H.; Brauns, S.C.A.; Dyason, K.; Milne, P.J. Biological activity of tyrosine-containing 2, 5-diketopiperazines. Die Pharm.-Int. J. Pharm. Sci. 2005, 60, 305–309. [Google Scholar]
- Bycroft, B.; Payne, D. Dictionary of Antibiotics and Related Substances: With CD-ROM; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Du, L.; Yang, X.; Zhu, T.; Wang, F.; Xiao, X.; Park, H.; Gu, Q. Diketopiperazine Alkaloids from a Deep Ocean Sediment Derived Fungus Penicillium sp. Chem. Pharm. Bull. 2009, 57, 873–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalský, J.; Čtvrtník, J.; Horáková, Z.; Bydžovský, V. Über die tuberkulostatische Aktivität von 2,5-Bis-(aminooxymethyl)-3,6-diketopiperazin, eines Umwandlungsproduktes des Cycloserins. Experientia 1962, 18, 217–218. [Google Scholar] [CrossRef]
- Miyazaki, K.; Yasutake, A.; Nishino, N.; Aoyagi, H.; Kato, T.; Izumiya, N. Cyclic peptides. X. Bitter taste and chymotryptic hydrolysis of cyclic depsidipeptides containing a tryptophan residue. Int. J. Pept. Protein Res. 1981, 17, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Deigin, V.; Ksenofontova, O.; Yatskin, O.; Goryacheva, A.; Ignatova, A.; Feofanov, A.; Ivanov, V. Novel platform for the preparation of synthetic orally active peptidomimetics with hemoregulating activity. II. Hemosuppressor activity of 2, 5-diketopiperazine-based. Int. Immunopharmacol. 2020, 81, 106185. [Google Scholar] [CrossRef] [PubMed]
- Deigin, V.; Ksenofontova, O.; Khrushchev, A.; Yatskin, O.; Goryacheva, A.; Ivanov, V. Chemical Platform for the Preparation of Synthetic Orally Active Peptidomimetics with Hemoregulating Activity. ChemMedChem 2016, 11, 1974–1977. [Google Scholar] [CrossRef] [PubMed]
- Deigin, V. Substituted Piperazin-2,5-diones and Their Use as Multifunctional Bioactive Compounds. Available online: https://patents.google.com/patent/US8637521 (accessed on 11 January 2022).
- Borthwick, A.D. 2,5-diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Smith, K.S.; Deveau, A.M.; Dieckhaus, C.M.; Johnson, M.A.; Macdonald, T.L.; Cook, J.M. Biological activity of the tryprostatins and their diastereomers on human carcinoma cell lines. J. Med. Chem. 2002, 45, 1559–1562. [Google Scholar] [CrossRef]
- Prasad, C. Bioactive cyclic dipeptides. Peptides 1995, 16, 151–164. [Google Scholar] [CrossRef]
- Shiosaki, K. Toward development of peptidomimetics: Diketopiperazine templates for the Trp-Met segment of CCK-4. Pept. Chem. Struct. Biol. 1990, 978–980. [Google Scholar]
- Grigor’eva, M.E.; Lyapina, L.A.; Kudryashov, B.A. Antithrombotic Protective Effects of Arg-Pro-Gly-Pro Peptide during Emotional Stress Provoked by Forced Swimming Test in Rats. Bull. Exp. Biol. Med. 2016, 162, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, S.V.; Mjasoedov, N.F. Physiological Effects of Selank and Its Fragments. Biol. Bull. 2019, 46, 407–414. [Google Scholar] [CrossRef]
- Lasukova, T.V.; Maslov, L.N.; Podoksenov, Y.K.; Podoksenov, A.Y.; Platonov, A.A.; Ovchinnikov, M.V.; Bespalova, Z.D. Effect of opiate peptide dalargin and des-Tyr-dalargin on cardiac pump function during ischemia-reperfusion. Bull. Exp. Biol. Med. 2004, 137, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Rushkovskii, S.R.; Chegrinets, S.E.; Bezrukov, V.F.; Khrapunov, S.N. The effect of thymogen on the chromosome aberration level in a culture of human peripheral blood lymphocytes. Tsitol. Genet. 1996, 30, 81–85. [Google Scholar]
- Zabrodsky, P.F. The effect of thymogen on the postintoxication immunodeficiency state induced by acute acetonitrile poisoning. Eksp. Klin. Farmakol. 1999, 62, 48–49. [Google Scholar]
- Jucker, M.; Walker, L.C. Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1341–1349. [Google Scholar] [CrossRef]
- Manzano, S.; Agüera, L.; Aguilar, M.; Olazarán, J. A Review on Tramiprosate (Homotaurine) in Alzheimer’s Disease and Other Neurocognitive Disorders. Front. Neurol. 2020, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Giulian, D.; Haverkamp, L.J.; Yu, J.; Karshin, W.; Tom, D.; Li, J.; Kazanskaia, A.; Kirkpatrick, J.; Roher, A.E. The HHQK Domain of β-Amyloid Provides a Structural Basis for the Immunopathology of Alzheimer’s Disease *. J. Biol. Chem. 1998, 273, 29719–29726. [Google Scholar] [CrossRef] [Green Version]
- Riccardi, C.; Napolitano, F.; Montesarchio, D.; Sampaolo, S.; Melone, M.A.B. Nanoparticle-Guided Brain Drug Delivery: Expanding the Therapeutic Approach to Neurodegenerative Diseases. Pharmaceutics 2021, 13, 1897. [Google Scholar] [CrossRef] [PubMed]
- Pederzoli, F.; Ruozi, B.; Duskey, J.; Hagmeyer, S.; Sauer, A.K.; Grabrucker, S.; Coelho, R.; Oddone, N.; Ottonelli, I.; Daini, E.; et al. Nanomedicine against Aβ Aggregation by β–Sheet Breaker Peptide Delivery: In Vitro Evidence. Pharmaceutics 2019, 11, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Istrate, A.N.; Kozin, S.A.; Zhokhov, S.S.; Mantsyzov, A.B.; Kechko, O.I.; Pastore, A.; Makarov, A.A.; Polshakov, V.I. Interplay of histidine residues of the Alzheimer’s disease Aβ peptide governs its Zn-induced oligomerization. Sci. Reports 2016, 6, 21734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khmeleva, S.A.; Radko, S.P.; Kozin, S.A.; Kiseleva, Y.Y.; Mezentsev, Y.V.; Mitkevich, V.A.; Kurbatov, L.K.; Ivanov, A.S.; Makarov, A.A. Zinc-Mediated Binding of Nucleic Acids to Amyloid-β Aggregates: Role of Histidine Residues. J. Alzheimer’s Dis. 2016, 54, 809–819. [Google Scholar] [CrossRef]
- Kozin, S.A.; Mezentsev, Y.V.; Kulikova, A.A.; Indeykina, M.I.; Golovin, A.V.; Ivanov, A.S.; Tsvetkov, P.O.; Makarov, A.A. Zinc-induced dimerization of the amyloid-β metal-binding domain 1–16 is mediated by residues 11–14. Mol. Biosyst. 2011, 7, 1053–1055. [Google Scholar] [CrossRef]
- Kozin, S.A.; Polshakov, V.I.; Mezentsev, Y.V.; Ivanov, A.S.; Zhokhov, S.S.; Yurinskaya, M.M.; Vinokurov, M.G.; Makarov, A.A.; Mitkevich, V.A. Enalaprilat Inhibits Zinc-Dependent Oligomerization of Metal-Binding Domain of Amyloid-beta Isoforms and Protects Human Neuroblastoma Cells from Toxic Action of these Isoforms. Mol. Biol. 2018, 52, 590–597. [Google Scholar] [CrossRef]
- Mezentsev, Y.V.; Medvedev, A.E.; Kechko, O.I.; Makarov, A.A.; Ivanov, A.S.; Mantsyzov, A.B.; Kozin, S.A. Zinc-induced heterodimer formation between metal-binding domains of intact and naturally modified amyloid-beta species: Implication to amyloid seeding in Alzheimer’s disease? J. Biomol. Struct. Dyn. 2016, 34, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Polshakov, V.I.; Mantsyzov, A.B.; Kozin, S.A.; Adzhubei, A.A.; Zhokhov, S.S.; van Beek, W.; Kulikova, A.A.; Indeykina, M.I.; Mitkevich, V.A.; Makarov, A.A. A Binuclear Zinc Interaction Fold Discovered in the Homodimer of Alzheimer’s Amyloid-β Fragment with Taiwanese Mutation D7H. Angew. Chem. 2017, 56, 11734–11739. [Google Scholar] [CrossRef] [PubMed]
- Radko, S.P.; Khmeleva, S.A.; Mantsyzov, A.B.; Kiseleva, Y.Y.; Mitkevich, V.A.; Kozin, S.A.; Makarov, A.A. Heparin Modulates the Kinetics of Zinc-Induced Aggregation of Amyloid-β Peptides. J. Alzheimer’s Dis. 2018, 63, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.O.; Kulikova, A.A.; Golovin, A.V.; Tkachev, Y.V.; Archakov, A.I.; Kozin, S.A.; Makarov, A.A. Minimal Zn2+ Binding Site of Amyloid-β. Biophys. J. 2010, 99, L84–L86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozin, S.A.; Makarov, A.A. The Convergence of Alzheimer’s Disease Pathogenesis Concepts. Mol. Biol. 2019, 53, 896–903. [Google Scholar] [CrossRef]
- Nisbet, R.M.; Nuttall, S.D.; Robert, R.; Caine, J.M.; Dolezal, O.; Hattarki, M.; Pearce, L.A.; Davydova, N.; Masters, C.L.; Varghese, J.N.; et al. Structural studies of the tethered N-terminus of the Alzheimer’s disease amyloid-β peptide. Proteins Struct. Funct. Bioinforma. 2013, 81, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Zirah, S.; Kozin, S.A.; Mazur, A.K.; Blond, A.; Cheminant, M.; Ségalas-Milazzo, I.; Debey, P.; Rebuffat, S. Structural changes of region 1–16 of the Alzheimer disease amyloid β-peptide upon zinc binding and in vitro aging. J. Biol. Chem. 2006, 281, 2151–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollmer, M.; Close, W.; Funk, L.; Rasmussen, J.; Bsoul, A.; Schierhorn, A.; Schmidt, M.; Sigurdson, C.J.; Jucker, M.; Fändrich, M. Cryo-EM structure and polymorphism of Aβ amyloid fibrils purified from Alzheimer’s brain tissue. Nat. Commun. 2019, 10, 4760. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, J.L.M.; Tong, M.; Alfulaij, N.; Sherrin, T.; Contarino, M.; White, M.M.; Bellinger, F.P.; Todorovic, C.; Nichols, R.A. Regulation of Presynaptic Ca2+, Synaptic Plasticity and Contextual Fear Conditioning by a N-terminal β-Amyloid Fragment. J. Neurosci. 2014, 34, 14210–14218. [Google Scholar] [CrossRef] [Green Version]
- Kozin, S.A.; Barykin, E.P.; Mitkevich, V.A.; Makarov, A.A. Anti-amyloid Therapy of Alzheimer’s Disease: Current State and Prospects. Biochemistry 2018, 83, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.O.; Cheglakov, I.B.; Ovsepyan, A.A.; Mediannikov, O.Y.; Morozov, A.O.; Telegin, G.B.; Kozin, S.A. Peripherally Applied Synthetic Tetrapeptides HAEE and RADD Slow Down the Development of Cerebral β-Amyloidosis in AβPP/PS1 Transgenic Mice. J. Alzheimer’s Dis. 2015, 46, 849–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribarič, S. Peptides as Potential Therapeutics for Alzheimer’s Disease. Molecules 2018, 23, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovejoy, D.A.; Hogg, D.W.; Dodsworth, T.L.; Jurado, F.R.; Read, C.C.; D’Aquila, A.L.; Barsyte-Lovejoy, D. Synthetic Peptides as Therapeutic Agents: Lessons Learned From Evolutionary Ancient Peptides and Their Transit Across Blood-Brain Barriers. Front. Endocrinol. 2019, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Zolotarev, Y.A.; Mitkevich, V.A.; Shram, S.I.; Adzhubei, A.A.; Tolstova, A.P.; Talibov, O.B.; Dadayan, A.K.; Myasoyedov, N.F.; Makarov, A.A.; Kozin, S.A. Pharmacokinetics and Molecular Modeling Indicate nAChRα4-Derived Peptide HAEE Goes through the Blood–Brain Barrier. Biomolecules 2021, 11, 909. [Google Scholar] [CrossRef]
- Andersson, U.; Ottestad, W.; Tracey, K.J. Extracellular HMGB1: A therapeutic target in severe pulmonary inflammation including COVID-19? Mol. Med. 2020, 26, 42. [Google Scholar] [CrossRef] [PubMed]
- Karkischenko, V.N.; Skvortsova, V.I.; Gasanov, M.T.; Fokin, Y.V.; Nesterov, M.S.; Petrova, N.V.; Alimkina, O.V.; Pomytkin, I.A. Inhaled [D-Ala2]-Dynorphin 1-6 Prevents Hyperacetylation and Release of High Mobility Group Box 1 in a Mouse Model of Acute Lung Injury. J. Immunol. Res. 2021, 2021, 4414544. [Google Scholar] [CrossRef] [PubMed]
- Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Sudarkina, O.Y.; Dmitrieva, V.G.; Gubsky, L.V.; Myasoedov, N.F.; Limborska, S.A.; et al. Novel Insights into the Protective Properties of ACTH(4-7)PGP (Semax) Peptide at the Transcriptome Level Following Cerebral Ischaemia–Reperfusion in Rats. Genes 2020, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Dergunova, L.V.; Dmitrieva, V.G.; Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Valieva, L.V.; Sudarkina, O.Y.; Gubsky, L.V.; et al. The Peptide Drug ACTH(4–7)PGP (Semax) Suppresses mRNA Transcripts Encoding Proinflammatory Mediators Induced by Reversible Ischemia of the Rat Brain. Mol. Biol. 2021, 55, 346–353. [Google Scholar] [CrossRef]





| # | Original Russian Peptide Preparation | Clinical or Pharmacological Group |
|---|---|---|
| 1 | Dalargin (tyrosyl-d-alanyl-glycyl-phenylalanyl-leucyl-arginine) | Antiulcer drug with antisecretory activity |
| 2 | Thymogen (Glu–Try) | Immunostimulatory drug |
| 3 | Semax (Met–Glu–His–Phe–Pro–Gly–Pro) | Nootropic drug |
| 4 | Licopid (glucosaminylmuramil dipeptide) | Immunomodulator |
| 5 | Immunofan (arginyl-alpha-aspartyl-lysyl-valyl-tyrosyl-arginine) | Immunomodulator |
| 6 | Thymodepressin (dipeptide disodium salt; γ-d-glutamyl-d-tryptophan) | Immunosuppressive drug |
| 7 | Gepon (Thr–Glu–Lys–Lys–Arg–Arg–Glu–Thr–Val–Glu–Arg–Glu–Lys–Glu | Antiviral agent |
| 8 | Sedatin (Arg–Tyr-d-Ala–Phe–Gly) | Stress protector (for veterinary use) |
| 9 | Bestim (thymogen analog; γ-d-glutamyl-l-tryptophan) | Immunomodulator |
| 10 | Noopept (ethyl ester of N–phenyl–acetyl–l–prolyl–glycine). | Nootropic drug |
| 11 | Deltaran (tryptophanyl-alanyl-glycyl-glycyl-aspartyl-alanyl-seryl-glycyl-glutamic acid) | Stress protector, treatment of alcohol addiction |
| 12 | Stemokin (Ile–Glu–Trp) | Hematopoietic stimulant |
| 13 | Selank (l-threonyl-l-lysyl-l-prolyl-l-arginyl-l-prolylglycyl-l-proline) | Anxiolytic |
| 14 | Allokin alpha (alloferon; His–Gly–Val–Ser–Gly–His–Gly–Glu–His–Gly–Val–His–Gly) | Immunomodulator |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deigin, V.I.; Poluektova, E.A.; Beniashvili, A.G.; Kozin, S.A.; Poluektov, Y.M. Development of Peptide Biopharmaceuticals in Russia. Pharmaceutics 2022, 14, 716. https://doi.org/10.3390/pharmaceutics14040716
Deigin VI, Poluektova EA, Beniashvili AG, Kozin SA, Poluektov YM. Development of Peptide Biopharmaceuticals in Russia. Pharmaceutics. 2022; 14(4):716. https://doi.org/10.3390/pharmaceutics14040716
Chicago/Turabian StyleDeigin, Vladislav I., Elena A. Poluektova, Allan G. Beniashvili, Sergey A. Kozin, and Yuri M. Poluektov. 2022. "Development of Peptide Biopharmaceuticals in Russia" Pharmaceutics 14, no. 4: 716. https://doi.org/10.3390/pharmaceutics14040716