Synthesis and Evaluation of the Antifungal and Toxicological Activity of Nitrofuran Derivatives
Abstract
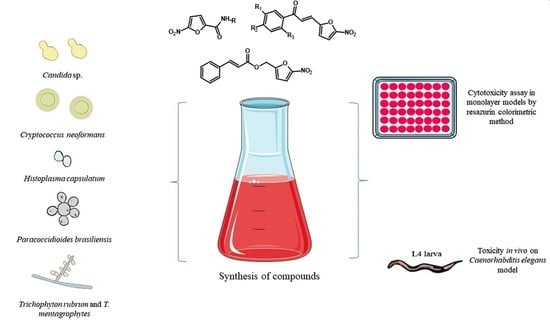
:1. Introduction
2. Materials and Methods
2.1. Chemical Synthesis
2.1.1. General Procedure for the Synthesis of Compounds N-(3-(1H-imidazol-1-yl)propyl)-5-nitrofuran-2-carboxamide (2) and 5-nitro-N-(2-(pyridin-2-yl)ethyl)furan-2-carboxamide (3)
N-(3-(1H-imidazol-1-yl)propyl)-5-nitrofuran-2-carboxamide (2)
5-nitro-N-(2-(pyridin-2-yl)ethyl)furan-2-carboxamide (3)
2.1.2. General Procedure for the Synthesis of Compounds (E)-1-(4-(methylsulfonyl)phenyl)-3-(5-nitrofuran-2-yl)prop-2-en-1-one (15), (E)-1-(2,4-dichlorophenyl)-3-(5-nitrofuran-2-yl)prop-2-en-1-one (16) and (E)-1-(2,4-dichloro-5-fluorophenyl)-3-(5-nitrofuran-2-yl)prop-2-en-1-one (17)
(E)-1-(4-(methylsulfonyl)phenyl)-3-(5-nitrofuran-2-yl)prop-2-en-1-one (15)
(E)-1-(2,4-dichlorophenyl)-3-(5-nitrofuran-2-yl)prop-2-en-1-one (16)
(E)-1-(2,4-dichloro-5-fluorophenyl)-3-(5-nitrofuran-2-yl)prop-2-en-1-one (17)
2.2. Antifungal Drugs and Nitrofuran Derivatives
2.3. Microorganisms and Culture Conditions
2.4. Fungal Susceptibility to Nitrofuran Derivates and Antifungal Drugs
2.4.1. Candida sp. and Cryptococcus neoformans
2.4.2. Histoplasma capsulatum
2.4.3. Paracoccidioides brasiliensis
2.4.4. Trichophyton rubrum and T. mentagrophytes
2.5. Determination of Minimum Fungicide Concentration (MFC)
2.6. Cell Line Maintenance
Cytotoxicity Assay in Monolayer Models by Resazurin Colorimetric Method
2.7. Toxicity In Vivo on C. elegans Model
2.8. Statistic Analysis
3. Results
3.1. Chemical Synthesis
3.2. Broad-Spectrum Antifungal Activity
3.3. Determination of MIC90 and MFC for Candida species and Cryptococcus neoformans
3.4. Determination of MIC and MFC for H. capsulatum and P. brasiliensis
3.5. Determination of MIC and MFC for T. mentagrophytes
3.6. Cytotoxicity Assay by the Resazurin Method and Selectivity Index
3.7. Toxicity on C. elegans Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar] [CrossRef]
- Basso, R.P.; Poester, V.R.; Benelli, J.L.; Stevens, D.A.; Zogbi, H.E.; da S. Vasconcellos, I.C.; Pasqualotto, A.C.; Xavier, M.O. COVID-19-Associated Histoplasmosis in an AIDS Patient. Mycopathologia 2021, 186, 109–112. [Google Scholar] [CrossRef]
- Rawson, T.M.; Wilson, R.C.; Holmes, A. Understanding the role of bacterial and fungal infection in COVID-19. Clin. Microbiol. Infect. 2021, 27, 9–11. [Google Scholar] [CrossRef]
- Song, G.; Liang, G.; Liu, W. Fungal Co-infections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia 2020, 185, 599–606. [Google Scholar] [CrossRef]
- Ivaskiene, M.; Matusevicius, A.P.; Grigonis, A.; Zamokas, G.; Babickaite, L. Efficacy of Topical Therapy with Newly Developed Terbinafine and Econazole Formulations in the Treatment of Dermatophytosis in Cats. Pol. J. Vet. Sci. 2016, 19, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Arya, N.R.; Rafiq, N.B. Candidiasis. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Garcia, L.M.; Costa-Orlandi, C.B.; Bila, N.M.; Vaso, C.O.; Gonçalves, L.N.C.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. A Two-Way Road: Antagonistic Interaction between Dual-Species Biofilms Formed by Candida albicans/Candida parapsilosis and Trichophyton rubrum. Front. Microbiol. 2020, 11, 1980. [Google Scholar] [CrossRef]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Paul, S.; Singh, S.; Sharma, D.; Chakrabarti, A.; Rudramurthy, S.M.; Ghosh, A.K. Dynamics of in vitro development of azole resistance in Candida tropicalis. J. Glob. Antimicrob. Resist. 2020, 22, 553–561. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Cantón, E.; Pemán, J. Antifungal Resistance among Less Prevalent Candida Non-albicans and Other Yeasts versus Established and under Development Agents: A Literature Review. J. Fungi 2021, 7, 24. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharm. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Sanglard, D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Front. Med. 2016, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.L.; Yu, S.J.; Heitman, J.; Wellington, M.; Chen, Y.L. New facets of antifungal therapy. Virulence 2017, 8, 222–236. [Google Scholar] [CrossRef] [Green Version]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Hagen, F.; Khayhan, K.; Theelen, B.; Kolecka, A.; Polacheck, I.; Sionov, E.; Falk, R.; Parnmen, S.; Lumbsch, H.T.; Boekhout, T. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 2015, 78, 16–48. [Google Scholar] [CrossRef] [Green Version]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.H.; Del Pozzo, J.; Salamanca, S.A.; Hernandez, H.; Martinez, L.R. Reduced phagocytosis and killing of Cryptococcus neoformans biofilm-derived cells by J774.16 macrophages is associated with fungal capsular production and surface modification. Fungal Genet. Biol. 2019, 132, 103258. [Google Scholar] [CrossRef]
- Goldman, D.L.; Lee, S.C.; Casadevall, A. Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect. Immun. 1995, 63, 3448–3453. [Google Scholar] [CrossRef] [Green Version]
- Bermas, A.; Geddes-McAlister, J. Combatting the evolution of antifungal resistance in Cryptococcus neoformans. Mol. Microbiol. 2020, 114, 721–734. [Google Scholar] [CrossRef]
- Mourad, A.; Perfect, J.R. Present and Future Therapy of Cryptococcus infections. J. Fungi 2018, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Srichatrapimuk, S.; Sungkanuparph, S. Integrated therapy for HIV and cryptococcosis. AIDS Res. Ther. 2016, 13, 42. [Google Scholar] [CrossRef] [Green Version]
- Dong, K.; You, M.; Xu, J. Genetic Changes in Experimental Populations of a Hybrid in the Cryptococcus neoformans Species Complex. Pathogens 2020, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Kauffman, C.A. Histoplasmosis. Clin. Chest Med. 2009, 30, 217–225. [Google Scholar] [CrossRef]
- Queiroz-Telles, F.; Escuissato, D.L. Pulmonary paracoccidioidomycosis. Semin. Respir. Crit. Care Med. 2011, 32, 764–774. [Google Scholar] [CrossRef] [Green Version]
- Wheat, L.J.; Azar, M.M.; Bahr, N.C.; Spec, A.; Relich, R.F.; Hage, C. Histoplasmosis. Infect. Dis. Clin. N. Am. 2016, 30, 207–227. [Google Scholar] [CrossRef]
- Bagatin, M.C.; Rozada, A.M.F.; Rodrigues, F.A.V.; Bueno, P.S.A.; Santos, J.L.; Canduri, F.; Kioshima, É.S.; Seixas, F.A.V.; Basso, E.A.; Gauze, G.F. New 4-methoxy-naphthalene derivatives as promisor antifungal agents for paracoccidioidomycosis treatment. Future Microbiol. 2019, 14, 235–245. [Google Scholar] [CrossRef]
- Martinez, R. New Trends in Paracoccidioidomycosis Epidemiology. J. Fungi 2017, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- do Carmo Silva, L.; de Oliveira, A.A.; de Souza, D.R.; Barbosa, K.L.; Freitas e Silva, K.S.; Carvalho Júnior, M.A.; Rocha, O.B.; Lima, R.M.; Santos, T.G.; Soares, C.M.; et al. Overview of Antifungal Drugs against Paracoccidioidomycosis: How Do We Start, Where Are We, and Where Are We Going? J. Fungi 2020, 6, 300. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; Queiroz-Telles, F. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017, 50, 715–740. [Google Scholar] [CrossRef]
- Azar, M.M.; Hage, C.A. Clinical Perspectives in the Diagnosis and Management of Histoplasmosis. Clin. Chest Med. 2017, 38, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Wheat, L.J.; Freifeld, A.G.; Kleiman, M.B.; Baddley, J.W.; McKinsey, D.S.; Loyd, J.E.; Kauffman, C.A. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 807–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azar, M.M.; Loyd, J.L.; Relich, R.F.; Wheat, L.J.; Hage, C.A. Current Concepts in the Epidemiology, Diagnosis, and Management of Histoplasmosis Syndromes. Semin. Respir. Crit. Care Med. 2020, 41, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Costa-Orlandi, C.B.; Serafim-Pinto, A.; da Silva, P.B.; Bila, N.M.; Bonatti, J.L.d.C.; Scorzoni, L.; Singulani, J.d.L.; dos Santos, C.T.; Nazaré, A.C.; Chorilli, M.; et al. Incorporation of Nonyl 3,4-Dihydroxybenzoate Into Nanostructured Lipid Systems: Effective Alternative for Maintaining Anti-Dermatophytic and Antibiofilm Activities and Reducing Toxicity at High Concentrations. Front. Microbiol. 2020, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Stec, N.; Summerbell, R.C.; Shear, N.H.; Piguet, V.; Tosti, A.; Piraccini, B.M. Onychomycosis: A review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1972–1990. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, W. The Changing Face of Dermatophytic Infections Worldwide. Mycopathologia 2017, 182, 77–86. [Google Scholar] [CrossRef]
- Bila, N.M.; Costa-Orlandi, C.B.; Vaso, C.O.; Bonatti, J.L.C.; de Assis, L.R.; Regasini, L.O.; Fontana, C.R.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. 2-Hydroxychalcone as a Potent Compound and Photosensitizer Against Dermatophyte Biofilms. Front. Cell. Infect. Microbiol. 2021, 11, 679470. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cooper, E.A. Update in Antifungal Therapy of Dermatophytosis. Mycopathologia 2008, 166, 353–367. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B.; Martinez, L.R.; Bila, N.M.; Friedman, J.M.; Friedman, A.J.; Mendes-Giannini, M.J.S.; Nosanchuk, J.D. Nitric Oxide-Releasing Nanoparticles Are Similar to Efinaconazole in Their Capacity to Eradicate Trichophyton rubrum Biofilms. Front. Cell. Infect. Microbiol. 2021, 11, 684150. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, W.; Quan, W.; Jiang, J.; Qu, B. Occurrence and levels of nitrofuran metabolites in sea cucumber from Dalian, China. Food Addit. Contam. Part A 2016, 33, 1672–1677. [Google Scholar] [CrossRef]
- Krasavin, M.; Parchinsky, V.; Kantin, G.; Manicheva, O.; Dogonadze, M.; Vinogradova, T.; Karge, B.; Brönstrup, M. New nitrofurans amenable by isocyanide multicomponent chemistry are active against multidrug-resistant and poly-resistant Mycobacterium tuberculosis. Bioorg. Med. Chem. 2017, 25, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Claussen, K.; Stocks, E.; Bhat, D.; Fish, J.; Rubin, C.D. How Common Are Pulmonary and Hepatic Adverse Effects in Older Adults Prescribed Nitrofurantoin? J. Am. Geriatr. Soc. 2017, 65, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Aldeek, F.; Hsieh, K.C.; Ugochukwu, O.N.; Gerard, G.; Hammack, W. Accurate Quantitation and Analysis of Nitrofuran Metabolites, Chloramphenicol, and Florfenicol in Seafood by Ultrahigh-Performance Liquid Chromatography-Tandem Mass Spectrometry: Method Validation and Regulatory Samples. J. Agric. Food Chem. 2018, 66, 5018–5030. [Google Scholar] [CrossRef] [PubMed]
- De Vita, D.; Friggeri, L.; D’Auria, F.D.; Pandolfi, F.; Piccoli, F.; Panella, S.; Palamara, A.T.; Simonetti, G.; Scipione, L.; Di Santo, R.; et al. Activity of caffeic acid derivatives against Candida albicans biofilm. Bioorg. Med. Chem. Lett. 2014, 24, 1502–1505. [Google Scholar] [CrossRef]
- Kamal, A.; Hussaini, S.M.; Sucharitha, M.L.; Poornachandra, Y.; Sultana, F.; Ganesh Kumar, C. Synthesis and antimicrobial potential of nitrofuran-triazole congeners. Org. Biomol. Chem. 2015, 13, 9388–9397. [Google Scholar] [CrossRef]
- De Vita, D.; Simonetti, G.; Pandolfi, F.; Costi, R.; Di Santo, R.; D’Auria, F.D.; Scipione, L. Exploring the anti-biofilm activity of cinnamic acid derivatives in Candida albicans. Bioorg. Med. Chem. Lett. 2016, 26, 5931–5935. [Google Scholar] [CrossRef] [Green Version]
- Tangallapally, R.P.; Yendapally, R.; Lee, R.E.; Hevener, K.; Jones, V.C.; Lenaerts, A.J.; McNeil, M.R.; Wang, Y.; Franzblau, S. Synthesis and evaluation of nitrofuranylamides as novel antituberculosis agents. J. Med. Chem. 2004, 47, 5276–5283. [Google Scholar] [CrossRef]
- Gallardo-Macias, R.; Kumar, P.; Jaskowski, M.; Richmann, T.; Shrestha, R.; Russo, R.; Singleton, E.; Zimmerman, M.D.; Ho, H.P.; Dartois, V.; et al. Optimization of N-benzyl-5-nitrofuran-2-carboxamide as an antitubercular agent. Bioorg. Med. Chem. Lett. 2019, 29, 601–606. [Google Scholar] [CrossRef]
- Arias, D.G.; Herrera, F.E.; Garay, A.S.; Rodrigues, D.; Forastieri, P.S.; Luna, L.E.; Bürgi, M.D.; Prieto, C.; Iglesias, A.A.; Cravero, R.M.; et al. Rational design of nitrofuran derivatives: Synthesis and valuation as inhibitors of Trypanosoma cruzi trypanothione reductase. Eur. J. Med. Chem. 2017, 125, 1088–1097. [Google Scholar] [CrossRef]
- Pandolfi, F.; D’Acierno, F.; Bortolami, M.; De Vita, D.; Gallo, F.; De Meo, A.; Di Santo, R.; Costi, R.; Simonetti, G.; Scipione, L. Searching for new agents active against Candida albicans biofilm: A series of indole derivatives, design, synthesis and biological evaluation. Eur. J. Med. Chem. 2019, 165, 93–106. [Google Scholar] [CrossRef]
- Jin, H.; Geng, Y.; Yu, Z.; Tao, K.; Hou, T. Lead optimization and anti-plant pathogenic fungi activities of daphneolone analogues from Stellera chamaejasme. L. Pestic. Biochem. Physiol. 2009, 93, 133–137. [Google Scholar] [CrossRef]
- Gomes, M.N.; Braga, R.C.; Grzelak, E.M.; Neves, B.J.; Muratov, E.; Ma, R.; Klein, L.L.; Cho, S.; Oliveira, G.R.; Franzblau, S.G.; et al. QSAR-driven design, synthesis and discovery of potent chalcone derivatives with antitubercular activity. Eur. J. Med. Chem. 2017, 137, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Press, J.B.; Wright, W.B., Jr.; Chan, P.S.; Haug, M.F.; Marsico, J.W.; Tomcufcik, A.S. Thromboxane synthetase inhibitors and antihypertensive agents. 3. N-[(1H-imidazol-1-yl)alkyl]heteroaryl amides as potent enzyme inhibitors. J. Med. Chem. 1987, 30, 1036–1040. [Google Scholar] [CrossRef]
- Tawari, N.R.; Bairwa, R.; Ray, M.K.; Rajan, M.G.R.; Degani, M.S. Design, synthesis, and biological evaluation of 4-(5-nitrofuran-2-yl)prop-2-en-1-one derivatives as potent antitubercular agents. Bioorg. Med. Chem. Lett. 2010, 20, 6175–6178. [Google Scholar] [CrossRef]
- Holla, B.S.; Veerendra, B.; Shivananda, M.K. Non-linear optical properties of new arylfuranylpropenones. J. Cryst. Growth 2004, 263, 532–535. [Google Scholar] [CrossRef]
- de Macedo, P.M.; Teixeira, M.M.; Barker, B.M.; Zancopé-Oliveira, R.M.; Almeida-Paes, R.; do Valle, A.C.F. Clinical features and genetic background of the sympatric species Paracoccidioides brasiliensis and Paracoccidioides americana. PLoS Negl. Trop. Dis. 2019, 13, e0007309. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts (M27-A3); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Baltazar, L.M.; Zamith-Miranda, D.; Burnet, M.C.; Choi, H.; Nimrichter, L.; Nakayasu, E.S.; Nosanchuk, J.D. Concentration-dependent protein loading of extracellular vesicles released by Histoplasma capsulatum after antibody treatment and its modulatory action upon macrophages. Sci. Rep. 2018, 8, 8065. [Google Scholar] [CrossRef]
- Gonçalves, L.N.C.; Costa-Orlandi, C.B.; Bila, N.M.; Vaso, C.O.; Da Silva, R.A.M.; Mendes-Giannini, M.J.S.; Taylor, M.L.; Fusco-Almeida, A.M. Biofilm Formation by Histoplasma capsulatum in Different Culture Media and Oxygen Atmospheres. Front. Microbiol. 2020, 11, 1455. [Google Scholar] [CrossRef]
- de Paula e Silva, A.C.A.; Oliveira, H.C.; Silva, J.F.; Sangalli-Leite, F.; Scorzoni, L.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Microplate alamarBlue Assay for Paracoccidioides Susceptibility Testing. J. Clin. Microbiol. 2013, 51, 1250–1252. [Google Scholar] [CrossRef] [Green Version]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Santos, C.T.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. In vitro characterization of Trichophyton rubrum and T. mentagrophytes biofilms. Biofouling 2014, 30, 719–727. [Google Scholar] [CrossRef]
- Li, R.K.; Ciblak, M.A.; Nordoff, N.; Pasarell, L.; Warnock, D.W.; McGinnis, M.R. In vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum. Antimicrob. Agents Chemother. 2000, 44, 1734–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheat, L.J.; Connolly, P.; Smedema, M.; Brizendine, E.; Hafner, R.; AIDS Clinical Trials Group and the Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. Emergence of Resistance to Fluconazole as a Cause of Failure during Treatment of Histoplasmosis in Patients with Acquired Immunodeficiency Disease Syndrome. Clin. Infect. Dis. 2001, 33, 1910–1913. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, S.; Singh, P.K.; Meis, J.F.; Chowdhary, A. In vitro antifungal susceptibility profile and correlation of mycelial and yeast forms of molecularly characterized Histoplasma capsulatum strains from India. Antimicrob. Agents Chemother. 2014, 58, 5613–5616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, N.; Fukuhara-Takaki, K.; Jono, T.; Nakajou, K.; Eto, N.; Horiuchi, S.; Takeya, M.; Nagai, R. Association of advanced glycation end products with A549 cells, a human pulmonary epithelial cell line, is mediated by a receptor distinct from the scavenger receptor family and RAGE. J. Biochem. 2006, 139, 821–829. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Y.; Wang, J.; Yu, W.; Wang, W.; Ma, X. Monitoring of Cell Viability and Proliferation in Hydrogel-Encapsulated System by Resazurin Assay. Appl. Biochem. Biotechnol. 2010, 162, 1996–2007. [Google Scholar] [CrossRef]
- Scorzoni, L.; Sangalli-Leite, F.; de Lacorte Singulani, J.; de Paula e Silva, A.C.A.; Costa-Orlandi, C.B.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Searching new antifungals: The use of in vitro and in vivo methods for evaluation of natural compounds. J. Microbiol. Methods 2016, 123, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, N.A.; Ja’afar, F.; Yasin, H.M.; Taha, H.; Petalcorin, M.I.R.; Mamit, M.H.; Kusrini, E.; Usman, A. Physicochemical analyses, antioxidant, antibacterial, and toxicity of propolis particles produced by stingless bee Heterotrigona itama found in Brunei Darussalam. Heliyon 2019, 5, e02476. [Google Scholar] [CrossRef] [Green Version]
- Medina-Alarcón, K.P.; Singulani, J.L.; Voltan, A.R.; Sardi, J.C.O.; Petrônio, M.S.; Santos, M.B.; Polaquini, C.R.; Regasini, L.O.; Bolzani, V.S.; da Silva, D.H.S.; et al. Alkyl Protocatechuate-Loaded Nanostructured Lipid Systems as a Treatment Strategy for Paracoccidioides brasiliensis and Paracoccidioides lutzii In Vitro. Front. Microbiol. 2017, 8, 1048. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Lucas, M.P.; Mesa-Arango, A.C.; Fusco-Almeida, A.M.; Lozano, E.; Cuenca-Estrella, M.; Mendes-Giannini, M.J.; Zaragoza, O. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS ONE 2013, 8, e60047. [Google Scholar] [CrossRef]
- Sun, S.; Hoy, M.J.; Heitman, J. Fungal pathogens. Curr. Biol. 2020, 30, R1163–R1169. [Google Scholar] [CrossRef]
- Gupta, A.K.; Stec, N. Recent advances in therapies for onychomycosis and its management. F1000Research 2019, 8, 968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grässer, U.; Bubel, M.; Sossong, D.; Oberringer, M.; Pohlemann, T.; Metzger, W. Dissociation of mono- and co-culture spheroids into single cells for subsequent flow cytometric analysis. Ann. Anat. 2018, 216, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, E.M.; Caputo, L.F.G.; Amendoeira, M.R.R. Conceitos e Métodos Para Formação de Profissionais em Laboratório de Saúde; Escola Politécnica de Saúde Joaquim Venâncio, I.O.C.: Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Singulani, J.L.; Scorzoni, L.; Gomes, P.C.; Nazaré, A.C.; Polaquini, C.R.; Regasini, L.O.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Activity of gallic acid and its ester derivatives in Caenorhabditis elegans and zebrafish (Danio rerio) models. Future Med. Chem. 2017, 9, 1863–1872. [Google Scholar] [CrossRef]
- Bagla, V.P.; McGaw, L.J.; Elgorashi, E.E.; Eloff, J.N. Antimicrobial activity, toxicity and selectivity index of two biflavonoids and a flavone isolated from Podocarpus henkelii (Podocarpaceae) leaves. BMC Complementary Altern. Med. 2014, 14, 383. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Pacheco, A.; Escalona Arranz, J.C.; Beaven, M.; Peres-Roses, R.; Gámez, Y.M.; Camacho-Pozo, M.I.; Maury, G.L.; de Macedo, M.B.; Cos, P.; Tavares, J.F.; et al. Bioassay-guided In vitro Study of the Antimicrobial and Cytotoxic Properties of the Leaves from Excoecaria Lucida Sw. Pharmacogn. Res. 2017, 9, 396–400. [Google Scholar] [CrossRef]
- Nielsen, K.; Vedula, P.; Smith, K.D.; Meya, D.B.; Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.J.; Boulware, D.R. Activity of VT-1129 against Cryptococcus neoformans clinical isolates with high fluconazole MICs. Med. Mycol. 2016, 55, 453–456. [Google Scholar] [CrossRef] [Green Version]
- May, R.C.; Stone, N.R.H.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar] [CrossRef]
- Smith, K.D.; Achan, B.; Hullsiek, K.H.; McDonald, T.R.; Okagaki, L.H.; Alhadab, A.A.; Akampurira, A.; Rhein, J.R.; Meya, D.B.; Boulware, D.R.; et al. Increased Antifungal Drug Resistance in Clinical Isolates of Cryptococcus neoformans in Uganda. Antimicrob. Agents Chemother. 2015, 59, 7197–7204. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-C.; Chang, T.-Y.; Liu, J.-W.; Chen, F.-J.; Chien, C.-C.; Lee, C.-H.; Lu, C.-H. Increasing trend of fluconazole-non-susceptible Cryptococcus neoformans in patients with invasive cryptococcosis: A 12-year longitudinal study. BMC Infect. Dis. 2015, 15, 277. [Google Scholar] [CrossRef] [Green Version]
- Pontón, J.; Rüchel, R.; Clemons, K.V.; Coleman, D.C.; Grillot, R.; Guarro, J.; Aldebert, D.; Ambroise-Thomas, P.; Cano, J.; Carrillo-Muñoz, A.J.; et al. Emerging pathogens. Med. Mycol. 2000, 38, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Wheat, L.J.; Connolly, P.; Smedema, M.; Durkin, M.; Brizendine, E.; Mann, P.; Patel, R.; McNicholas, P.M.; Goldman, M. Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole. J. Antimicrob. Chemother. 2006, 57, 1235–1239. [Google Scholar] [CrossRef]






| C. albicans | C. krusei | C. glabrata | C. neoformans | |||||
|---|---|---|---|---|---|---|---|---|
| Compounds | MIC90 | MFC | MIC90 | MFC | MIC90 | MFC | MIC90 | MFC |
| 1 | 3.90 | 125.00 | 31.25 | 31.25 | 7.81 | >250 | 31.25 | 31.25 |
| 2 | >250 | >250 | >250 | >250 | >250 | >250 | ≥250 | ≥250 |
| 3 | >250 | >250 | 250.00 | >250 | >250 | >250 | 31.25 | 62.50 |
| 4 | 250.00 | >250 | 250.00 | 250.00 | >250 | >250 | 31.25 | 62.50 |
| 5 | 125.00 | 250.00 | 62.50 | 62.50 | 125.00 | 125.00 | 7.81 | 15.62 |
| 6 | >250 | >250 | >250 | >250 | >250 | >250 | 250.00 | 250.00 |
| 7 | 250.00 | >250 | 250.00 | >250 | >250 | >250 | 62.5 | 62.5 |
| 8 | 7.80 | >250 | 250.00 | >250 | 7.81 | >250 | 31.25 | 31.25 |
| 9 | 15.62 | 31.25 | 250.00 | >250 | 31.25 | >250 | 7.81 | 15.62 |
| 10 | 250.00 | >250 | >250 | >250 | >250 | >250 | 250.00 | ≥250 |
| 11 | 125.00 | 250.00 | 250.00 | >250 | 250.00 | >250 | ≥250 | ≥250 |
| 12 | 250.00 | >250 | 250.00 | >250 | 250.00 | >250 | 62.50 | 125.00 |
| 13 | >250 | >250 | >250 | >250 | >250 | >250 | ≥250 | ≥250 |
| 14 | 15.60 | 62.50 | 15.60 | 31.25 | 7.81 | 7.81 | 3.90 | 3.90 |
| 15 | >250 | >250 | >250 | >250 | >250 | >250 | ≥250 | ≥250 |
| 16 | 250.00 | >250 | >250 | >250 | >250 | >250 | ≥250 | ≥250 |
| 17 | >250 | >250 | >250 | >250 | >250 | >250 | ≥250 | ≥250 |
| AmB | - | - | 1 | - | - | - | 0.06 | - |
| H. capsulatum | P. brasiliensis | |||
|---|---|---|---|---|
| Compounds | MIC90 | MFC | MIC90 | MFC |
| 1 | >250 | >250 | 3.90 | 62.50 |
| 2 | 250.00 | >250 | 31.25 | 125.00 |
| 3 | 3.90 | 3.90 | 0.48 | 0.98 |
| 4 | 3.90 | 3.90 | 3.90 | 7.81 |
| 5 | 1.95 | 1.95 | 15.62 | 31.25 |
| 6 | >250 | >250 | 31.25 | 31.25 |
| 7 | 0.98 | 0.98 | 1.95 | 1.95 |
| 8 | 15.62 | 15.62 | 0.98 | 1.95 |
| 9 | 3.90 | 3.90 | 0.48 | 1.95 |
| 10 | 7.81 | 15.62 | 0.98 | 0.98 |
| 11 | 0.48 | 0.48 | 0.98 | 0.98 |
| 12 | 7.81 | 15.62 | 3.90 | 3.90 |
| 13 | 125.00 | 125.00 | 0.98 | 0.98 |
| 14 | 7.81 | 7.81 | 1.95 | 1.95 |
| 15 | >250 | >250 | 3.90 | 7.81 |
| 16 | 62.50 | 62.50 | 1.95 | 1.95 |
| 17 | >250 | >250 | 1.95 | 3.90 |
| AmB | 0.03 | - | 0.13 | - |
| T. rubrum | T. mentagrophytes | T. rubrum ATCC MYA-4438 | |||
|---|---|---|---|---|---|
| Compounds | MIC90 | MFC | MIC90 | MFC | MIC90 |
| 1 | >250 | >250 | 15.60 | 15.60 | - |
| 2 | 125.00 | >250 | 125.00 | 125.00 | - |
| 3 | 125.00 | 125.00 | 62.50 | 250.00 | - |
| 4 | 125.00 | 125.00 | 31.25 | 250.00 | - |
| 5 | 15.65 | 31.25 | 31.25 | 62.50 | - |
| 6 | >250 | >250 | >250 | >250 | - |
| 7 | 125.00 | >250 | 125.00 | >250 | - |
| 8 | 0.98 | 1.95 | 0.98 | 1.95 | - |
| 9 | 0.98 | 1.95 | 1.95 | 1.95 | - |
| 10 | 3.90 | 7.81 | 3.90 | 7.81 | - |
| 11 | 7.80 | 15.60 | 7.80 | 7.80 | - |
| 12 | 0.98 | 1.95 | 1.95 | 1.95 | - |
| 13 | 0.98 | 1.95 | 0.98 | 0.98 | - |
| 14 | 1.95 | 3.9 | 1.95 | 1.95 | - |
| 15 | 7.8 | 15.6 | 7.8 | 15.6 | - |
| 16 | 7.80 | 31.25 | 7.80 | 7.80 | - |
| 17 | >250 | >250 | >250 | >250 | - |
| TRB | 0.03 | ||||
| Calculated SI | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | A549 IC50 (µg/mL) | C. albicans | C. krusei | C. glabrata | C. neoformans | H. capsulatum | P. brasiliensis | T. rubrum | T. mentagrophytes |
| 1 | 250 | 64.10 | 8.00 | 32.01 | 8.00 | 1.00 | 64.10 | 1.00 | 16.02 |
| 2 | 49.1 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 1.57 | 0.39 | 0.39 |
| 3 | 231.2 | 0.92 | 0.92 | 0.92 | 7.39 | 59.28 | 481.66 | 1.84 | 3.69 |
| 4 | 29.5 | 0.11 | 0.11 | 0.11 | 1.89 | 7.56 | 7.56 | 0.23 | 0.94 |
| 5 | 30.11 | 0.24 | 0.48 | 0.24 | 7.72 | 15.44 | 1.92 | 1.92 | 0.96 |
| 6 | 13.21 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.42 | 0.05 | 0.05 |
| 7 | 8.58 | 0.03 | 0.03 | 0.03 | 0.13 | 8.75 | 4.40 | 0.06 | 0.06 |
| 8 | 53.8 | 6.89 | 0.21 | 6.88 | 1.72 | 3.44 | 55.46 | 54.89 | 54.89 |
| 9 | 23.41 | 1.49 | 0.09 | 0.74 | 1.50 | 6.00 | 48.77 | 23.88 | 12.00 |
| 10 | 38.67 | 0.15 | 0.15 | 0.15 | 0.15 | 4.95 | 39.86 | 9.91 | 9.91 |
| 11 | 12.24 | 0.09 | 0.04 | 0.04 | 0.04 | 25.50 | 12.61 | 1.56 | 1.56 |
| 12 | 250 | 1.00 | 1.00 | 1.00 | 1.00 | 32.01 | 64.10 | 255.10 | 128.20 |
| 13 | 17.9 | 0.07 | 0.07 | 0.07 | 0.07 | 0.14 | 18.26 | 18.26 | 18.26 |
| 14 | 33.44 | 2.14 | 2.14 | 4.28 | 8.57 | 4.28 | 17.14 | 17.14 | 17.14 |
| 15 | 34.23 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 8.77 | 4.38 | 4.38 |
| 16 | 35.58 | 0.14 | 0.14 | 0.14 | 0.14 | 0.56 | 17.96 | 4.56 | 4.56 |
| 17 | 68.18 | 0.27 | 0.27 | 0.27 | 0.27 | 0.27 | 34.96 | 0.27 | 0.27 |
| Calculated SI | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | MRC5 IC50 (µg/mL) | C. albicans | C. krusei | C. glabrata | C. neoformans | H. capsulatum | P. brasiliensis | T. rubrum | T. mentagrophytes |
| 1 | 240.5 | 61.66 | 7.69 | 30.79 | 7.69 | 0.96 | 61.66 | 0.96 | 15.41 |
| 2 | 90.75 | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 | 2.90 | 0.72 | 0.72 |
| 3 | 250.00 | 1.00 | 1.00 | 1.00 | 8.00 | 64.10 | 520.83 | 2.00 | 4.00 |
| 4 | 135.2 | 0.54 | 0.54 | 0.54 | 8.66 | 34.66 | 34.66 | 1.08 | 4.32 |
| 5 | 39.76 | 0.31 | 0.63 | 0.31 | 10.19 | 20.38 | 2.54 | 2.54 | 1.27 |
| 6 | 15.6 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.49 | 0.06 | 0.06 |
| 7 | 71.24 | 0.28 | 0.28 | 0.28 | 1.13 | 72.69 | 36.53 | 0.56 | 0.56 |
| 8 | 6.66 | 0.85 | 0.02 | 0.85 | 0.21 | 0.42 | 6.86 | 6.79 | 6.79 |
| 9 | 47.23 | 3.02 | 0.18 | 1.51 | 3.02 | 12.11 | 98.39 | 48.19 | 24.22 |
| 10 | 215.4 | 0.86 | 0.86 | 0.86 | 0.86 | 27.58 | 222.06 | 55.23 | 55.23 |
| 11 | 62.56 | 0.50 | 0.25 | 0.25 | 0.25 | 130.33 | 64.49 | 8.02 | 8.02 |
| 12 | 228 | 0.91 | 0.91 | 0.91 | 0.91 | 29.19 | 58.46 | 232.65 | 116.92 |
| 13 | 4.15 | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 | 4.23 | 4.23 | 4.23 |
| 14 | 26.8 | 1.71 | 1.71 | 3.43 | 6.87 | 3.43 | 13.74 | 13.74 | 13.74 |
| 15 | 11.51 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 2.95 | 1.47 | 1.47 |
| 16 | 29.89 | 0.11 | 0.11 | 0.11 | 0.11 | 0.47 | 15.09 | 3.83 | 3.83 |
| 17 | 1.56 | 0.006 | 0.006 | 0.006 | 0.006 | 0.006 | 0.80 | 0.006 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaso, C.O.; Pandolfi, F.; Bila, N.M.; De Vita, D.; Bortolami, M.; Mendes-Giannini, M.J.S.; Tudino, V.; Costi, R.; Costa-Orlandi, C.B.; Fusco-Almeida, A.M.; et al. Synthesis and Evaluation of the Antifungal and Toxicological Activity of Nitrofuran Derivatives. Pharmaceutics 2022, 14, 593. https://doi.org/10.3390/pharmaceutics14030593
Vaso CO, Pandolfi F, Bila NM, De Vita D, Bortolami M, Mendes-Giannini MJS, Tudino V, Costi R, Costa-Orlandi CB, Fusco-Almeida AM, et al. Synthesis and Evaluation of the Antifungal and Toxicological Activity of Nitrofuran Derivatives. Pharmaceutics. 2022; 14(3):593. https://doi.org/10.3390/pharmaceutics14030593
Chicago/Turabian StyleVaso, Carolina Orlando, Fabiana Pandolfi, Níura Madalena Bila, Daniela De Vita, Martina Bortolami, Maria José Soares Mendes-Giannini, Valeria Tudino, Roberta Costi, Caroline Barcelos Costa-Orlandi, Ana Marisa Fusco-Almeida, and et al. 2022. "Synthesis and Evaluation of the Antifungal and Toxicological Activity of Nitrofuran Derivatives" Pharmaceutics 14, no. 3: 593. https://doi.org/10.3390/pharmaceutics14030593