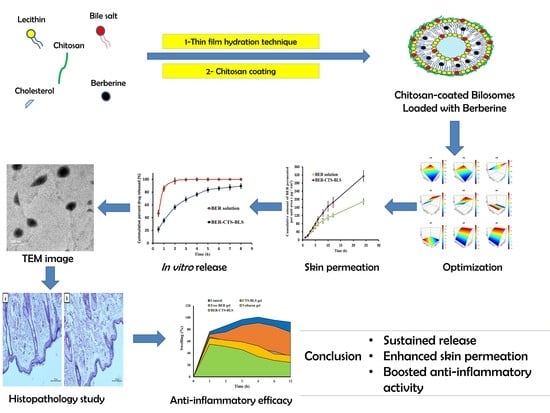
Surface-Modified Bilosomes Nanogel Bearing a Natural Plant Alkaloid for Safe Management of Rheumatoid Arthritis Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Design and Optimization of Experiments
2.2.2. Preparation of Berberine-Loaded Chitosan-Coated Bilosomes (BER-CTS-BLS)
2.2.3. Chromatographic Conditions
2.2.4. Characterization of the Experimental Runs
Particle Size and Surface Charge Analysis
BER Entrapment
2.2.5. Optimized BER-CTS-BLS Characterization
Ex Vivo Skin Permeation Study
In Vitro Release Evaluation
Morphological Evaluation
Stability Study of the Optimized BER-CTS-BLS
2.2.6. Formulation of BER-CTS-BLS-Based Gel
2.2.7. Animal Experiment
Histopathological Investigation of the BER-CTS-BLS-Based Gel
Anti-Inflammatory Effectiveness
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Bilosomes Formulation
3.2. Experimental Design and Statistical Analysis
3.2.1. Effect of Independent Variables on PS
3.2.2. Effect of Independent Variables on EE
3.2.3. Effect of Independent Variables on ZP
3.2.4. Formulation Optimization
3.3. Optimized BER-CTS-BLS Characterization
3.3.1. Ex Vivo Skin Permeation Study
3.3.2. In Vitro Release Evaluation
3.3.3. Morphological Evaluation
3.3.4. Stability Study of the Optimized BER-CTS-BLS
3.4. Histopathological Study
3.5. Anti-Inflammatory Effectiveness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Quan, L.-D.; Thiele, G.; Tian, J.; Wang, D. The development of novel therapies for rheumatoid arthritis. Expert Opin. Ther. Patents 2008, 18, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Müller-Ladner, U.; Pap, T.; E Gay, R.; Neidhart, M.; Gay, S. Mechanisms of Disease: The molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat. Clin. Pr. Rheumatol. 2005, 1, 102–110. [Google Scholar] [CrossRef]
- Fan, X.-X.; Xu, M.-Z.; Leung, E.L.-H.; Jun, C.; Yuan, Z.; Liu, L. ROS-Responsive Berberine Polymeric Micelles Effectively Suppressed the Inflammation of Rheumatoid Arthritis by Targeting Mitochondria. Nano-Micro Lett. 2020, 12, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ćalasan, M.B.; Bosch, O.F.v.D.; Creemers, M.C.; Custers, M.; Heurkens, A.H.; Van Woerkom, J.M.; Wulffraat, N.M. Prevalence of methotrexate intolerance in rheumatoid arthritis and psoriatic arthritis. Arthritis Res. Ther. 2013, 15, R217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGarry, T.; Fearon, U. Cell metabolism as a potentially targetable pathway in RA. Nat. Rev. Rheumatol. 2018, 15, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Feist, E.; Burmester, G.R. Small molecules targeting JAKs—A new approach in the treatment of rheumatoid arthritis. Rheumatologya 2013, 52, 1352–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.-L.; Chi, C.-W.; Liu, T.-Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004, 203, 127–137. [Google Scholar] [CrossRef]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef]
- Vita, A.A.; Pullen, N.A. The influence of berberine on co-stimulatory molecule expression and T cell activation. Am. Assoc. Immnol. 2018, 200, 175–211. [Google Scholar]
- Fan, X.-X.; Leung, E.L.-H.; Xie, Y.; Liu, Z.Q.; Zheng, Y.F.; Yao, X.J.; Lu, L.L.; Wu, J.L.; He, J.-X.; Yuan, Z.-W.; et al. Suppression of Lipogenesis via Reactive Oxygen Species–AMPK Signaling for Treating Malignant and Proliferative Diseases. Antioxidants Redox Signal. 2018, 28, 339–357. [Google Scholar] [CrossRef]
- Hu, Z.; Jiao, Q.; Ding, J.; Liu, F.; Liu, R.; Shan, L.; Zeng, H.; Zhang, J.; Zhang, W. Berberine induces dendritic cell apoptosis and has therapeutic potential for rheumatoid arthritis. Arthritis Care Res. 2010, 63, 949–959. [Google Scholar] [CrossRef]
- Ranade, V.V. Drug Delivery Systems. 6. Transdermal Drug Delivery. J. Clin. Pharmacol. 1991, 31, 401–418. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, A.; Oh, Y.-K.; Kim, C.-K. Effect of edge activators on the formation and transfection efficiency of ultradeformable liposomes. Biomaterials 2005, 26, 205–210. [Google Scholar] [CrossRef]
- Honeywell-Nguyen, P.L.; Bouwstra, J.A. Vesicles as a tool for transdermal and dermal delivery. Drug Discov. Today Technol. 2005, 2, 67–74. [Google Scholar] [CrossRef]
- El Menshawe, S.F.; Aboud, H.M.; Elkomy, M.H.; Kharshoum, R.M.; Abdeltwab, A.M. A novel nanogel loaded with chitosan decorated bilosomes for transdermal delivery of terbutaline sulfate: Artificial neural network optimization, in vitro characterization and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 10, 471–485. [Google Scholar] [CrossRef]
- Waglewska, E.; Pucek-Kaczmarek, A.; Bazylińska, U. Novel Surface-Modified Bilosomes as Functional and Biocompatible Nanocarriers of Hybrid Compounds. Nanomaterials 2020, 10, 2472. [Google Scholar] [CrossRef]
- Stojančević, M.; Pavlović, N.; Goločorbin-Kon, S.; Mikov, M. Application of bile acids in drug formulation and delivery. Front. Life Sci. 2013, 7, 112–122. [Google Scholar] [CrossRef]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Bilosomes as Promising Nanovesicular Carriers for Improved Transdermal Delivery: Construction, in vitro Optimization, ex vivo Permeation and in vivo Evaluation. Int. J. Nanomed. 2020, 15, 9783–9798. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef]
- Khalil, R.M.; Abdelbary, A.; El-Arini, S.K.; Basha, M.; El-Hashemy, H.A. Evaluation of bilosomes as nanocarriers for transdermal delivery of tizanidine hydrochloride: In vitro and ex vivo optimization. J. Liposome Res. 2019, 29, 171–182. [Google Scholar] [CrossRef]
- Ammar, H.O.; Mohamed, M.I.; Tadros, M.I.; Fouly, A.A. Transdermal Delivery of Ondansetron Hydrochloride via Bilosomal Systems: In Vitro, Ex Vivo, and In Vivo Characterization Studies. AAPS PharmSciTech 2018, 19, 2276–2287. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Ali, A.A.; Ali, A.M.A.; Eissa, E.M.; Hassan, R.M.; El-Ela, F.I.A.; Hassan, A.H. Potential Use of Tailored Citicoline Chitosan-Coated Liposomes for Effective Wound Healing in Diabetic Rat Model. Int. J. Nanomed. 2022, 17, 555–575. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Eid, H.; Elkomy, M.; Khames, A.; Hassan, R.; El-Ela, F.A.; Yassin, H. Berberine Encapsulated Lecithin–Chitosan Nanoparticles as Innovative Wound Healing Agent in Type II Diabetes. Pharmaceutics 2021, 13, 1197. [Google Scholar] [CrossRef]
- El-Enin, H.A.A.; Elkomy, M.H.; Naguib, I.A.; Ahmed, M.F.; Alsaidan, O.A.; Alsalahat, I.; Ghoneim, M.M.; Eid, H.M. Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route. Pharmaceutics 2022, 15, 281. [Google Scholar] [CrossRef]
- Gibis, M.; Ruedt, C.; Weiss, J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef]
- Hasanovic, A.; Hollick, C.; Fischinger, K.; Valenta, C. Improvement in physicochemical parameters of DPPC liposomes and increase in skin permeation of aciclovir and minoxidil by the addition of cationic polymers. Eur. J. Pharm. Biopharm. 2010, 75, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Han, S.; Qu, Z.; Zhao, J.; Chen, Y.; Chen, Z.; Duan, J.; Pan, Y.; Tang, X. Penetration enhancement of lidocaine hydrochlorid by a novel chitosan coated elastic liposome for transdermal drug delivery. J. Biomed. Nanotechnol. 2011, 7, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Elkomy, M.H.; El Menshawe, S.F.; Salem, H.F. Development, Optimization, and In Vitro/In Vivo Characterization of Enhanced Lipid Nanoparticles for Ocular Delivery of Ofloxacin: The Influence of Pegylation and Chitosan Coating. AAPS PharmSciTech 2019, 20, 183. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.; Junginger, H. Chitosan and its derivatives as intestinal absorption enhancers. Adv. Drug Deliv. Rev. 2001, 50, S91–S101. [Google Scholar] [CrossRef]
- Kang, M.L.; Cho, C.S.; Yoo, H.S. Application of chitosan microspheres for nasal delivery of vaccines. Biotechnol. Adv. 2009, 27, 857–865. [Google Scholar] [CrossRef]
- He, W.; Guo, X.; Xiao, L.; Feng, M. Study on the mechanisms of chitosan and its derivatives used as transdermal penetration enhancers. Int. J. Pharm. 2009, 382, 234–243. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, I.M.; Fernande, D.C.; Cengiz, I.F.; Reis, R.L.; Oliveira, J.M. Hydrogels in the treatment of rheumatoid arthritis: Drug delivery systems and artificial matrices for dynamic in vitro models. J. Mater. Sci. Mater. Med. 2021, 32, 1–13. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, M.; Barros, L.B.; Palma, J.; González-Rodríguez, P.L.; Rabasco, A.M. Application of statistical experimental design to study the formulation variables influencing the coating process of lidocaine liposomes. Int. J. Pharm. 2007, 337, 336–345. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Wang, S.; Liu, R.; Wu, Z.; Wang, C.; Wang, Y.; Chen, M. Enhancing the Antitumor Activity of Berberine Hydrochloride by Solid Lipid Nanoparticle Encapsulation. AAPS PharmSciTech 2014, 15, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Eid, H.M.; Elkomy, M.H.; El Menshawe, S.F.; Salem, H.F. Transfersomal nanovesicles for nose-to-brain delivery of ofloxacin for better management of bacterial meningitis: Formulation, optimization by Box-Behnken design, characterization and in vivo pharmacokinetic study. J. Drug Deliv. Sci. Technol. 2019, 54, 101304. [Google Scholar] [CrossRef]
- Elkomy, M.H.; El Menshawe, S.F.; Eid, H.M.; Ali, A.M.A. Development of a nanogel formulation for transdermal delivery of tenoxicam: A pharmacokinetic–pharmacodynamic modeling approach for quantitative prediction of skin absorption. Drug Dev. Ind. Pharm. 2017, 43, 531–544. [Google Scholar] [CrossRef]
- Eid, H.M.; Naguib, I.A.; Alsantali, R.I.; Alsalahat, I.; Hegazy, A.M. Novel chitosan-coated niosomal formulation for improved management of bacterial conjunctivitis: A highly permeable and efficient ocular nanocarrier for azithromycin. J. Pharm. Sci. 2021, 110, 3027–3036. [Google Scholar] [CrossRef]
- Mahmoud, M.O.; Aboud, H.M.; Hassan, A.H.; Ali, A.A.; Johnston, T.P. Transdermal delivery of atorvastatin calcium from novel nanovesicular systems using polyethylene glycol fatty acid esters: Ameliorated effect without liver toxicity in poloxamer 407-induced hyperlipidemic rats. J. Control. Release 2017, 254, 10–22. [Google Scholar] [CrossRef]
- Aboud, H.; Ali, A.; El-Menshawe, S.F.; Elbary, A.A. Nanotransfersomes of carvedilol for intranasal delivery: Formulation, characterization and in vivo evaluation. Drug Deliv. 2015, 23, 2471–2481. [Google Scholar] [CrossRef] [Green Version]
- Elkomy, M.H.; Elmenshawe, S.F.; Eid, H.; Ali, A.M.A. Topical ketoprofen nanogel: Artificial neural network optimization, clustered bootstrap validation, and in vivo activity evaluation based on longitudinal dose response modeling. Drug Deliv. 2016, 23, 3294–3306. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. Methods Mol. Biol. 2003, 225, 115–121. [Google Scholar] [CrossRef] [PubMed]
- El-Badry, M.; Fetih, G.; Fathalla, D.; Shakeel, F. Transdermal delivery of meloxicam using niosomal hydrogels: In vitro and pharmacodynamic evaluation. Pharm. Dev.Technol. 2015, 20, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Hashim, I.I.A.; El-Magd, N.F.A.; El-Sheakh, A.R.; Hamed, M.F.; El-Gawad, A.E.H.A. Pivotal role of Acitretin nanovesicular gel for effective treatment of psoriasis: Ex vivo–in vivo evaluation study. Int. J. Nanomed. 2018, 13, 1059. [Google Scholar] [CrossRef] [Green Version]
- Salem, H.F.; Kharshoum, R.M.; Sayed, O.M.; Hakim, L.F.A. Formulation design and optimization of novel soft glycerosomes for enhanced topical delivery of celecoxib and cupferron by Box–Behnken statistical design. Drug Dev. Ind. Pharm. 2018, 44, 1871–1884. [Google Scholar] [CrossRef]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.A. Preparation of transfersomes encapsulating sildenafil aimed for transdermal drug delivery: Plackett–Burman design and characterization. J. Liposome Res. 2015, 25, 1–10. [Google Scholar] [CrossRef]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A new tool for effective dermal and transdermal drug delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-Mahallawi, A.M. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: In vitro characterization, ex vivo permeation and in vivo safety assessment. Int. J. Pharm. 2016, 513, 688–696. [Google Scholar] [CrossRef]
- Janga, K.Y.; Tatke, A.; Balguri, S.P.; Lamichanne, S.P.; Ibrahim, M.M.; Maria, D.N.; Jablonski, M.M.; Majumdar, S. Ion-sensitive in situ hydrogels of natamycin bilosomes for enhanced and prolonged ocular pharmacotherapy: In vitro permeability, cytotoxicity and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1039–1050. [Google Scholar] [CrossRef] [Green Version]
- Salama, H.; Mahmoud, A.; Kamel, A.O.; Hady, M.A.; A Awad, G. Brain delivery of olanzapine by intranasal administration of transfersomal vesicles. J. Liposome Res. 2012, 22, 336–345. [Google Scholar] [CrossRef]
- Aziz, D.E.; Abdelbary, A.A.; Elassasy, A.I. Investigating superiority of novel bilosomes over niosomes in the transdermal delivery of diacerein: In vitro characterization, ex vivo permeation and in vivo skin deposition study. J. Liposome Res. 2019, 29, 73–85. [Google Scholar] [CrossRef]
- Dora, C.P.; Singh, S.K.; Kumar, S.; Datusalia, A.K.; Deep, A. Development and characterization of nanoparticles of glibenclamide by solvent displacement method. Acta Pol. Pharm. Drug Res. 2010, 67, 283–290. [Google Scholar]
- Guo, J.; Ping, Q.; Jiang, G.; Huang, L.; Tong, Y. Chitosan-coated liposomes: Characterization and interaction with leuprolide. Int. J. Pharm. 2003, 260, 167–173. [Google Scholar] [CrossRef]
- Chaudhary, H.; Kohli, K.; Kumar, V. Nano-transfersomes as a novel carrier for transdermal delivery. Int. J. Pharm. 2013, 454, 367–380. [Google Scholar] [CrossRef]
- Aburahma, M.H. Bile salts-containing vesicles: Promising pharmaceutical carriers for oral delivery of poorly water-soluble drugs and peptide/protein-based therapeutics or vaccines. Drug Deliv. 2014, 23, 1–21. [Google Scholar] [CrossRef]
- Mandal, U.; Mahmood, S.; Taher, M. Experimental design and optimization of raloxifene hydrochloride loaded nanotransfersomes for transdermal application. Int. J. Nanomed. 2014, 9, 4331–4346. [Google Scholar] [CrossRef] [Green Version]
- Niu, M.; Tan, Y.; Guan, P.; Hovgaard, L.; Lu, Y.; Qi, J.; Lian, R.; Li, X.; Wu, W. Enhanced oral absorption of insulin-loaded liposomes containing bile salts: A mechanistic study. Int. J. Pharm. 2014, 460, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.P.; Vaz, A.F.M.; Correia, M.T.S.; Cerqueira, M.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Quercetin-Loaded Lecithin/Chitosan Nanoparticles for Functional Food Applications. Food Bioprocess Technol. 2014, 7, 1149–1159. [Google Scholar] [CrossRef] [Green Version]
- Abdellatif, M.M.; Khalil, I.; Khalil, M.A. Sertaconazole nitrate loaded nanovesicular systems for targeting skin fungal infection: In-vitro, ex-vivo and in-vivo evaluation. Int. J. Pharm. 2017, 527, 1–11. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, M.; Arroyo, C.; Cózar-Bernal, M.; Gonzalez-R, P.L.; León, J.; Calle, M.; Canca, D.; Rabasco, A. Deformability properties of timolol-loaded transfersomes based on the extrusion mechanism. Statistical optimization of the process. Drug Dev. Ind. Pharm. 2016, 42, 1683–1694. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.S.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef]
- Taveira, S.F.; Nomizo, A.; Lopez, R.F. Effect of the iontophoresis of a chitosan gel on doxorubicin skin penetration and cytotoxicity. J. Control. Release 2009, 134, 35–40. [Google Scholar] [CrossRef]
- Smith, J.; Wood, E.; Dornish, M. Effect of Chitosan on Epithelial Cell Tight Junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Elmowafy, M.; Shalaby, K.; Azmy, A.F.; Ahmad, N.; Zafar, A.; Eid, H.M. Development and machine-learning optimization of mucoadhesive nanostructured lipid carriers loaded with fluconazole for treatment of oral candidiasis. Drug Dev. Ind. Pharm. 2021, 47, 246–258. [Google Scholar] [CrossRef]
- O’Brien, R.W. Electroacoustic studies of moderately concentrated colloidal suspensions. Faraday Dis. Chem. Soc. 1990, 90, 301–312. [Google Scholar] [CrossRef]
- Zubairu, Y.; Negi, L.M.; Iqbal, Z.; Talegaonkar, S. Design and development of novel bioadhesive niosomal formulation for the transcorneal delivery of anti-infective agent: In-vitro and ex-vivo investigations. Asian J. Pharm. Sci. 2015, 10, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Moghimipour, E.; Ameri, A.; Handali, S. Absorption-Enhancing Effects of Bile Salts. Molecules 2015, 20, 14451–14473. [Google Scholar] [CrossRef] [Green Version]








| Independent Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1: Lipid concentration (%w/v) | 2.5 | 3.75 | 5 |
| X2: SDC concentration in lipid mixture (%w/w) | 5 | 10 | 15 |
| X3: CTS concentration (%w/v) | 0 | 0.125 | 0.25 |
| F | Lipid (% w/v) | SDC in Lipid Mixture (% w/w) | CTS (% w/v) | PS (nm) | EE (%) | ZP (mV) |
|---|---|---|---|---|---|---|
| 1 | 2.50 | 10 | 0.250 | 413.2 ± 5.45 | 51.3 ± 2.6 | (+) 30.9 ± 1.2 |
| 2 | 3.75 | 15 | 0.250 | 491.4 ± 3.58 | 65.8 ± 3.2 | (+) 27.5 ± 0.6 |
| 3 | 3.75 | 10 | 0.125 | 355.3 ± 7.30 | 77.3 ± 4.7 | (+) 20.3 ± 1.1 |
| 4 | 3.75 | 10 | 0.125 | 349.4 ± 16.12 | 73.5 ± 3.8 | (+) 23.6 ± 0.3 |
| 5 | 5.00 | 5 | 0.125 | 189.0 ± 2.29 | 86.2 ± 5.3 | (+) 17.6 ± 0.5 |
| 6 | 2.50 | 5 | 0.125 | 358.9 ± 6.31 | 60.2 ± 3.4 | (+) 24.4 ± 1.7 |
| 7 | 3.75 | 5 | 0.000 | 279.8 ± 13.24 | 83.4 ± 5.2 | (−) 30.2 ± 0.8 |
| 8 | 5.00 | 10 | 0.000 | 100.3 ± 1.06 | 89.0 ± 6.1 | (−) 33.1 ± 2.3 |
| 9 | 3.75 | 5 | 0.250 | 391.1 ± 8.36 | 69.3 ± 2.2 | (+) 35.1 ± 1.9 |
| 10 | 2.50 | 10 | 0.000 | 443.5 ± 11.93 | 63.2 ± 2.9 | (−) 32.1 ± 2.7 |
| 11 | 3.75 | 15 | 0.000 | 321.0 ± 15.28 | 81.3 ± 3.7 | (−) 34.9 ± 3.4 |
| 12 | 5.00 | 10 | 0.250 | 251.2 ± 9.87 | 84.0 ± 2.5 | (+) 28.4 ± 1.4 |
| 13 | 2.50 | 15 | 0.125 | 539.1 ± 25.28 | 57.2 ± 3.1 | (+) 20.5 ± 0.4 |
| 14 | 5.00 | 15 | 0.125 | 388.7 ± 12.34 | 85.3 ± 5.6 | (+) 16.7 ± 0.3 |
| 15 | 3.75 | 10 | 0.125 | 301.0 ± 10.29 | 72.9 ± 3.6 | (+) 21.0 ± 0.7 |
| 16 | 3.75 | 10 | 0.125 | 373.4 ± 5.92 | 75.3 ± 2.4 | (+) 22.6 ± 1.1 |
| 17 | 3.75 | 10 | 0.125 | 289.5 ± 7.27 | 70.3 ± 4.8 | (+) 22.2 ± 1.9 |
| Source | Size (nm) | EE% | ZP (mV) | |||
|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | F | p-Value | |
| Model | 14.45 | 0.0002 | 90.62 | <0.0001 | 795.32 | <0.0001 |
| X1: Lipid concentration (%w/v) | 26.43 | 0.0002 | 230.84 | <0.0001 | 7.85 | 0.0160 |
| X2: SDC concentration in lipid mixture (%w/w) | 10.60 | 0.0063 | 1.64 | 0.2223 | 11.55 | 0.0053 |
| X3: CTS concentration (%w/v) | 6.33 | 0.0258 | 39.37 | <0.0001 | 2511.95 | <0.0001 |
| X32 | 649.94 | <0.0001 | ||||
| Lack of Fit | 3.06 | 0.1471 | 0.9911 | 0.5482 | 2.28 | 0.2221 |
| Model | Linear | Linear | Quadratic | |||
| Adjusted R2 | 0.7161 | 0.9438 | 0.9950 | |||
| R2 | 0.7693 | 0.9544 | 0.9962 | |||
| %CV | 16.53 | 3.58 | 16.76 | |||
| Predicted R2 | 0.5478 | 0.9190 | 0.9920 | |||
| Adequate precision | 12.2408 | 31.2946 | 69.7784 | |||
| SD | 56.72 | 2.62 | 1.78 | |||
| Response Variables | Experimental Value | Expected Value | Prediction Error (%) * |
|---|---|---|---|
| Particle size (nm) | 202.3 | 188.5 | 6.8 |
| Entrapment (%) | 83.8 | 86.9 | 3.7 |
| Zeta potential (mV) | 30.8 | 28.3 | 8.1 |
| Formulation | Cumulative BER Permeated at 24 h (μg/cm2) | Permeability Coefficient (cm/h) | Flux Jss (µg·cm−2 h−1) |
|---|---|---|---|
| BER-CTS-BLS | 314.5 ± 26.87 | 0.0037 ± 0.00025 | 3.69 ± 0.56 |
| BER solution | 189.5 ± 14.12 | 0.0026 ± 0.00032 | 2.71 ± 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkomy, M.H.; Alruwaili, N.K.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Ahmad, N.; Alsalahat, I.; Ghoneim, M.M.; Eissa, E.M.; Eid, H.M. Surface-Modified Bilosomes Nanogel Bearing a Natural Plant Alkaloid for Safe Management of Rheumatoid Arthritis Inflammation. Pharmaceutics 2022, 14, 563. https://doi.org/10.3390/pharmaceutics14030563
Elkomy MH, Alruwaili NK, Elmowafy M, Shalaby K, Zafar A, Ahmad N, Alsalahat I, Ghoneim MM, Eissa EM, Eid HM. Surface-Modified Bilosomes Nanogel Bearing a Natural Plant Alkaloid for Safe Management of Rheumatoid Arthritis Inflammation. Pharmaceutics. 2022; 14(3):563. https://doi.org/10.3390/pharmaceutics14030563
Chicago/Turabian StyleElkomy, Mohammed H., Nabil K. Alruwaili, Mohammed Elmowafy, Khaled Shalaby, Ameeduzzafar Zafar, Naveed Ahmad, Izzeddin Alsalahat, Mohammed M. Ghoneim, Essam M. Eissa, and Hussein M. Eid. 2022. "Surface-Modified Bilosomes Nanogel Bearing a Natural Plant Alkaloid for Safe Management of Rheumatoid Arthritis Inflammation" Pharmaceutics 14, no. 3: 563. https://doi.org/10.3390/pharmaceutics14030563