Novel Cocrystals of Vonoprazan: Machine Learning-Assisted Discovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
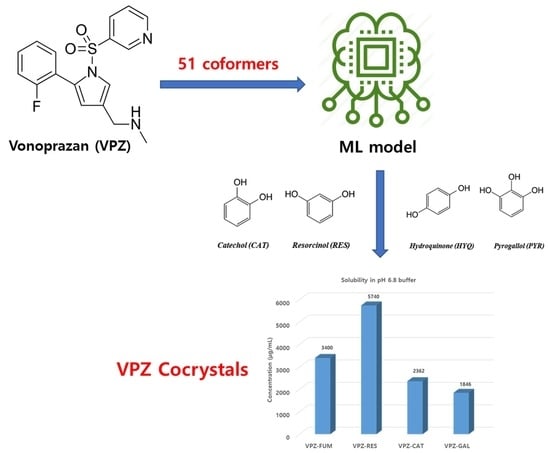
2.2. Prediction of Cocrystal Formation of VPZ with 51 Coformers
2.3. Experimental Screening of Cocrystals
2.4. Bulk Synthesis Using Reaction Crystallization
2.5. Solid-State Characterization
2.6. Solubility and Intrinsic Dissolution Rate (IDR)
3. Results and Discussion
3.1. Virtual and Experimental Screening
3.2. Characterization of VPZ Cocrystals
3.3. Solubility and IDR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Inatomi, N.; Matsukawa, J.; Sakurai, Y.; Otake, K. Potassium-competitive Acid Blockers: Advanced Therapeutic Option for Acid-Related Diseases. Pharmacol. Ther. 2016, 168, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.; Imanishi, A.; Matsukawa, J.; Tsukimi, Y.; Nishida, H.; Arikawa, Y.; Hirase, K.; Kajino, M.; Inatomi, N. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J. Pharmacol. Exp. Ther. 2010, 335, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.; Suzuki, H. Role of Acid Suppression in Acid-Related Diseases: Proton Pump Inhibitor and Potassium-Competitive Acid Blocker. J. Neurogastroenterol. Motil. 2019, 25, 6–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.; Ko, J.-W.; Lee, H.; Kim, S.; Kim, B.; Song, G.S.; Kim, J. Co-Administration of Vonoprazan, Not Tegoprazan, Affects the Pharmacokinetics of Atorvastatin in Healthy Male Subjects. Front. Pharmacol. 2021, 12, 754849. [Google Scholar] [CrossRef]
- Okuyama, M.; Nakahara, K.; Iwakura, N.; Hasegawa, T.; Oyama, M.; Inoue, A.; Ishizu, H.; Satoh, H.; Fujiwara, Y. Factors Associated with Potassium-Competitive Acid Blocker Non-Response in Patients with Proton Pump Inhibitor-Refractory Gastroesophageal Reflux Disease. Digestion 2017, 95, 281–287. [Google Scholar] [CrossRef]
- Sakurai, Y.; Mori, Y.; Okamoto, H.; Nishimura, A.; Komura, E.; Araki, T.; Shiramoto, M. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects—A randomised open-label cross-over study. Aliment. Pharmacol. Ther. 2015, 42, 719–730. [Google Scholar] [CrossRef]
- Garnock-Jones, K.P. Vonoprazan: First Global Approval. Drugs 2015, 75, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, Y.; Oda, K.; Funao, N.; Nishimura, A.; Soen, S.; Kawai, T.; Ashida, K.; Sugano, K. Vonoprazan prevents ulcer recurrence during long-term NSAID therapy: Randomised, lansoprazole-controlled non-inferiority and single-blind extension study. Gut 2018, 67, 1042–1051. [Google Scholar] [CrossRef] [Green Version]
- Phathom Pharmaceuticals. Available online: https://www.globenewswire.com/news-release/2021/09/08/2293340/0/en/Phathom-Pharmaceuticals-Submits-Two-NDAs-to-U-S-FDA-for-Vonoprazan-based-Treatment-Regimens-for-the-Treatment-of-H-pylori-Infection.html (accessed on 14 December 2021).
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical cocrystals: New solid phase modification approaches for the formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating cocrystals: A review of pharmaceutical cocrystal preparation routes and applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Lipinski, C.A. Poor aqueous solubility—An industry wide problem in drug discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- Kumar Bandaru, R.; Rout, S.R.; Kenguva, G.; Gorain, B.; Alhakamy, N.A.; Kesharwani, P.; Dandela, R. Recent Advances in Pharmaceutical Cocrystals: From Bench to Market. Front. Pharmacol. 2021, 12, 780582. [Google Scholar] [CrossRef]
- Sarmah, K.K.; Sarma, A.; Roy, K.; Rao, D.R.; Thakuria, R. Olanzapine Salts and Diversity in Molecular Packing. Cryst. Growth Des. 2016, 16, 1047–1055. [Google Scholar] [CrossRef]
- Nechipadappu, S.K.; Reddy, I.R.; Tarafder, K.; Trivedi, D.R. Salt/Cocrystal of AntiFibrinolytic Hemostatic Drug Tranexamic Acid: Structural, DFT, and Stability Study of Salt/Cocrystal with GRAS Molecules. Cryst. Growth Des. 2019, 19, 347–361. [Google Scholar] [CrossRef]
- Gong, W.; Mondal, P.K.; Ahmadi, S.; Wu, Y.; Rohani, S. Cocrystals, Salts, and Salt-Solvates of olanzapine; selection of coformers and improved solubility. Int. J. Pharm. 2021, 608, 121063. [Google Scholar] [CrossRef]
- Sanphui, P.; Bolla, G.; Nangia, A. High solubility piperazine salts of the nonsteroidal anti-inflammatory drug (NSAID) meclofenamic acid. Cryst. Growth Des. 2012, 12, 2023–2036. [Google Scholar] [CrossRef]
- Guan, D.; Xuan, B.; Wang, C.; Long, R.; Jiang, Y.; Mao, L.; Kang, J.; Wang, Z.; Chow, S.F.; Zhou, Q. Improving the Physicochemical and Biopharmaceutical Properties of Active Pharmaceutical Ingredients Derived from Traditional Chinese Medicine through Cocrystal Engineering. Pharmaceutics 2021, 13, 2160. [Google Scholar] [CrossRef]
- Food and Drug Administration. Regulatory Classification of Pharmaceutical Co-Crystals Guidance for Industry; Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
- Wong, S.N.; Chen, Y.C.S.; Xuan, B.; Sun, C.C.; Chow, S.F. Cocrystal Engineering of Pharmaceutical Solids: Therapeutic Potentials and Challenges. CrystEngComm 2021, 23, 7005–7038. [Google Scholar] [CrossRef]
- Wood, P.A.; Feeder, N.; Furlow, M.; Galek, P.T.A.; Groom, C.R.; Pidcock, E. Knowledge-based approaches to co-crystal design. CrystEngComm 2014, 16, 5839–5848. [Google Scholar] [CrossRef]
- Abramov, Y.A. (Ed.) Computational Pharmaceutical Solid State Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Sarkar, N.; Aakeröy, C.B. Evaluating hydrogen-bond propensity, hydrogen-bond coordination and hydrogen-bond energy as tools for predicting the outcome of attempted co-crystallisations. Supramol. Chem. 2020, 32, 81–90. [Google Scholar] [CrossRef]
- Grecu, T.; Hunter, C.A.; Gardiner, E.J.; McCabe, J.F. Validation of a Computational Cocrystal Prediction Tool: Comparison of Virtual and Experimental Cocrystal Screening Results. Cryst. Growth Des. 2014, 14, 165–171. [Google Scholar] [CrossRef]
- Barbas, R.; Font-Bardia, M.; Paradkar, A.; Hunter, C.A.; Prohens, R. Combined Virtual/Experimental Multicomponent Solid Forms Screening of Sildenafil: New Salts, Cocrystals, and Hybrid Salt–Cocrystals. Cryst. Growth Des. 2018, 18, 7618–7627. [Google Scholar] [CrossRef]
- Salem, A.; Nagy, S.; Pál, S.; Széchenyi, A. Reliability of the Hansen solubility parameters as co-crystal formation prediction tool. Int. J. Pharm. 2019, 558, 319–327. [Google Scholar] [CrossRef]
- Abramov, Y.A.; Loschen, C.; Klamt, A. Rational Coformer or Solvent Selection for Pharmaceutical Cocrystallization or Desolvation. J. Pharm. Sci. 2012, 101, 3687–3697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, G.; Jin, Y.; Li, S.; Yang, Z.; Shi, B.; Chang, C.; Abramov, Y.A. Virtual Coformer Screening by Crystal Structure Predictions: Crucial Role of Crystallinity in Pharmaceutical Cocrystallization. J. Phys. Chem. Lett. 2020, 11, 8832–8838. [Google Scholar] [CrossRef] [PubMed]
- Ratkova, E.L.; Abramov, Y.A.; Baskin, I.I.; Livingstone, D.J.; Fedorov, M.V.; Withnall, M.; Tetko, I.V. Empirical and Physics-Based Calculations of Physical−Chemical Properties. In Comprehensive Medicinal Chemistry III, 3rd ed.; Chackalamannil, S., Rotella, D., Ward, S.E., Eds.; Elsevier: Oxford, UK, 2017; pp. 393–428. [Google Scholar]
- Wicker, J.G.; Crowley, L.M.; Robshaw, O.; Little, E.J.; Stokes, S.P.; Cooper, R.I.; Lawrence, S.E. Will they co-crystallize? CrystEngComm 2017, 19, 5336–5340. [Google Scholar] [CrossRef] [Green Version]
- Przybyłek, M.; Jeliński, T.; Słabuszewska, J.; Ziółkowska, D.; Mroczyńska, K.; Cysewski, P. Application of multivariate adaptive regression splines (MARSplines) Methodology for screening of dicarboxylic acid cocrystal using 1D and 2D molecular descriptors. Cryst. Growth Des. 2019, 19, 3876–3887. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Z.; Zhu, B.; Mei, X.; Luo, X. Machine-learning-guided cocrystal prediction based on large data base. Cryst. Growth Des. 2020, 20, 6610–6621. [Google Scholar] [CrossRef]
- Devogelaer, J.J.; Charpentier, M.D.; Tijink, A.; Dupray, V.; Coquerel, G.; Johnston, K.; Meekes, H.; Tinnemans, P.; Vlieg, E.; ter Horst, J.H.; et al. Cocrystals of Praziquantel: Discovery by Network-Based Link Prediction. Cryst. Growth Des. 2021, 21, 3428–3437. [Google Scholar] [CrossRef]
- Mswahili, M.E.; Lee, M.-J.; Martin, G.L.; Kim, J.; Kim, P.; Choi, G.J.; Jeong, Y.-S. Cocrystal Prediction Using Machine Learning Models and Descriptors. Appl. Sci. 2021, 11, 1323. [Google Scholar] [CrossRef]
- Cherukuvada, S.; Kaur, R.; Row, T.N.G. Co-crystallization and small molecule crystal form diversity: From pharmaceutical to materials applications. CrystEngComm 2016, 18, 8528–8555. [Google Scholar] [CrossRef]
- Roca-Paixão, L.; Correia, N.T.; Affouard, F. Affinity prediction computations and mechanosynthesis of carbamazepine based cocrystals. CrystEngComm 2019, 21, 6991–7001. [Google Scholar] [CrossRef]
- Hori, Y.; Matsukawa, J.; Takeuchi, T.; Nishida, H.; Kajino, M.; Inatomi, N. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J. Pharmacol. Exp. Ther. 2011, 337, 797–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, S.L.; Stahly, G.P.; Park, A. The Salt−Cocrystal Continuum: The Influence of Crystal Structure on Ionization State. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.N.; Hu, S.; Ng, W.W.; Xu, X.; Lai, K.L.; Lee, W.Y.T.; Chow, A.H.L.; Sun, C.C.; Chow, S.F. Cocrystallization of Curcumin with Benzenediols and Benzenetriols via Rapid Solvent Removal. Cryst. Growth Des. 2018, 18, 5534–5546. [Google Scholar] [CrossRef]
- Garg, U.; Azim, Y.; Kar, A.; Pradeep, C.P. Cocrystals/salt of 1-naphthaleneacetic acid and utilizing Hirshfeld surface calculations for acid–aminopyrimidine synthons. CrystEngComm 2020, 22, 2978–2989. [Google Scholar] [CrossRef]
- Burger, A.; Ramberger, R. On the polymorphism of pharmaceuticals and other molecular crystals. I. Microchim. Acta 1979, 72, 259–271. [Google Scholar] [CrossRef]
- Mayo, D.W.; Miller, F.A.; Hannah, R.W. Course Notes on the Interpretation of Infrared and Raman Spectra; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Gunasekaran, S.; Sailatha, E.; Seshadri, S.; Kumaresan, S. FTIR, FT Raman spectra and molecular structural confirmation of isoniazid. Indian J. Pure Appl. Phys. 2009, 47, 12–18. [Google Scholar]
- Qiao, Y.; Zhao, J.; Yue, X.; Zhang, Y.; Zhang, R.; Xu, Y.; Tang, X.; Liu, X.; Wang, Q. Study on pharmacokinetics and bioequivalence of Vonoprazan pyroglutamate in rats by liquid chromatography with tandem mass spectrometry. J. Chromatogr. B 2017, 1059, 56–65. [Google Scholar] [CrossRef]








| Group | Coformer | LAG Result a | pKab | ΔpKa | Prediction |
|---|---|---|---|---|---|
| Pyrocatechol | √ | 9.64 | −0.24 | cocrystal | |
| Hydroquinone | √ | 9.98 | −0.58 | cocrystal | |
| Phenols | Resorcinol | √ | 9.57 | −0.17 | cocrystal |
| Pyrogallol | √ | 9.1 | 0.3 | continuum | |
| Methyl-hydroquinone | – | 10.1 | −0.7 | cocrystal | |
| Acids | Benzoic acid Succinic acid Pyroglutamic acid 4-hydroxybenzoic acid Pyrrole-2-carboxylic acid Oxamic acid saccharin | – √ √ x x x x | 4.08 3.86 3.61 4.38 3.62 2.48 1.94 | 5.32 5.54 5.79 5.02 5.78 6.92 7.46 | salt salt salt salt salt salt salt |
Amide /Amine | Acetamide Glycolamide Benzamide Nicotinamide Isonicotinamide Urea | x x x x x x | 16.75 13.65 14.56 13.39 13.71 16.3 | −7.35 −4.25 −5.16 −3.99 −4.31 −6.9 | cocrystal cocrystal cocrystal cocrystal cocrystal cocrystal |
| Piperazine | x | 9.26 (base) | - | - |
| Wavenumber (cm−1) | Peak Assignment [40,>42,43,44] | ||||||
|---|---|---|---|---|---|---|---|
| VPZ | RES | VPZ- RES | CAT | VPZ- CAT | GAL | VPZ- GAL | |
| 3321 | 3296 | 3306 | N-H stretching | ||||
| 3188 | 3129 | 3444 3320 | 3126 | 3534 3229 | 3426 3060 | O-H stretching | |
| 1623 | 1606 | 1618 | 1622 | 1620 | 1605 | C=C stretching | |
| 1573 | 1582 1563 | 1578 1568 | 1585 1567 | C=N stretching | |||
| 1374 1179 | 1377 1183 | 1378 1177 | 1378 1184 | S=O stretching | |||
| 1313 | 1325 1302 | 1330 1305 | 1332 1306 | Aromatic C-N stretching | |||
| Solid Form | Equilibrium Solubility after 24 h in pH 1.2 (mg/mL) | Equilibrium Solubility after 24 h in pH 6.8 (mg/mL) | IDR in pH 6.8 (mg/cm2∙min) |
|---|---|---|---|
| VPZ-FUM | 27.71 ±0.21 | 3.4 ±0.45 | 1.35 |
| VPZ-RES | 17.53 ±0.49 | 5.74 ±0.13 | 0.99 |
| VPZ-CAT | 17.42 ±0.46 | 2.09 ±0.87 | 0.9 |
| VPZ-GAL | 22.68 ±0.5 | 1.85 ±0.31 | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-J.; Kim, J.-Y.; Kim, P.; Lee, I.-S.; Mswahili, M.E.; Jeong, Y.-S.; Choi, G.J. Novel Cocrystals of Vonoprazan: Machine Learning-Assisted Discovery. Pharmaceutics 2022, 14, 429. https://doi.org/10.3390/pharmaceutics14020429
Lee M-J, Kim J-Y, Kim P, Lee I-S, Mswahili ME, Jeong Y-S, Choi GJ. Novel Cocrystals of Vonoprazan: Machine Learning-Assisted Discovery. Pharmaceutics. 2022; 14(2):429. https://doi.org/10.3390/pharmaceutics14020429
Chicago/Turabian StyleLee, Min-Jeong, Ji-Yoon Kim, Paul Kim, In-Seo Lee, Medard E. Mswahili, Young-Seob Jeong, and Guang J. Choi. 2022. "Novel Cocrystals of Vonoprazan: Machine Learning-Assisted Discovery" Pharmaceutics 14, no. 2: 429. https://doi.org/10.3390/pharmaceutics14020429