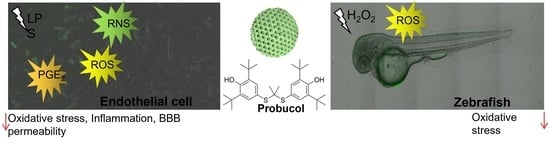
Enhanced Antioxidant Effects of the Anti-Inflammatory Compound Probucol When Released from Mesoporous Silica Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Synthesis of AMS-6
2.3. Scanning Electron Microscopy
2.4. Dynamic Light Scattering (DLS)
2.5. Powder X-ray Diffraction (XRD)
2.6. Fourier Transform Infrared Spectroscopy
2.7. Nitrogen Adsorption/Desorption Isotherm
2.8. Drug Loading
2.9. Thermogravimetric Analysis (TGA)
2.10. Differential Scanning Calorimetry (DSC)
2.11. Drug Dissolution and Release
2.12. Cells and Cell Culture
2.13. Oxidative Stress and Cell Viability Assays
2.14. DCFDA Cellular Reactive Oxygen Stress Measurement
2.15. Mitochondria Hydroxyl Assay
2.16. Nitric Oxide Assay
2.17. Peroxynitrite Assay
2.18. COX Enzyme Activity
2.19. PGE2 Measurement
2.20. TNF-α Assay
2.21. Flow Cytometry
2.22. Preparation of Endothelial Cells for Cellular Uptake of Probucol
2.23. Preparation of Standard Samples
2.24. HPLC Conditions
2.25. Data Analysis
2.26. TEER and FITC Dextran Permeability Measurements
2.27. FITC Dextran Permeability Coefficient Measurements and Calculations
2.28. Calculation of the Permeability Coefficient (Pe)
2.29. Zebrafish Experiments
2.30. Measurement of ROS Concentration in Zebrafish
2.31. Statistical Analysis
3. Results
3.1. Material Characterization
3.2. Drug Loading and Dissolution Kinetics
3.3. In Vitro Attenuation of Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engelhardt, B.; Sorokin, L. The blood–brain and the blood–cerebrospinal fluid barriers: Function and dysfunction. Semin. Immunopathol. 2009, 31, 497–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pober, J.S.; Tellides, G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012, 33, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kukreja, R.C.; Kontos, H.A.; Hess, M.L.; Ellis, E.F. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ. Res. 1986, 59, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001, 21, 2–14. [Google Scholar] [CrossRef]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol.—Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [Green Version]
- Minghetti, L. Cyclooxygenase-2 (COX-2) in Inflammatory and Degenerative Brain Diseases. J. Neuropathol. Exp. Neurol. 2004, 63, 901–910. [Google Scholar] [CrossRef] [Green Version]
- Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. J. Virtual Libr. 2008, 13, 7210. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, L.B.; Dohgu, S.; Sultana, R.; Lynch, J.L.; Owen, J.B.; Erickson, M.A.; Shah, G.N.; Price, T.O.; Fleegal-Demotta, M.A.; Butterfiled, D.A. Lipopolysaccharide alters the blood–brain barrier transport of amyloid β protein: A mechanism for inflammation in the progression of Alzheimer’s disease. Brain Behav. Immun. 2009, 23, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Nagyőszi, P.; Wilhelm, I.; Farkas, A.E.; Fazakas, C.; Dung, N.T.K.; Haskó, J.; Krizbai, I.A. Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem. Int. 2010, 57, 556–564. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef] [Green Version]
- Engström, L.; Ruud, J.; Eskilsson, A.; Larsson, A.; Mackerlova, L.; Kugelberg, U.; Qian, H.; Vasilache, A.M.; Larsson, P.; Engblom, D. Lipopolysaccharide-induced fever depends on prostaglandin E2 production specifically in brain endothelial cells. Endocrinology 2012, 153, 4849–4861. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, Y.; Matsumura, K.; Ballou, L.; Morham, S.; Blatteis, C. The febrile response to lipopolysaccharide is blocked in cyclooxygenase-2−/−, but not in cyclooxygenase-1−/− mice. Brain Res. 1999, 825, 86–94. [Google Scholar] [CrossRef]
- Jaworowicz, D.J., Jr.; Korytko, P.J.; Lakhman, S.S.; Boje, K.M. Nitric oxide and prostaglandin E2 formation parallels blood–brain barrier disruption in an experimental rat model of bacterial meningitis. Brain Res. Bull. 1998, 46, 541–546. [Google Scholar] [CrossRef]
- Banks, W.A.; Gray, A.M.; Erickson, M.A.; Salameh, T.S.; Damodarasamy, M.; Sheibani, N.; Meabon, J.S.; Wing, E.E.; Morofuji, Y.; Cook, D.G. Lipopolysaccharide-induced blood-brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflamm. 2015, 12, 223. [Google Scholar] [CrossRef] [Green Version]
- Wilhelms, D.B.; Kirilov, M.; Mirrasekhian, E.; Eskilsson, A.; Kugelberg, U.Ö.; Klar, C.; Ridder, D.A.; Herschman, H.R.; Schwaninger, M.; Blomqvist, A. Deletion of prostaglandin E2 synthesizing enzymes in brain endothelial cells attenuates inflammatory fever. J. Neurosci. 2014, 34, 11684–11690. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-L.; Jin, J.-M.; Yang, C.; Wang, P.; Huang, F.; Wu, H.; Zhang, B.-B.; Shi, H.-L.; Wu, X.-J. Isoastragaloside I suppresses LPS-induced tight junction disruption and monocyte adhesion on bEnd.3 cells via an activating Nrf2 antioxidant defense system. RSC Adv. 2018, 8, 464–471. [Google Scholar] [CrossRef] [Green Version]
- Ramilo, O.; Sáez-Llorens, X.; Mertsola, J.; Jafari, H.; Olsen, K.D.; Hansen, E.J.; Yoshinaga, M.; Ohkawara, S.; Nariuchi, H.; McCracken, G., Jr. Tumor necrosis factor alpha/cachectin and interleukin 1 beta initiate meningeal inflammation. J. Exp. Med. 1990, 172, 497–507. [Google Scholar] [CrossRef]
- Zucoloto, A.Z.; Manchope, M.F.; Staurengo-Ferrari, L.; Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Saraiva, A.L.; Cecílio, N.T.; Alves-Filho, J.C.; Cunha, T.M.; Menezes, G.B. Probucol attenuates lipopolysaccharide-induced leukocyte recruitment and inflammatory hyperalgesia: Effect on NF-κB activation and cytokine production. Eur. J. Pharmacol. 2017, 809, 52–63. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, J.H.; Kim, H.; Kim, S.Y.; Hwang, J.Y.; Hong, K.W.; Bae, S.S.; Choi, B.T.; Lee, S.-W.; Shin, H.K. Probucol inhibits LPS-induced microglia activation and ameliorates brain ischemic injury in normal and hyperlipidemic mice. Acta Pharmacol. Sin. 2016, 37, 1031–1044. [Google Scholar] [CrossRef] [Green Version]
- Takechi, R.; Galloway, S.; Pallebage-Gamarallage, M.M.; Lam, V.; Dhaliwal, S.S.; Mamo, J.C. Probucol prevents blood–brain barrier dysfunction in wild-type mice induced by saturated fat or cholesterol feeding. Clin. Exp. Pharmacol. Physiol. 2013, 40, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, M.; Naito, M.; Funaki, C.; Hayashi, T.; Asai, K.; Kuzuya, F. Probucol prevents oxidative injury to endothelial cells. J. Lipid Res. 1991, 32, 197–204. [Google Scholar] [CrossRef]
- Barnhart, R.L.; Busch, S.J.; Jackson, R.L. Concentration-dependent antioxidant activity of probucol in low density lipoproteins in vitro: Probucol degradation precedes lipoprotein oxidation. J. Lipid Res. 1989, 30, 1703–1710. [Google Scholar] [CrossRef]
- Witting, P.K.; Wu, B.J.; Raftery, M.; Southwell-Keely, P.; Stocker, R. Probucol protects against hypochlorite-induced endothelial dysfunction identification of a novel pathway of probucol oxidation to a biologically active intermediate. J. Biol. Chem. 2005, 280, 15612–15618. [Google Scholar] [CrossRef] [Green Version]
- Mamo, J.C.; Lam, V.; Brook, E.; Mooranian, A.; Al-Salami, H.; Fimognari, N.; Nesbit, M.; Takechi, R. Probucol prevents blood–brain barrier dysfunction and cognitive decline in mice maintained on pro-diabetic diet. Diabetes Vasc. Dis. Res. 2019, 16, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Mo, J.; He, L.; Ma, B.; Chen, T. Tailoring particle size of mesoporous silica nanosystem to antagonize glioblastoma and overcome blood–brain barrier. ACS Appl. Mater. Interfaces 2016, 8, 6811–6825. [Google Scholar] [CrossRef]
- He, L.; Lai, H.; Chen, T. Dual-function nanosystem for synergetic cancer chemo-/radiotherapy through ROS-mediated signaling pathways. Biomaterials 2015, 51, 30–42. [Google Scholar] [CrossRef]
- Karimzadeh, M.; Rashidi, L.; Ganji, F. Mesoporous silica nanoparticles for efficient rivastigmine hydrogen tartrate delivery into SY5Y cells. Drug Dev. Ind. Pharm. 2017, 43, 628–636. [Google Scholar] [CrossRef]
- Mendiratta, S.; Hussein, M.; Nasser, H.A.; Ali, A.A.A. Multidisciplinary Role of Mesoporous Silica Nanoparticles in Brain Regeneration and Cancers: From Crossing the Blood–Brain Barrier to Treatment. Part. Part. Syst. Charact. 2019, 36, 1900195. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, O.; Ohsuna, T.; Liu, Z.; Sakamoto, Y.; Garcia-Bennett, A.E. Structural study of mesoporous materials by electron microscopy. In Studies in Surface Science and Catalysis; Terasaki, O., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 148, pp. 261–288. [Google Scholar]
- Wan, Y.; Zhao, D. On the Controllable Soft-Templating Approach to Mesoporous Silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef]
- Atluri, R.; Hedin, N.; Garcia-Bennett, A.E. Hydrothermal Phase Transformation of Bicontinuous Cubic Mesoporous Material AMS-6. Chem. Mater. 2008, 20, 3857–3866. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Meinander, A.; Peuhu, E.; Niemi, R.; Eriksson, J.E.; Sahlgren, C.; Linden, M. Targeting of porous hybrid silica nanoparticles to cancer cells. ACS Nano 2009, 3, 197–206. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. New developments in ordered mesoporous materials for drug delivery. J. Mater. Chem. 2010, 20, 5593. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Sahlgren, C.; Linden, M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles—Opportunities & challenges. Nanoscale 2010, 2, 1870–1883. [Google Scholar] [CrossRef]
- Stromme, M.; Brohede, U.; Atluri, R.; Garcia-Bennett, A.E. Mesoporous silica-based nanomaterials for drug delivery: Evaluation of structural properties associated with release rate. Wiley Interdiscip. Rev.—Nanomed. Nanobiotechnol. 2009, 1, 140–148. [Google Scholar] [CrossRef]
- Kjellman, T.; Xia, X.; Alfredsson, V.; Garcia-Bennett, A.E. Influence of microporosity in SBA-15 on the release properties of anticancer drug dasatinib. J. Mater. Chem. B 2014, 2, 5265. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Shen, Y.; Cao, B.; Snyder, N.R.; Woeppel, K.M.; Eles, J.R.; Cui, X.T. ROS responsive resveratrol delivery from LDLR peptide conjugated PLA-coated mesoporous silica nanoparticles across the blood-brain barrier. J. Nanobiotechnology 2018, 16, 13. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Bennett, A.; Che, S.; Miyasaka, K.; Sakamoto, Y.; Ohsuna, T.; Liu, Z.; Terasaki, O.; Sayari, A.; Jaroniec, M. Studies of anionic surfactant templated mesoporous structures by electron microscopy. In Nanoporous Materials IV, Proceedings of the 4th International Symposium on Nanoporous Materials, Niagara Falls, ON, Canada, 7–10 June 2005; Abdelhamid, S., Mietek, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 156, pp. 11–18. [Google Scholar]
- Garcia-Bennett, A.; Terasaki, O.; Che, S.; Tatsumi, T. Structural investigations of AMS-n mesoporous materials by transmission electron microscopy. Chem. Mater. 2004, 16, 813–821. [Google Scholar] [CrossRef]
- Ulrika, B.; Rambabu, A.; Alfonso, E.G.-B.; Maria, S. Sustained Release from Mesoporous Nanoparticles: Evaluation of Structural Properties Associated with Release Rate. Curr. Drug Deliv. 2008, 5, 177–185. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarmah, S.; Marrs, J.A. Zebrafish as a vertebrate model system to evaluate effects of environmental toxicants on cardiac development and function. Int. J. Mol. Sci. 2016, 17, 2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svahn, A.J.; Don, E.K.; Badrock, A.P.; Cole, N.J.; Graeber, M.B.; Yerbury, J.J.; Chung, R.; Morsch, M. Nucleo-cytoplasmic transport of TDP-43 studied in real time: Impaired microglia function leads to axonal spreading of TDP-43 in degenerating motor neurons. Acta Neuropathol. 2018, 136, 445–459. [Google Scholar] [CrossRef] [Green Version]
- Radford, R.A.; Morsch, M.; Rayner, S.L.; Cole, N.J.; Pountney, D.L.; Chung, R.S. The established and emerging roles of astrocytes and microglia in amyotrophic lateral sclerosis and frontotemporal dementia. Front. Cell. Neurosci. 2015, 9, 414. [Google Scholar] [CrossRef] [Green Version]
- Morsch, M.; Radford, R.; Lee, A.; Don, E.; Badrock, A.; Hall, T.; Cole, N.; Chung, R. In vivo characterization of microglial engulfment of dying neurons in the zebrafish spinal cord. Front. Cell. Neurosci. 2015, 9, 321. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Morsch, M.; Shami, G.J.; Chung, R.S.; Braet, F. Observation and characterisation of macrophages in zebrafish liver. Micron 2020, 132, 102851. [Google Scholar] [CrossRef]
- Radford, R.A.W.; Vidal-Itriago, A.; Scherer, N.M.; Lee, A.; Graeber, M.; Chung, R.S.; Morsch, M. Evidence for a Growing Involvement of Glia in Amyotrophic Lateral Sclerosis. In Spectrums of Amyotrophic Lateral Sclerosis; Shaw, C.A., Morrice, J.R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 123–142. [Google Scholar]
- Jia, J.; Zhang, Y.; Yuan, X.; Qin, J.; Yang, G.; Yu, X.; Wang, B.; Sun, C.; Li, W. Reactive oxygen species participate in liver function recovery during compensatory growth in zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2018, 499, 285–290. [Google Scholar] [CrossRef]
- Ko, E.-Y.; Cho, S.-H.; Kwon, S.-H.; Eom, C.-Y.; Jeong, M.S.; Lee, W.; Kim, S.-Y.; Heo, S.-J.; Ahn, G.; Lee, K.P. The roles of NF-κB and ROS in regulation of pro-inflammatory mediators of inflammation induction in LPS-stimulated zebrafish embryos. Fish Shellfish. Immunol. 2017, 68, 525–529. [Google Scholar] [CrossRef]
- Sullivan, C.; Kim, C.H. Zebrafish as a model for infectious disease and immune function. Fish Shellfish. Immunol. 2008, 25, 341–350. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2′, 7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free. Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef]
- Formella, I.; Svahn, A.J.; Radford, R.A.W.; Don, E.K.; Cole, N.J.; Hogan, A.; Lee, A.; Chung, R.S.; Morsch, M. Real-time visualization of oxidative stress-mediated neurodegeneration of individual spinal motor neurons in vivo. Redox Biol. 2018, 19, 226–234. [Google Scholar] [CrossRef]
- Gaillard, P.J.; de Boer, A.B.G.; Breimer, D.D. Pharmacological investigations on lipopolysaccharide-induced permeability changes in the blood–brain barrier in vitro. Microvasc. Res. 2003, 65, 24–31. [Google Scholar] [CrossRef]
- Atluri, R.; Sakamoto, Y.; Garcia-Bennett, A.E. Co-structure directing agent induced phase transformation of mesoporous materials. Langmuir 2009, 25, 3189–3195. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, C.; Che, S. Amino and quaternary ammonium group functionalized mesoporous silica: An efficient ion-exchange method to remove anionic surfactant from AMS. Microporous Mesoporous Mater. 2008, 116, 299–307. [Google Scholar] [CrossRef]
- Witasp, E.; Kupferschmidt, N.; Bengtsson, L.; Hultenby, K.; Smedman, C.; Paulie, S.; Garcia-Bennett, A.E.; Fadeel, B. Efficient internalization of mesoporous silica particles of different sizes by primary human macrophages without impairment of macrophage clearance of apoptotic or antibody-opsonized target cells. Toxicol. Appl. Pharmacol. 2009, 239, 306–319. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Lastoskie, C.; Gubbins, K.E.; Quirke, N. Pore size distribution analysis of microporous carbons: A density functional theory approach. J. Phys. Chem. 1993, 97, 4786–4796. [Google Scholar] [CrossRef]
- Oparka, M.; Walczak, J.; Malinska, D.; van Oppen, L.M.; Szczepanowska, J.; Koopman, W.J.; Wieckowski, M.R. Quantifying ROS levels using CM-H2DCFDA and HyPer. Methods 2016, 109, 3–11. [Google Scholar] [CrossRef]
- Watson, P.M.D.; Paterson, J.C.; Thom, G.; Ginman, U.; Lundquist, S.; Webster, C.I. Modelling the endothelial blood-CNS barriers: A method for the production of robust in vitro models of the rat blood-brain barrier and blood-spinal cord barrier. BMC Neurosci. 2013, 14, 59. [Google Scholar] [CrossRef] [Green Version]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish Danio (“Brachydanio rerio”); University of Oregon: Eugene, OR, USA, 2007. [Google Scholar]
- Li, Z.; Su, K.; Cheng, B.; Deng, Y. Organically modified MCM-type material preparation and its usage in controlled amoxicillin delivery. J. Colloid Interface Sci. 2010, 342, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Marler, B.; Oberhagemann, U.; Vortmann, S.; Gies, H. Influence of the sorbate type on the XRD peak intensities of loaded MCM-41. Microporous Mater. 1996, 6, 375–383. [Google Scholar] [CrossRef]
- Wang, L.; Roitberg, A.; Meuse, C.; Gaigalas, A.K. Raman and FTIR spectroscopies of fluorescein in solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 1781–1791. [Google Scholar] [CrossRef]
- Garcia-Bennett, A.E.; Lau, M.; Bedford, N. Probing the Amorphous State of Pharmaceutical Compounds Within Mesoporous Material Using Pair Distribution Function Analysis. J. Pharm. Sci. 2018, 107, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.; Giri, K.; Garcia-Bennett, A.E. Antioxidant properties of probucol released from mesoporous silica. Eur. J. Pharm. Sci. 2019, 138, 105038. [Google Scholar] [CrossRef] [PubMed]
- Heeg, J.F.; Hiser, M.F.; Satonin, D.K.; Rose, J.Q. Pharmacokinetics of Probucol in Male Rats. J. Pharm. Sci. 1984, 73, 1758–1763. [Google Scholar] [CrossRef] [PubMed]
- Hewett, S.J.; Bell, S.C.; Hewett, J.A. Contributions of cyclooxygenase-2 to neuroplasticity and neuropathology of the central nervous system. Pharmacol. Ther. 2006, 112, 335–357. [Google Scholar] [CrossRef] [PubMed]
- Kupferschmidt, N.; Qazi, K.R.; Kemi, C.; Vallhov, H.; Garcia-Bennett, A.E.; Gabrielsson, S.; Scheynius, A. Mesoporous silica particles potentiate antigen-specific T-cell responses. Nanomedicine 2014, 9, 1835–1846. [Google Scholar] [CrossRef]
- Garcia-Bennett, A.E.; Kozhevnikova, M.; Konig, N.; Zhou, C.; Leao, R.; Knopfel, T.; Pankratova, S.; Trolle, C.; Berezin, V.; Bock, E.; et al. Delivery of differentiation factors by mesoporous silica particles assists advanced differentiation of transplanted murine embryonic stem cells. Stem Cells Transl. Med. 2013, 2, 906–915. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Mayhan, W.G.; Heistad, D.D. Permeability of blood-brain barrier to various sized molecules. Am. J. Physiol.—Heart Circ. Physiol. 1985, 248, H712–H718. [Google Scholar] [CrossRef]
- Nag, S. Blood-brain barrier permeability using tracers and immunohistochemistry. In The Blood-Brain Barrier; Springer: Berlin/Heidelberg, Germany, 2003; pp. 133–144. [Google Scholar]
- De Vries, H.E.; Blom-Roosemalen, M.; de Boer, A.G.; Van Berkel, T.; Breimer, D.D.; Kuiper, J. Effect of endotoxin on permeability of bovine cerebral endothelial cell layers in vitro. J. Pharmacol. Exp. Ther. 1996, 277, 1418–1423. [Google Scholar]
- Ziylan, Y.Z.; Robinson, P.J.; Rapoport, S.I. Blood-brain barrier permeability to sucrose and dextran after osmotic opening. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1984, 247, R634–R638. [Google Scholar] [CrossRef]
- Liu, T.-P.; Wu, S.-H.; Chen, Y.-P.; Chou, C.-M.; Chen, C.-T. Biosafety evaluations of well-dispersed mesoporous silica nanoparticles: Towards in vivo-relevant conditions. Nanoscale 2015, 7, 6471–6480. [Google Scholar] [CrossRef]
- Zhu, B.; He, W.; Hu, S.; Kong, R.; Yang, L. The fate and oxidative stress of different sized SiO2 nanoparticles in zebrafish (Danio rerio) larvae. Chemosphere 2019, 225, 705–712. [Google Scholar] [CrossRef]
- Han, M.; Pendem, S.; Teh, S.L.; Sukumaran, D.K.; Wu, F.; Wilson, J.X. Ascorbate protects endothelial barrier function during septic insult: Role of protein phosphatase type 2A. Free. Radic. Biol. Med. 2010, 48, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Wilson, J.X. Peroxynitrite-dependent activation of protein phosphatase type 2A mediates microvascular endothelial barrier dysfunction. Cardiovasc. Res. 2009, 81, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Predescu, D.; Predescu, S.; Shimizu, J.; Miyawaki-Shimizu, K.; Malik, A.B. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L371–L381. [Google Scholar] [CrossRef]
- Bucci, M.; Roviezzo, F.; Posadas, I.; Yu, J.; Parente, L.; Sessa, W.C.; Ignarro, L.J.; Cirino, G. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 904–908. [Google Scholar] [CrossRef] [Green Version]
- Kurose, I.; Kubes, P.; Wolf, R.; Anderson, D.; Paulson, J.; Miyasaka, M.; Granger, D. Inhibition of nitric oxide production. Mechanisms of vascular albumin leakage. Circ. Res. 1993, 73, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Heller, R.; Münscher-Paulig, F.; Gräbner, R.; Till, U. L-Ascorbic acid potentiates nitric oxide synthesis in endothelial cells. J. Biol. Chem. 1999, 274, 8254–8260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.L.; Li, N.s.; Li, Y.J.; Deng, H.W. Probucol preserves endothelial function by reduction of the endogenous nitric oxide synthase inhibitor level. Br. J. Pharmacol. 2002, 135, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Westendorp, R.G.; Draijer, R.; Meinders, E.; van Hinsbergh, V.W. Cyclic-GMP-mediated decrease in permeability of human umbilical and pulmonary artery endothelial cell monolayers. J. Vasc. Res. 1994, 31, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Draijer, R.; Atsma, D.E.; van der Laarse, A.; van Hinsbergh, V.W. cGMP and nitric oxide modulate thrombin-induced endothelial permeability: Regulation via different pathways in human aortic and umbilical vein endothelial cells. Circ. Res. 1995, 76, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, G.L.; Jin, X.; Pryor, W.A. Stopped-flow kinetic study of the reaction of ascorbic acid with peroxynitrite. Arch. Biochem. Biophys. 1995, 322, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Nakaoke, R.; Dohgu, S.; Banks, W.A. Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain Behav. Immun. 2006, 20, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Mark, K.S.; Miller, D.W. Increased permeability of primary cultured brain microvessel endothelial cell monolayers following TNF-α exposure. Life Sci. 1999, 64, 1941–1953. [Google Scholar] [CrossRef]
- Sheng, L.; Jiao, B.; Shao, L.; Bi, S.; Cheng, C.; Zhang, J.; Jiang, Y. Probucol inhibits hydrogen peroxide to induce apoptosis of vascular smooth muscle cells. Mol. Med. Rep. 2013, 7, 1185–1190. [Google Scholar] [CrossRef]
- Iqbal, M.; Sharma, S.D.; Okada, S. Probucol as a potent inhibitor of oxygen radical-induced lipid peroxidation and DNA damage: In vitro studies. Redox Rep. 2004, 9, 167–172. [Google Scholar] [CrossRef]
- Goton, N.; Shimizu, K.; Komuro, E.; Tsuchiya, J.; Noguchi, N.; Niki, E. Antioxidant activities of probucol against lipid peroxidations. Biochim. Biophys. Acta BBA—Lipids Lipid Metab. 1992, 1128, 147–154. [Google Scholar] [CrossRef]
- Santos, D.B.; Colle, D.; Moreira, E.L.; Santos, A.A.; Hort, M.A.; Santos, K.; Oses, J.P.; Razzera, G.; Farina, M. Probucol Protects Neuronal Cells Against Peroxide-Induced Damage and Directly Activates Glutathione Peroxidase-1. Mol. Neurobiol. 2020, 57, 2345–3257. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, C.; Chen, S.; Zhao, H.; Zhou, K.; Wang, W.; Yuan, Y.; Li, Z.; Guo, Y.; Shen, Z. Activation of the Nrf2/ARE signaling pathway by probucol contributes to inhibiting inflammation and neuronal apoptosis after spinal cord injury. Oncotarget 2017, 8, 52078. [Google Scholar] [CrossRef] [Green Version]
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta BBA—Gen. Subj. 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Beyer, R.E. The role of ascorbate in antioxidant protection of biomembranes: Interaction with vitamin E and coenzyme Q. J. Bioenerg. Biomembr. 1994, 26, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Francis, S.; Delgoda, R.; Young, R. Effects of embryonic exposure to α-lipoic acid or ascorbic acid on hatching rate and development of zebrafish (Danio rerio). Aquac. Res. 2012, 43, 777–788. [Google Scholar] [CrossRef]
- Kupferschmidt, N.; Xia, X.; Labrador, R.H.; Atluri, R.; Ballell, L.; Garcia-Bennett, A.E. In vivo oral toxicological evaluation of mesoporous silica particles. Nanomedicine 2013, 8, 57–64. [Google Scholar] [CrossRef]
- Tan, A.; Eskandar, N.G.; Rao, S.; Prestidge, C.A. First in man bioavailability and tolerability studies of a silica–lipid hybrid (Lipoceramic) formulation: A Phase I study with ibuprofen. Drug Deliv. Transl. Res. 2014, 4, 212–221. [Google Scholar] [CrossRef]
- Lungare, S.; Hallam, K.; Badhan, R.K.S. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016, 513, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Simon, B.C.; Haudenschild, C.C.; Cohen, R.A. Preservation of endothelium-dependent relaxation in atherosclerotic rabbit aorta by probucol. J. Cardiovasc. Pharmacol. 1993, 21, 893–901. [Google Scholar] [CrossRef]
- Inoue, N.; Ohara, Y.; Fukai, T.; Harrison, D.G.; Nishida, K.i. Probucol improves endothelial-dependent relaxation and decreases vascular superoxide production in cholesterol-fed rabbits. Am. J. Med. Sci. 1998, 315, 242–247. [Google Scholar]
- Kita, T.; Nagano, Y.; Yokode, M.; Ishii, K.; Kume, N.; Ooshima, A.; Yoshida, H.; Kawai, C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc. Natl. Acad. Sci. USA 1987, 84, 5928–5931. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Material | aa (Å) | SABET b (m2/g) | Tvol (cm3/g) | PSD (Å) | DT c (°C) | Tm d (°C) | ΔHm (J/g) |
|---|---|---|---|---|---|---|---|
| CAL-AMS-6 | 114.8 | 777.3 | 0.75 | 46.7 | - | - | - |
| PB | - | - | - | - | 210.4 | 124.2 | 54.5 |
| AMS-6 PB (29.5%) | 113.9 | 197.7 | 0.21 | 40.3 | 256.5 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, M.; Sealy, B.; Combes, V.; Morsch, M.; Garcia-Bennett, A.E. Enhanced Antioxidant Effects of the Anti-Inflammatory Compound Probucol When Released from Mesoporous Silica Particles. Pharmaceutics 2022, 14, 502. https://doi.org/10.3390/pharmaceutics14030502
Lau M, Sealy B, Combes V, Morsch M, Garcia-Bennett AE. Enhanced Antioxidant Effects of the Anti-Inflammatory Compound Probucol When Released from Mesoporous Silica Particles. Pharmaceutics. 2022; 14(3):502. https://doi.org/10.3390/pharmaceutics14030502
Chicago/Turabian StyleLau, Michael, Benjamin Sealy, Valery Combes, Marco Morsch, and Alfonso E. Garcia-Bennett. 2022. "Enhanced Antioxidant Effects of the Anti-Inflammatory Compound Probucol When Released from Mesoporous Silica Particles" Pharmaceutics 14, no. 3: 502. https://doi.org/10.3390/pharmaceutics14030502