Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances
Abstract
:1. Introduction
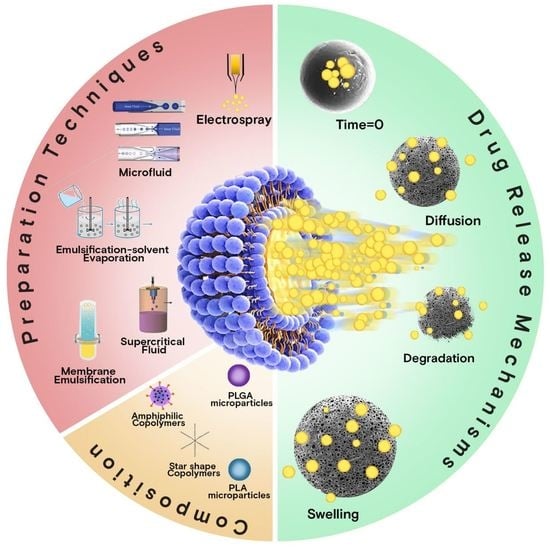
2. MP Preparation Techniques at a Glance
3. Overview of Release Mechanisms
3.1. Diffusion
3.2. Erosion
3.3. Swelling
4. Recent Advances in Microparticle-Based Delivery Systems
4.1. PLA Microparticles
4.2. PLGA Microparticles
4.3. Microparticles Based on Amphiphilic PLA/PEG and PLGA/PEG Copolymers
4.4. PLCL Microparticles
4.5. Formulations Based on Star-Shaped PLA or PLGA
4.6. MPs Prepared by PLA/Poly(Alkylene Adipate) Matrices
4.7. PLA/PAsp Copolymer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Jamaledin, R.; Sartorius, R.; Di Natale, C.; Vecchione, R.; De Berardinis, P.; Netti, P.A. Recombinant Filamentous Bacteriophages Encapsulated in Biodegradable Polymeric Microparticles for Stimulation of Innate and Adaptive Immune Responses. Microorganisms 2020, 8, 650. [Google Scholar] [CrossRef] [PubMed]
- Blasi, P. Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid)-Based Microparticles: An Overview. J. Pharm. Investig. 2019, 49, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A Promising Technique for Controlled Drug Delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar]
- Lagreca, E.; Onesto, V.; Di Natale, C.; La Manna, S.; Netti, P.A.; Vecchione, R. Recent Advances in the Formulation of PLGA Microparticles for Controlled Drug Delivery. Prog. Biomater. 2020, 9, 153–174. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. An Overview of Clinical and Commercial Impact of Drug Delivery Systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Pagels, R.F.; Prud’Homme, R.K. Polymeric Nanoparticles and Microparticles for the Delivery of Peptides, Biologics, and Soluble Therapeutics. J. Control. Release 2015, 219, 519–535. [Google Scholar] [CrossRef]
- Iqbal, M.; Zafar, N.; Fessi, H.; Elaissari, A. Double Emulsion Solvent Evaporation Techniques Used for Drug Encapsulation. Int. J. Pharm. 2015, 496, 173–190. [Google Scholar] [CrossRef]
- Beslikas, T.; Gigis, I.; Goulios, V.; Christoforides, J.; Papageorgiou, G.Z.; Bikiaris, D.N. Crystallization Study and Comparative in Vitro-in Vivo Hydrolysis of PLA Reinforcement Ligament. Int. J. Mol. Sci. 2011, 12, 6597–6618. [Google Scholar] [CrossRef] [Green Version]
- Mazzara, J.M.; Ochyl, L.J.; Hong, J.K.Y.; Moon, J.J.; Prausnitz, M.R.; Schwendeman, S.P. Self-Healing Encapsulation and Controlled Release of Vaccine Antigens from PLGA Microparticles Delivered by Microneedle Patches. Bioeng. Transl. Med. 2019, 4, 116–128. [Google Scholar] [CrossRef] [Green Version]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in Modern Medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Lopez-Mendez, T.B.; Santos-Vizcaino, E.; Pedraz, J.L.; Hernandez, R.M.; Orive, G. Cell Microencapsulation Technologies for Sustained Drug Delivery: Clinical Trials and Companies. Drug Discov. Today 2021, 26, 852–861. [Google Scholar] [CrossRef]
- Molavi, F.; Barzegar-Jalali, M.; Hamishehkar, H. Polyester Based Polymeric Nano and Microparticles for Pharmaceutical Purposes: A Review on Formulation Approaches. J. Control. Release 2020, 320, 265–282. [Google Scholar] [CrossRef]
- Wan, F.; Yang, M. Design of PLGA-Based Depot Delivery Systems for Biopharmaceuticals Prepared by Spray Drying. Int. J. Pharm. 2016, 498, 82–95. [Google Scholar] [CrossRef]
- Rodrigues, S.; da Costa, A.M.R.; Flórez-Fernández, N.; Torres, M.D.; Faleiro, M.L.; Buttini, F.; Grenha, A. Inhalable Spray-Dried Chondroitin Sulphate Microparticles: Effect of Different Solvents on Particle Properties and Drug Activity. Polymers 2020, 12, 425. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Kim, Y.J.; Yang, M.S.; Shin, D.H.; Kim, D.W.; Park, I.Y.; Park, C.W. Co-Spray Dried Nafamostat Mesylate with Lecithin and Mannitol as Respirable Microparticles for Targeted Pulmonary Delivery: Pharmacokinetics and Lung Distribution in Rats. Pharmaceutics 2021, 13, 1519. [Google Scholar] [CrossRef]
- Morais, A.Í.S.; Vieira, E.G.; Afewerki, S.; Sousa, R.B.; Honorio, L.M.C.; Cambrussi, A.N.C.O.; Santos, J.A.; Bezerra, R.D.S.; Furtini, J.A.O.; Silva-Filho, E.C.; et al. Fabrication of Polymeric Microparticles by Electrospray: The Impact of Experimental Parameters. J. Funct. Biomater. 2020, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Tasci, M.E.; Dede, B.; Tabak, E.; Gur, A.; Sulutas, R.B.; Cesur, S.; Ilhan, E.; Lin, C.C.; Paik, P.; Ficai, D.; et al. Production, Optimization and Characterization of Polylactic Acid Microparticles Using Electrospray with Porous Structure. Appl. Sci. 2021, 11, 5090. [Google Scholar] [CrossRef]
- Damiati, S.A.; Damiati, S. Microfluidic Synthesis of Indomethacin-Loaded PLGA Microparticles Optimized by Machine Learning. Front. Mol. Biosci. 2021, 8, 677547. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Xie, R.; Ju, X.; Liu, Z.; Jiang, L.; Chen, Q.; Chu, L. Controllable Microfluidic Strategies for Fabricating Microparticles Using Emulsions as Templates. Particuology 2016, 24, 18–31. [Google Scholar] [CrossRef]
- Ramazani, F.; Chen, W.; Van Nostrum, C.F.; Storm, G.; Kiessling, F.; Lammers, T.; Hennink, W.E.; Kok, R.J. Strategies for Encapsulation of Small Hydrophilic and Amphiphilic Drugs in PLGA Microspheres: State-of-the-Art and Challenges. Int. J. Pharm. 2016, 499, 358–367. [Google Scholar] [CrossRef]
- Tanhaei, A.; Mohammadi, M.; Hamishehkar, H.; Hamblin, M.R. Electrospraying as a Novel Method of Particle Engineering for Drug Delivery Vehicles. J. Control. Release 2021, 330, 851–865. [Google Scholar] [CrossRef]
- Duncanson, W.J.; Lin, T.; Abate, A.R.; Seiffert, S.; Shah, R.K.; Weitz, D.A. Microfluidic Synthesis of Advanced Microparticles for Encapsulation and Controlled Release. Lab Chip 2012, 12, 2135–2145. [Google Scholar] [CrossRef]
- Teekamp, N.; Duque, L.F.; Frijlink, H.W.; Hinrichs, W.L.; Olinga, P. Production Methods and Stabilization Strategies for Polymer-Based Nanoparticles and Microparticles for Parenteral Delivery of Peptides and Proteins. Expert Opin. Drug Deliv. 2015, 12, 1311–1331. [Google Scholar] [CrossRef]
- Girotra, P.; Singh, S.K.; Nagpal, K. Supercritical Fluid Technology: A Promising Approach in Pharmaceutical Research. Pharm. Dev. Technol. 2013, 18, 22–38. [Google Scholar] [CrossRef]
- Soh, S.H.; Lee, L.Y. Microencapsulation and Nanoencapsulation Using Supercritical Fluid (SCF) Techniques. Pharmaceutics 2019, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- Gangapurwala, G.; Vollrath, A.; De San Luis, A.; Schubert, U.S. PLA/PLGA-Based Drug Delivery Systems Produced with Supercritical CO2—A Green Future for Particle Formulation? Pharmaceutics 2020, 12, 1118. [Google Scholar] [CrossRef]
- Wu, J.; Fan, Q.; Xia, Y.; Ma, G. Uniform-Sized Particles in Biomedical Field Prepared by Membrane Emulsification Technique. Chem. Eng. Sci. 2015, 125, 85–97. [Google Scholar] [CrossRef]
- Petersen, R.S.; Boisen, A.; Keller, S.S. Micromechanical Punching: A Versatile Method for Non-Spherical Microparticle Fabrication. Polymers 2021, 13, 83. [Google Scholar] [CrossRef]
- Boni, F.I.; Cury, B.S.F.; Ferreira, N.N.; Gremião, M.P.D. Ionic Cross-Linking as a Strategy Tomodulate the Properties of Oral Mucoadhesive Microparticles Based on Polysaccharide Blends. Pharmaceutics 2021, 13, 407. [Google Scholar] [CrossRef]
- Wang, F.J.; Lu, F.S.; Cui, M.; Shao, Z.Q. Biocompatible Microcapsule of Carboxymethyl Cellulose/Chitosan as Drug Carrier. Adv. Mater. Res. 2015, 1118, 227–236. [Google Scholar] [CrossRef]
- Yoshida, K.; Ono, T.; Kashiwagi, Y.; Takahashi, S.; Sato, K.; Anzai, J.I. PH-Dependent Release of Insulin from Layer-by-Layer-Deposited Polyelectrolyte Microcapsules. Polymers 2015, 7, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Kranz, H.; Ubrich, N.; Maincent, P.; Bodmeier, R. Physicomechanical Properties of Biodegradable Poly(D,L-Lactide) and Poly(D,L-Lactide-Co-Glycolide) Films in the Dry and Wet States. J. Pharm. Sci. 2000, 89, 1558–1566. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The Mechanisms of Drug Release in Poly(Lactic-Co-Glycolic Acid)-Based Drug Delivery Systems—A Review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Emami, F.; Mostafavi Yazdi, S.J.; Na, D.H. Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid) Particulate Carriers for Pulmonary Drug Delivery. J. Pharm. Investig. 2019, 49, 427–442. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, S.S.; Faraj, J.A.; DeLuca, P.P. A Model-Dependent Approach to Correlate Accelerated with Real-Time Release from Biodegradable Microspheres. AAPS PharmSciTech 2005, 6, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Mollo, A.R.; Corrigan, O.I. Effect of Poly-Hydroxy Aliphatic Ester Polymer Type on Amoxycillin Release from Cylindrical Compacts. Int. J. Pharm. 2003, 268, 71–79. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Drug Transport Mechanisms and in Vitro Release Kinetics of Vancomycin Encapsulated Chitosan-Alginate Polyelectrolyte Microparticles as a Controlled Drug Delivery System. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef]
- Asmatulu, R.; Fakhari, A.; Wamocha, H.L.; Chu, H.Y.; Chen, Y.Y.; Eltabey, M.M.; Hamdeh, H.H.; Ho, J.C. Drug-Carrying Magnetic Nanocomposite Particles for Potential Drug Delivery Systems. J. Nanotechnol. 2009, 2009, 238536. [Google Scholar] [CrossRef] [Green Version]
- Mirakabad, F.S.T.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y.; et al. PLGA-Based Nanoparticles as Cancer Drug Delivery Systems. Asian Pacific J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef] [Green Version]
- Siepmann, J.; Siepmann, F. Modeling of Diffusion Controlled Drug Delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, X.; Fu, H.; Wen, X.; Ma, C.; Zhang, J.; Wu, C.; Huang, Y.; Pan, X.; Wu, C. Formation Mechanism and In Vitro Evaluation of Risperidone-Containing PLGA Microspheres Fabricated by Ultrafine Particle Processing System. J. Pharm. Sci. 2017, 106, 3363–3371. [Google Scholar] [CrossRef]
- Yu, B.; Meng, L.; Fu, S.; Zhao, Z.; Liu, Y.; Wang, K.; Fu, Q. Morphology and Internal Structure Control over PLA Microspheres by Compounding PLLA and PDLA and Effects on Drug Release Behavior. Colloids Surf. B Biointerfaces 2018, 172, 105–112. [Google Scholar] [CrossRef]
- Doty, A.C.; Weinstein, D.G.; Hirota, K.; Olsen, K.F.; Ackermann, R.; Wang, Y.; Choi, S.; Schwendeman, S.P. Mechanisms of in Vivo Release of Triamcinolone Acetonide from PLGA Microspheres. J. Control. Release 2017, 256, 19–25. [Google Scholar] [CrossRef]
- Shenderova, A.; Burke, T.G.; Schwendeman, S.P. The acidic microclimate in poly (lactide-co-glycolide) microspheres stabilizes camptothecins. Pharm. Res. 1999, 16, 241–248. [Google Scholar] [CrossRef]
- Klose, D.; Siepmann, F.; Elkharraz, K.; Siepmann, J. PLGA-Based Drug Delivery Systems: Importance of the Type of Drug and Device Geometry. Int. J. Pharm. 2008, 354, 95–103. [Google Scholar] [CrossRef]
- Li, S.; McCarthy, S. Further Investigations on the Hydrolytic Degradation of Poly (DL-Lactide). Biomaterials 1999, 20, 35–44. [Google Scholar] [CrossRef]
- Park, T.G. Degradation of Poly(d,l-Lactic Acid) Microspheres: Effect of Molecular Weight. J. Control. Release 1994, 30, 161–173. [Google Scholar] [CrossRef]
- Arifin, D.Y.; Lee, L.Y.; Wang, C.H. Mathematical Modeling and Simulation of Drug Release from Microspheres: Implications to Drug Delivery Systems. Adv. Drug Deliv. Rev. 2006, 58, 1274–1325. [Google Scholar] [CrossRef]
- Rapier, C.E.; Shea, K.J.; Lee, A.P. Investigating PLGA Microparticle Swelling Behavior Reveals an Interplay of Expansive Intermolecular Forces. Sci. Rep. 2021, 11, 14512. [Google Scholar] [CrossRef]
- Bode, C.; Kranz, H.; Fivez, A.; Siepmann, F.; Siepmann, J. Often Neglected: PLGA/PLA Swelling Orchestrates Drug Release: HME Implants. J. Control. Release 2019, 306, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer Degradation and Drug Delivery in PLGA-Based Drug–Polymer Applications: A Review of Experiments and Theories. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Trang, T.T.T.; Mariatti, M.; Badrul, H.Y.; Masakazu, K.; Nguyen, X.T.T.; Zuratul, A.A.H. Drug Release Profile Study of Gentamicin Encapsulated Poly(Lactic Acid) Microspheres for Drug Delivery. Mater. Today Proc. 2019, 17, 836–845. [Google Scholar] [CrossRef]
- Guan, Q.; Chen, W.; Hu, X. Development of Lovastatin-Loaded Poly(Lactic Acid) Microspheres for Sustained Oral Delivery: In Vitro and Ex Vivo Evaluation. Drug Des. Dev. Ther. 2015, 9, 791–798. [Google Scholar] [CrossRef] [Green Version]
- Loca, D.; Sevostjanovs, E.; Makrecka, M.; Zharkova-Malkova, O.; Berzina-Cimdina, L.; Tupureina, V.; Sokolova, M. Microencapsulation of Mildronate in Biodegradable and Non-Biodegradable Polymers. J. Microencapsul. 2014, 31, 246–253. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric Micelles in Cancer Therapy: State of the Art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Xu, J.; Bai, Y.; Li, X.; Wei, Z.; Sun, L.; Yu, H.; Xu, H. Porous Core/Dense Shell PLA Microspheres Embedded with High Drug Loading of Bupivacaine Crystals for Injectable Prolonged Release. AAPS PharmSciTech 2021, 22, 27. [Google Scholar] [CrossRef]
- Chaiyasat, P.; Chaiyasat, A.; Teeka, P.; Noppalit, S.; Srinorachun, U. Preparation of Poly(l-Lactic Acid) Microencapsulated Vitamin E. Energy Procedia 2013, 34, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Nanaki, S.; Tseklima, M.; Terzopoulou, Z.; Nerantzaki, M.; Giliopoulos, D.J.; Triantafyllidis, K.; Kostoglou, M.; Bikiaris, D.N. Use of Mesoporous Cellular Foam (MCF) in Preparation of Polymeric Microspheres for Long Acting Injectable Release Formulations of Paliperidone Antipsychotic Drug. Eur. J. Pharm. Biopharm. 2017, 117, 77–90. [Google Scholar] [CrossRef]
- Nanaki, S.; Tseklima, M.; Christodoulou, E.; Triantafyllidis, K.; Kostoglou, M.; Bikiaris, D.N. Thiolated Chitosan Masked Polymeric Microspheres with Incorporated Mesocellular Silica Foam (MCF) for Intranasal Delivery of Paliperidone. Polymers 2017, 9, 617. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fei, S.; Yu, M.; Guo, Y.; He, H.; Zhang, Y.; Yin, T.; Xu, H.; Tang, X. Injectable Sustained Release PLA Microparticles Prepared by Solvent Evaporation-Media Milling Technology. Drug Dev. Ind. Pharm. 2018, 44, 1591–1597. [Google Scholar] [CrossRef]
- Vergara-Mendoza, M.D.S.; Ortiz-Estrada, C.H.; González-Martínez, J.; Quezada-Gallo, J.A. Microencapsulation of Coenzyme Q 10 in Poly(Ethylene Glycol) and Poly(Lactic Acid) with Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2012, 51, 5840–5846. [Google Scholar] [CrossRef]
- Ito, F.; Yamada, H. Physical Properties of Microspheres Prepared by Blending Poly(Lactide-Co-Glycolide) and Poly Lactide. Bull. Mater. Sci. 2021, 44, 1–10. [Google Scholar] [CrossRef]
- Kudryavtseva, V.; Boi, S.; Read, J.; Gould, D.; Szewczyk, P.K.; Stachewicz, U.; Kiryukhin, M.V.; Pastorino, L.; Sukhorukov, G.B. Micro-Sized “Pelmeni”—A Universal Microencapsulation Approach Overview. Mater. Des. 2021, 202, 109527. [Google Scholar] [CrossRef]
- Ma, G. Microencapsulation of Protein Drugs for Drug Delivery: Strategy, Preparation, and Applications. J. Control. Release 2014, 193, 324–340. [Google Scholar] [CrossRef]
- Icart, L.P.; de Souza, F.G.; Lima, L.M.T.R. Sustained Release and Pharmacologic Evaluation of Human Glucagon-like Peptide-1 and Liraglutide from Polymeric Microparticles. J. Microencapsul. 2019, 36, 747–758. [Google Scholar] [CrossRef]
- Kong, Y.; Zhao, Y.; Li, D.; Shen, H.; Yan, M. Dual Delivery of Encapsulated BM-MSCs and BMP-2 Improves Osteogenic Differentiation and New Bone Formation. J. Biomed. Mater. Res.-Part A 2019, 107, 2282–2295. [Google Scholar] [CrossRef]
- Butreddy, A.; Gaddam, R.P.; Kommineni, N.; Dudhipala, N.; Voshavar, C. Plga/Pla-Based Long-Acting Injectable Depot Microspheres in Clinical Use: Production and Characterization Overview for Protein/Peptide Delivery. Int. J. Mol. Sci. 2021, 22, 8884. [Google Scholar] [CrossRef]
- Damiati, S.A.; Rossi, D.; Joensson, H.N.; Damiati, S. Artificial Intelligence Application for Rapid Fabrication of Size-Tunable PLGA Microparticles in Microfluidics. Sci. Rep. 2020, 10, 19517. [Google Scholar] [CrossRef]
- Nurdin, D.; Hardiansyah, A.; Chaldun, E.R.; Fikkriyah, A.K.; Dharsono, H.D.A.; Kurnia, D.; Satari, M.H. Preparation and Characterization of Terpenoid-Encapsulated Plga Microparticles and Its Antibacterial Activity against Enterococcus Faecalis. Key Eng. Mater. 2020, 829, 263–269. [Google Scholar] [CrossRef]
- Chen, W.; Palazzo, A.; Hennink, W.E.; Kok, R.J. Effect of Particle Size on Drug Loading and Release Kinetics of Gefitinib-Loaded PLGA Microspheres. Mol. Pharm. 2017, 14, 459–467. [Google Scholar] [CrossRef]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable PLGA-Based Microparticles for Producing Sustained-Release Drug Formulations and Strategies for Improving Drug Loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef] [Green Version]
- Di Natale, C.; Onesto, V.; Lagreca, E.; Vecchione, R.; Netti, P.A. Tunable Release of Curcumin with an in Silico-Supported Approach from Mixtures of Highly Porous PLGA Microparticles. Materials 2020, 13, 1807. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.; Kundukad, B.; Somasundar, A.; Vijayan, S.; Khan, S.A.; Doyle, P.S. Design of Mucoadhesive PLGA Microparticles for Ocular Drug Delivery. ACS Appl. Bio Mater. 2018, 1, 561–571. [Google Scholar] [CrossRef]
- Angkawinitwong, U.; Courtenay, A.J.; Rodgers, A.M.; Larrañeta, E.; Mccarthy, H.O.; Brocchini, S.; Donnelly, R.F.; Williams, G.R. A Novel Transdermal Protein Delivery Strategy via Electrohydrodynamic Coating of PLGA Microparticles onto Microneedles. ACS Appl. Mater. Interfaces 2020, 12, 12478–12488. [Google Scholar] [CrossRef]
- Sato, H.; Tabata, A.; Moritani, T.; Morinaga, T.; Mizumoto, T.; Seto, Y.; Onoue, S. Design and Characterizations of Inhalable Poly(Lactic-Co-Glycolic Acid) Microspheres Prepared by the Fine Droplet Drying Process for a Sustained Effect of Salmon Calcitonin. Molecules 2020, 25, 1311. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, X.; Xiu, B.; Duan, C.; Li, J.; Zhang, X.; Yang, X.; Dai, W.; Johnson, H.; Zhang, H.; et al. A Novel and Simple Preparative Method for Uniform-Sized PLGA Microspheres: Preliminary Application in Antitubercular Drug Delivery. Colloids Surf. B Biointerfaces 2016, 145, 679–687. [Google Scholar] [CrossRef]
- Gaspar, M.C.; Pais, A.A.C.C.; Sousa, J.J.S.; Brillaut, J.; Olivier, J.C. Development of Levofloxacin-Loaded PLGA Microspheres of Suitable Properties for Sustained Pulmonary Release. Int. J. Pharm. 2019, 556, 117–124. [Google Scholar] [CrossRef]
- Shi, X.; Li, C.; Gao, S.; Zhang, L.; Han, H.; Zhang, J.; Shi, W.; Li, Q. Combination of Doxorubicin-Based Chemotherapy and Polyethylenimine/P53 Gene Therapy for the Treatment of Lung Cancer Using Porous PLGA Microparticles. Colloids Surf. B Biointerfaces 2014, 122, 498–504. [Google Scholar] [CrossRef]
- Hu, Y.; Li, M.; Zhang, M.; Jin, Y. Inhalation Treatment of Idiopathic Pulmonary Fibrosis with Curcumin Large Porous Microparticles. Int. J. Pharm. 2018, 551, 212–222. [Google Scholar] [CrossRef]
- Wu, D.; Wang, C.; Yang, J.; Wang, H.; Han, H.; Zhang, A.; Yang, Y.; Li, Q. Improving the Intracellular Drug Concentration in Lung Cancer Treatment through the Codelivery of Doxorubicin and MiR-519c Mediated by Porous PLGA Microparticle. Mol. Pharm. 2016, 13, 3925–3933. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, H.; Xu, E.Y.; Moehwald, M.; Chen, L.; Zhang, X.; Mao, S. Inhalable PLGA Microspheres: Tunable Lung Retention and Systemic Exposure via Polyethylene Glycol Modification. Acta Biomater. 2021, 123, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Marcianes, P.; Negro, S.; Barcia, E.; Montejo, C.; Fernández-Carballido, A. Potential Active Targeting of Gatifloxacin to Macrophages by Means of Surface-Modified PLGA Microparticles Destined to Treat Tuberculosis. AAPS PharmSciTech 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Shahin, H.; Vinjamuri, B.P.; Mahmoud, A.A.; Mansour, S.M.; Chougule, M.B.; Chablani, L. Formulation and Optimization of Sildenafil Citrate-Loaded PLGA Large Porous Microparticles Using Spray Freeze-Drying Technique: A Factorial Design and in-Vivo Pharmacokinetic Study. Int. J. Pharm. 2021, 597, 120320. [Google Scholar] [CrossRef] [PubMed]
- Maghrebi, S.; Joyce, P.; Jambhrunkar, M.; Thomas, N.; Prestidge, C.A. Poly(Lactic-Co-Glycolic) Acid-Lipid Hybrid Microparticles Enhance the Intracellular Uptake and Antibacterial Activity of Rifampicin. ACS Appl. Mater. Interfaces 2020, 12, 8030–8039. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.F.O.; Nojosa, J.S.; Alencar, C.A.A.; Alcantara, A.P.M.P.; Araújo, R.S.; Yamauti, M.; Rodrigues, L.K.A. Design and Characterization of Digluconate and Diacetate Chlorhexidine Loaded-PLGA Microparticles for Dental Applications. J. Drug Deliv. Sci. Technol. 2021, 62, 102361. [Google Scholar] [CrossRef]
- Ali, S.W.; Mangrio, F.A.; Li, F.; Dwivedi, P.; Rajput, M.U.; Ali, R.; Khan, M.I.; Ding, W.; Xu, R.X. Co-Delivery of Artemether and Piperine via Core-Shell Microparticles for Enhanced Sustained Release. J. Drug Deliv. Sci. Technol. 2021, 63, 102505. [Google Scholar] [CrossRef]
- Aina, A.; Gupta, M.; Boukari, Y.; Morris, A.; Billa, N.; Doughty, S. Monitoring Model Drug Microencapsulation in PLGA Scaffolds Using X-Ray Powder Diffraction. Saudi Pharm. J. 2016, 24, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, P.; Tawre, M.; Dere, M.; Khan, A. Microencapsulation of Extracts from Corn Hair: A Study on Drug Release and Anticancer Activity. J. Drug Des. Med. Chem. 2021, 7, 12. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Y.; Meng, S.; Xing, C.; Ma, M.; Liu, Z.; Yang, C.; Kong, T. Micro-/Nano-Structures on Biodegradable Magnesium@PLGA and Their Cytotoxicity, Photothermal, and Anti-Tumor Effects. Small Methods 2021, 5, 2000920. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Ali, Z.N.; Tafaghodi, M.; Khameneh, B.; Kamali, H.; Hadizadeh, F. In Vitro Evaluation of PLGA-PEG-PLGA Microspheres for Sustained Release of Insulin In Vitro Evaluation of PLGA-PEG-PLGA Microspheres for Sustained Release of Insulin. Int. J. ChemTech Res. 2017, 10, 1018–1025. [Google Scholar]
- Nanaki, S.; Siafaka, P.I.; Zachariadou, D.; Nerantzaki, M.; Giliopoulos, D.J.; Triantafyllidis, K.S.; Kostoglou, M.; Nikolakaki, E.; Bikiaris, D.N. PLGA/SBA-15 Mesoporous Silica Composite Microparticles Loaded with Paclitaxel for Local Chemotherapy. Eur. J. Pharm. Sci. 2017, 99, 32–44. [Google Scholar] [CrossRef]
- Stefaniak, K.; Masek, A. Green Copolymers Based on Poly(Lactic Acid)—Short Review. Materials 2021, 14, 5254. [Google Scholar] [CrossRef]
- Ding, A.; Zhou, Y.; Chen, P.; Nie, W. Ibuprofen-Loaded Micelles Based on Star-Shaped Erythritol-Core PLLA-PEG Copolymer: Effect of Molecular Weights of PEG. Colloid Polym. Sci. 2017, 295, 1609–1619. [Google Scholar] [CrossRef]
- Rafat, M.; Cléroux, C.A.; Fong, W.G.; Baker, A.N.; Leonard, B.C.; O’Connor, M.D.; Tsilfidis, C. PEG-PLA Microparticles for Encapsulation and Delivery of Tat-EGFP to Retinal Cells. Biomaterials 2010, 31, 3414–3421. [Google Scholar] [CrossRef]
- Liu, H.J.; Chu, H.C.; Lin, L.H.; Hsu, S.Y. Preparation and Drug Release of Aspirin-Loaded PLGA-PEG-PLGA/Montmorillonite Microparticles. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 7–14. [Google Scholar] [CrossRef]
- Gaignaux, A.; Réeff, J.; Siepmann, F.; Siepmann, J.; De Vriese, C.; Goole, J.; Amighi, K. Development and Evaluation of Sustained-Release Clonidine-Loaded PLGA Microparticles. Int. J. Pharm. 2012, 437, 20–28. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R.H. “Stealth” Corona-Core Nanoparticles Surface Modified by Polyethylene Glycol (PEG): Influences of the Corona (PEG Chain Length and Surface Density) and of the Core Composition on Phagocytic Uptake and Plasma Protein Adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Avgoustakis, K.; Beletsi, A.; Panagi, Z.; Klepetsanis, P.; Karydas, A.G.; Ithakissios, D.S. PLGA-MPEG Nanoparticles of Cisplatin: In Vitro Nanoparticle Degradation, in Vitro Drug Release and in Vivo Drug Residence in Blood Properties. J. Control. Release 2002, 79, 123–135. [Google Scholar] [CrossRef]
- Avgoustakis, K. Pegylated Poly(Lactide) and Poly(Lactide-Co-Glycolide) Nanoparticles: Preparation, Properties and Possible Applications in Drug Delivery. Curr. Drug Deliv. 2004, 1, 13. [Google Scholar] [CrossRef]
- Avgoustakis, K.; Beletsi, A.; Panagi, Z.; Klepetsanis, P.; Livaniou, E.; Evangelatos, G.; Ithakissios, D.S. Effect of Copolymer Composition on the Physicochemical Characteristics, in Vitro Stability, and Biodistribution of PLGA-MPEG Nanoparticles. Int. J. Pharm. 2003, 259, 115–127. [Google Scholar] [CrossRef]
- Theunis, C.H.; Pierson, E.S.; Cresti, M.; Plant Reprod, S.; Kranz, E.; Bautor, J.; Lorz, H.; Faure, J.; Mogensen, H.L.; Dumas, C.; et al. Biodegradable Long-Circulating Polymeric Nanospheres. Science (80-) 1994, 263, 1600. [Google Scholar]
- Gryparis, E.C.; Hatziapostolou, M.; Papadimitriou, E.; Avgoustakis, K. Anticancer Activity of Cisplatin-Loaded PLGA-MPEG Nanoparticles on LNCaP Prostate Cancer Cells. Eur. J. Pharm. Biopharm. 2007, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mattheolabakis, G.; Taoufik, E.; Haralambous, S.; Roberts, M.L.; Avgoustakis, K. In Vivo Investigation of Tolerance and Antitumor Activity of Cisplatin-Loaded PLGA-MPEG Nanoparticles. Eur. J. Pharm. Biopharm. 2009, 71, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, A.; Voulgari, E.; Diamanti, E.K.; Gournis, D.; Avgoustakis, K. Graphene Oxide Stabilized by PLA-PEG Copolymers for the Controlled Delivery of Paclitaxel. Eur. J. Pharm. Biopharm. 2015, 93, 18–26. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Han, Y.; Guan, J.; Chung, S.; Wang, C.; Li, D. Poly(Ethylene Glycol)-Polylactide Micelles for Cancer Therapy. Front. Pharmacol. 2018, 9, 202. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zhang, Y.; Yin, G.; Pu, X.; Liao, X.; Huang, Z.; Chen, X.; Yao, Y. Tumor-Targeted Paclitaxel-Loaded Folate Conjugated Poly(Ethylene Glycol)-Poly(l-Lactide) Microparticles Produced by Supercritical Fluid Technology. J. Mater. Sci. Mater. Med. 2015, 26, 95. [Google Scholar] [CrossRef]
- Wang, M.; Long, J.; Zhang, S.; Liu, F.; Zhang, X.; Zhang, X.; Sun, L.; Ma, L.; Yu, C.; Wei, H. Folate-Targeted Anticancer Drug Delivery via a Combination Strategy of a Micelle Complex and Reducible Conjugation. ACS Biomater. Sci. Eng. 2020, 6, 1565–1572. [Google Scholar] [CrossRef]
- Ouyang, P.; Kang, Y.Q.; Yin, G.F.; Huang, Z.B.; Yao, Y.D.; Liao, X.M. Fabrication of Hydrophilic Paclitaxel-Loaded PLA-PEG-PLA Microparticles via SEDS Process. Front. Mater. Sci. China 2009, 3, 15–24. [Google Scholar] [CrossRef]
- Chen, F.; Yin, G.; Liao, X.; Yang, Y.; Huang, Z.; Gu, J.; Yao, Y.; Chen, X.; Gao, H. Preparation, Characterization and in Vitro Release Properties of Morphine-Loaded PLLA-PEG-PLLA Microparticles via Solution Enhanced Dispersion by Supercritical Fluids. J. Mater. Sci. Mater. Med. 2013, 24, 1693–1705. [Google Scholar] [CrossRef]
- Peña Icart, L.; Fernandes dos Santos, E.; Agüero Luztonó, L.; Zaldívar Silva, D.; Andrade, L.; Lopes Dias, M.; Trambaioli da Rocha e Lima, L.M.; Gomes de Souza, F. Paclitaxel-Loaded PLA/PEG/Magnetite Anticancer and Hyperthermic Agent Prepared From Materials Obtained by the Ugi’s Multicomponent Reaction. Macromol. Symp. 2018, 380, 1800094. [Google Scholar] [CrossRef]
- Ruan, G.; Feng, S.S. Preparation and Characterization of Poly(Lactic Acid)-Poly(Ethylene Glycol)-Poly(Lactic Acid) (PLA-PEG-PLA) Microspheres for Controlled Release of Paclitaxel. Biomaterials 2003, 24, 5037–5044. [Google Scholar] [CrossRef]
- Xiong, L.; He, Z. Preparation and In-Vitro Properties of MPEG/PLA Microspheres Loaded 5-Fluorouracil for Controlled Release. Polym.-Plast. Technol. Eng. 2013, 52, 268–272. [Google Scholar] [CrossRef]
- Andrade, L.N.; Cavendish, M.S.S.; Costa, S.P.M.; Amaral, R.G.; Corrêa, C.B.; Oliveira, D.S.; Morsink, M.; Gokce, E.H.; de Albuquerque Junior, R.L.C.; Souto, E.B.; et al. Perillyl Alcohol in Solid Lipid Nanoparticles (SLN-PA): Cytotoxicity and Antitumor Potential in Sarcoma 180 Mice Model. Precis. Nanomed. 2020, 3, 685–698. [Google Scholar] [CrossRef]
- Marson, B.M.; Picheth, G.; Kuczera Pereira, T.; Kita, D.H.; Valdameri, G.; Rocha Alencar Menezes, L.; Gioppo Nunes, G.; Alves de Freitas, R.; Pontarolo, R. Effect of Different Tensoactives on the Morphology and Release Kinetics of PLA-b-PEG Microcapsules Loaded With the Natural Anticancer Compound Perillyl Alcohol. J. Pharm. Sci. 2019, 108, 860–869. [Google Scholar] [CrossRef]
- Li, X.; Min, S.; Zhao, X.; Lu, Z.; Jin, A. Optimization of Entrapping Conditions to Improve the Release of BMP-2 from PELA Carriers by Response Surface Methodology. Biomed. Mater. 2015, 10, 15002. [Google Scholar] [CrossRef]
- Pascual-Gil, S.; Simón-Yarza, T.; Garbayo, E.; Prosper, F.; Blanco-Prieto, M.J. Tracking the in Vivo Release of Bioactive NRG from PLGA and PEG-PLGA Microparticles in Infarcted Hearts. J. Control. Release 2015, 220, 388–396. [Google Scholar] [CrossRef]
- Kirby, G.T.S.; White, L.J.; Rahman, C.V.; Cox, H.C.; Rose, F.R.A.J.; Hutmacher, D.W.; Shakesheff, K.M.; Woodruff, M.A. PLGA-Based Microparticles for the Sustained Release of BMP-2. Polymers 2011, 3, 571–586. [Google Scholar] [CrossRef] [Green Version]
- Tran, V.T.; Karam, J.P.; Garric, X.; Coudane, J.; Benoît, J.P.; Montero-Menei, C.N.; Venier-Julienne, M.C. Protein-Loaded PLGA-PEG-PLGA Microspheres: A Tool for Cell Therapy. Eur. J. Pharm. Sci. 2012, 45, 128–137. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, B.K.; Park, S.H.; Kim, M.G.; Lee, J.W.; Lee, H.Y.; Lee, H.B.; Kim, J.H.; Kim, M.S. Preparation of Biodegradable and Elastic Poly(ε-Caprolactone-Co-Lactide) Copolymers and Evaluation as a Localized and Sustained Drug Delivery Carrier. Int. J. Mol. Sci. 2017, 18, 671. [Google Scholar] [CrossRef] [Green Version]
- Jelonek, K.; Kasperczyk, J.; Li, S.; Dobrzynski, P.; Janeczek, H.; Jarzabek, B. Novel Poly(l-Lactide-Co-ε-Caprolactone) Matrices Obtained with the Use of Zr[Acac]as Nontoxic Initiator for Long-Term Release of Immunosuppressive Drugs. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Edlund, U.; Albertsson, A.C. Degradable Polymer Microspheres for Controlled Drug Delivery. Adv. Polym. Sci. 2002, 157, 67–112. [Google Scholar] [CrossRef]
- Hitzman, C.J.; Elmquist, W.F.; Wattenberg, L.W.; Wiedmann, T.S. Development of a Respirable, Sustained Release Microcarrier for 5-Fluorouracil I: In Vitro Assessment of Liposomes, Microspheres, and Lipid Coated Nanoparticles. J. Pharm. Sci. 2006, 95, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, K.J.; Zhang, J.X.; Jiang, H.L.; Liu, J.H.; Hao, Y.L.; Yasuda, H.; Ichimaru, A.; Yamamoto, K. In Vitro and in Vivo Studies of Cyclosporin A-Loaded Microspheres Based on Copolymers of Lactide and ε-Caprolactone: Comparison with Conventional PLGA Microspheres. Int. J. Pharm. 2005, 295, 67–76. [Google Scholar] [CrossRef]
- Cha, Y.; Pitt, C.G. A One-Week Subdermal Delivery System for l-Methadone Based on Biodegradable Microcapsules. J. Control. Release 1988, 7, 69–78. [Google Scholar] [CrossRef]
- Buntner, B.; Nowak, M.; Kasperczyk, J.; Ryba, M.; Grieb, P.; Walski, M.; Dobrzyñski, P.; Bero, M. The Application of Microspheres from the Copolymers of Lactide and ε-Caprolactone to the Controlled Release of Steroids. J. Control. Release 1998, 56, 159–167. [Google Scholar] [CrossRef]
- Kassab, R.; Yammine, P.; Moussa, D.; Dobrzyñski, P. Preparation and Characterization of Antifungal Drug-Loaded Poly(Dl-Lactide-Co-Caprolactone) and Poly(l-Lactide-Co-Caprolactone-Co-Glycolide) Microspheres. Int. J. Nov. Drug Deliv. Technol. 2011, 1, 213–219. [Google Scholar]
- Zhu, K.J.; Li, Y.; Jiang, H.L.; Yasuda, H.; Ichimaru, A.; Yamamoto, K.; Lecomte, P.; Jerome, R. Preparation, Characterization and in Vitro Release Properties of Ibuprofen-Loaded Microspheres Based on Polylactide, Poly(ε-Caprolactone) and Their Copolymers. J. Microencapsul. 2005, 22, 25–36. [Google Scholar] [CrossRef]
- Teng, L.; Nie, W.; Zhou, Y.; Chen, P. Synthesis and Characterization of Star-Shaped Poly(l-Lactide)s with an Erythritol Core and Evaluation of Their Rifampicin-Loaded Microspheres for Controlled Drug Delivery. Polym. Bull. 2016, 73, 97–112. [Google Scholar] [CrossRef]
- Dai, X.H.; Wang, Z.M.; Liu, W.; Dong, C.M.; Pan, J.M.; Yuan, S.S.; Yan, Y.S.; Liu, D.M.; Sun, L. Biomimetic Star-Shaped Porphyrin-Cored Poly(L-Lactide)-b-Glycopolymer Block Copolymers for Targeted Photodynamic Therapy. Colloid Polym. Sci. 2014, 292, 2111–2122. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Dijkstra, P.J.; Calucci, L.; Forte, C.; Feijen, J. Influence of Amide versus Ester Linkages on the Properties of Eight-Armed PEG-PLA Star Block Copolymer Hydrogels. Biomacromolecules 2010, 11, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Corneillie, S.; Smet, M. PLA Architectures: The Role of Branching. Polym. Chem. 2015, 6, 850–867. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Zhao, Y.; Bei, J.; Xi, F.; Wang, S. Synthesis and Properties of Star-Shaped Polylactide Attached to Poly(Amidoamine) Dendrimer. Biomacromolecules 2003, 4, 828–834. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.G.; Unal, S.; Wilkes, G.L.; Long, T.E. Branched Polyesters: Recent Advances in Synthesis and Performance. Prog. Polym. Sci. 2005, 30, 507–539. [Google Scholar] [CrossRef]
- Mooguee, M.; Omidi, Y.; Davaran, S. Synthesis and In Vitro Release of Adriamycin from Star-Shaped Poly(Lactide-Co-Glycolide) Nano- and Microparticles. J. Pharm. Sci. 2010, 99, 2386–2398. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.; Nakayama, A.; Kawasaki, N.; Yamamoto, N. Novel Star-Shaped Polylactide with Glycerol Using Stannous Octoate or Tetraphenyl Tin as Catalyst: 1. Synthesis, Characterization and Study of Their Biodegradability. Polymer 1995, 36, 2947–2956. [Google Scholar] [CrossRef]
- Hadar, J.; Skidmore, S.; Garner, J.; Park, H.; Park, K.; Wang, Y.; Qin, B.; Jiang, X. Characterization of Branched Poly(Lactide-Co-Glycolide) Polymers Used in Injectable, Long-Acting Formulations. J. Control. Release 2019, 304, 75–89. [Google Scholar] [CrossRef]
- Burke, J.; Donno, R.; D’Arcy, R.; Cartmell, S.; Tirelli, N. The Effect of Branching (Star Architecture) on Poly(d, l -Lactide) (PDLLA) Degradation and Drug Delivery. Biomacromolecules 2017, 18, 728–739. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Skidmore, S.; Hadar, J.; Garner, J.; Park, H.; Otte, A.; Soh, B.K.; Yoon, G.; Yu, D.; Yun, Y.; et al. Injectable, Long-Acting PLGA Formulations: Analyzing PLGA and Understanding Microparticle Formation. J. Control. Release 2019, 304, 125–134. [Google Scholar] [CrossRef]
- Ding, A.; Teng, L.; Zhou, Y.; Chen, P.; Nie, W. Synthesis and Characterization of Bovine Serum Albumin-Loaded Microspheres Based on Star-Shaped PLLA with a Xylitol Core and Their Drug Release Behaviors. Polym. Bull. 2018, 75, 2917–2931. [Google Scholar] [CrossRef]
- Teng, L.; Xu, X.; Nie, W.; Zhou, Y.; Song, L.; Chen, P. Synthesis and Degradability of a Star-Shaped Polylactide Based on l-Lactide and Xylitol. J. Polym. Res. 2015, 22, 83. [Google Scholar] [CrossRef]
- Brzeziński, M.; Biela, T. Micro- and Nanostructures of Polylactide Stereocomplexes and Their Biomedical Applications. Polym. Int. 2015, 64, 1667–1675. [Google Scholar] [CrossRef]
- Nagahama, K.; Nishimura, Y.; Ohya, Y.; Ouchi, T. Impacts of Stereoregularity and Stereocomplex Formation on Physicochemical, Protein Adsorption and Cell Adhesion Behaviors of Star-Shaped 8-Arms Poly(Ethylene Glycol)-Poly(Lactide) Block Copolymer Films. Polymer 2007, 48, 2649–2658. [Google Scholar] [CrossRef]
- Li, W.; Fan, X.; Wang, X.; Shang, X.; Wang, Q.; Lin, J.; Hu, Z.; Li, Z. Stereocomplexed Micelle Formation through Enantiomeric PLA-Based Y-Shaped Copolymer for Targeted Drug Delivery. Mater. Sci. Eng. C 2018, 91, 688–695. [Google Scholar] [CrossRef]
- Michalski, A.; Makowski, T.; Biedroń, T.; Brzeziński, M.; Biela, T. Controlling Polylactide Stereocomplex (Sc-PLA) Self-Assembly: From Microspheres to Nanoparticles. Polymer 2016, 90, 242–248. [Google Scholar] [CrossRef]
- Isono, T.; Kondo, Y.; Otsuka, I.; Nishiyama, Y.; Borsali, R.; Kakuchi, T.; Satoh, T. Synthesis and Stereocomplex Formation of Star-Shaped Stereoblock Polylactides Consisting of Poly(l-Lactide) and Poly(d-Lactide) Arms. Macromolecules 2013, 46, 8509–8518. [Google Scholar] [CrossRef]
- Jie, P.; Venkatraman, S.S.; Min, F.; Freddy, B.Y.C.; Huat, G.L. Micelle-like Nanoparticles of Star-Branched PEO-PLA Copolymers as Chemotherapeutic Carrier. J. Control. Release 2005, 110, 20–33. [Google Scholar] [CrossRef]
- Jeong, B.; Choi, Y.K.; Bae, Y.H.; Zentner, G.; Kim, S.W. New Biodegradable Polymers for Injectable Drug Delivery Systems. J. Control. Release 1999, 62, 109–114. [Google Scholar] [CrossRef]
- Moo Huh, K.; Woo Cho, Y.; Park, K. LGA-PEG Block Copolymers for Drug Formulations. pp. 1–11. Available online: http://www.drugdeliverytech.com/cgi-bin/articles.cgi?idArticle=152 (accessed on 22 October 2021).
- Wang, T.; Zhu, D.; Liu, G.; Tao, W.; Cao, W.; Zhang, L.; Wang, L.; Chen, H.; Mei, L.; Huang, L.; et al. DTX-Loaded Star-Shaped TAPP-PLA-b-TPGS Nanoparticles for Cancer Chemical and Photodynamic Combination Therapy. RSC Adv. 2015, 5, 50617–50627. [Google Scholar] [CrossRef]
- Tang, X.; Cai, S.; Zhang, R.; Liu, P.; Chen, H.; Zheng, Y.; Sun, L. Paclitaxel-Loaded Nanoparticles of Star-Shaped Cholic Acid-Core Pla-Tpgs Copolymer for Breast Cancer Treatment. Nanoscale Res. Lett. 2013, 8, 420. [Google Scholar] [CrossRef] [Green Version]
- Zorba, T.; Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Synthesis, Characterization and Thermal Degradation Mechanism of Three Poly(Alkylene Adipate)s: Comparative Study. Polym. Degrad. Stab. 2007, 92, 222–230. [Google Scholar] [CrossRef]
- Karava, V.; Siamidi, A.; Vlachou, M.; Christodoulou, E.; Zamboulis, A.; Bikiaris, D.N.; Kyritsis, A.; Klonos, P.A. Block Copolymers Based on Poly(Butylene Adipate) and Poly(l-Lactic Acid) for Biomedical Applications: Synthesis, Structure and Thermodynamical Studies. Soft Matter 2021, 17, 2439–2453. [Google Scholar] [CrossRef]
- Karava, V.; Siamidi, A.; Vlachou, M.; Christodoulou, E.; Bikiaris, N.D.; Zamboulis, A.; Kostoglou, M.; Gounari, E.; Barmpalexis, P. Poly(L-Lactic Acid)-Co-Poly(Butylene Adipate) New Block Copolymers for the Preparation of Drug-Loaded Long Acting Injectable Microparticles. Pharmaceutics 2021, 13, 930. [Google Scholar] [CrossRef]
- NaNanaki, S.; Viziridou, A.; Zamboulis, A.; Kostoglou, M.; Papageorgiou, G.Z.; Bikiaris, D.N. New Biodegradable Poly(l-lactide)-Block-Poly(propylene adipate) Copolymer Microparticles for Long-Acting Injectables of Naltrexone Drug. Polymers 2020, 12, 852. [Google Scholar] [CrossRef] [Green Version]
- Bikiaris, D.N.; Papageorgiou, G.Z.; Giliopoulos, D.J.; Stergiou, C.A. Correlation between Chemical and Solid-State Structures and Enzymatic Hydrolysis in Novel Biodegradable Polyesters. The Case of Poly(Propylene Alkanedicarboxylate)S. Macromol. Biosci. 2008, 8, 728–740. [Google Scholar] [CrossRef]
- Nanaki, S.; Barmpalexis, P.; Iatrou, A.; Christodoulou, E.; Kostoglou, M.; Bikiaris, D.N. Risperidone Controlled Release Microspheres Based on Poly(Lactic Acid)-Poly(Propylene Adipate) Novel Polymer Blends Appropriate for Long Acting Injectable Formulations. Pharmaceutics 2018, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic Acid (PLA) Synthesis and Modifications: A Review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Tudorachi, N.; Lipsa, R.; Vasile, C.; Mustata, F. Poly(Lactic Acid)-Co-Aspartic Acid Copolymers: Possible Utilization in Drug Delivery Systems. J. Polym. Environ. 2013, 21, 1064–1071. [Google Scholar] [CrossRef]


















| SCF Technique | Advantages | Limitations |
|---|---|---|
| RESS, RESOLV |
|
|
| SAS, GAS, SEDS |
|
|
| PGSS |
|
|
| SFEE |
|
|
| Particles | Drug | Treatment | Reference |
|---|---|---|---|
| PLGA MPs | Rifampicin | Tuberculosis | [77] |
| PLGA MPs | Levofloxacin | Cystic fibrosis | [78] |
| Large porous PLGA MPs | Doxorubicin and p53 gene | Lung cancer | [79] |
| Large porous PLGA MPs | Curcumin | Idiopathic pulmonary fibrosis | [80] |
| Porous PLGA MPs | Doxorubicin and miR-519c | Lung Cancer | [81] |
| PEG-modified PLGA | Budesonide | Inflammation in bronchial wall | [82] |
| PLGA MPS | Salmon Calcitonin | Paget’s disease, Osteoporosis | [76] |
| Surface-Modified PLGA Microparticles | Gatifloxacin | Tuberculosis | [83] |
| Large porous PLGA MPs | Sildenafil citrate | Pulmonary arterial hypertension | [84] |
| PLGA−lipid hybrid MPs | Rifampicin | Antibacterial activity | [85] |
| PLGA MPs | Rifampicin | Tuberculosis | [77] |
| Particles | Drug | Treatment | Reference |
|---|---|---|---|
| PLLA-PEG MPs | Ibuprofen | Aches and Pains | [94] |
| PLLA-PEG-Folic acid MPs | Paclitaxel and Doxorubicin | Cancer | [107] |
| PLA-PEG-PLA MPs | Paclitaxel | Ovarian, breast, colon, head and neck and non-small cell lung cancers | [109] |
| PLLA-PEG-PLLA | Morphine | Cancer pains | [110] |
| PLA-PEG-Magnetite MPs | Paclitaxel | Cancer-Hypothermia | [111] |
| PLA-PEG MPs | Perillyl Alcohol | Glioma | [115] |
| PEG-PLA MPs | Tat-EGFP | Retinal diseases | [95] |
| PLA-PEG-PLA MPs | Bone Morphogenetic protein | Bone regeneration | [116] |
| mPEG-PLA Microspheres | 5-Fluorouracil | Cancer | [113] |
| PLA-PEG-PLA triblock copolymer microspheres | Paclitaxel | Cancer | [112] |
| Particles | Drug | Treatment | Reference |
|---|---|---|---|
| PLGA-PEG-PLGA -montmorillonite MPs | Aspirin | Inflammations, fever and pain reduction | [96] |
| PLGA-PEG MPs | Neuregulin (NRG) | Myocardial infarction | [117] |
| PLGA-PEG-PLGA MPs | Bone morphogenetic protein 2 (BMP-2) | Bone regeneration | [118] |
| PLGA-PEG | Clonidine | Arterial pressure | [97] |
| PLGA-PEG | Lysozyme | Tissue engineering | [119] |
| PLGA-PEG-PLGA MPs | Insulin | Diabetes | [91] |
| Encapsulated Substance | Application | Encapsulation Method | LA/CL Molar Ratio | Advantages | Reference |
|---|---|---|---|---|---|
| 5-fluorouracil (5-FU) | chemotherapeutic agent | spray drying |
| Release rates of 5-FU from PLCL somewhat greater than that observed with PLGA/PLA microspheres | [123] |
| Cyclosporin A (CyA) |
| oil-in-water (O/W) emulsion solvent evaporation |
| Higher blood level of CyA during the initial 2 days, as well as constant levels for several weeks regarding in vivo evaluation | [124] |
| Nystatin | antifungal drug | oil-in-water (o/w) emulsion solvent evaporation | 86 mol% DL-lactide | Stable microspheres and no degradation observed during the period of study of two months | [127] |
| Ibuprofen |
| oil-in-water (o/w) solvent evaporation | 78.7/21.3 | The complete ibuprofen release duration from the microspheres exceeded 1 month | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. https://doi.org/10.3390/pharmaceutics14020359
Vlachopoulos A, Karlioti G, Balla E, Daniilidis V, Kalamas T, Stefanidou M, Bikiaris ND, Christodoulou E, Koumentakou I, Karavas E, et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics. 2022; 14(2):359. https://doi.org/10.3390/pharmaceutics14020359
Chicago/Turabian StyleVlachopoulos, Antonios, Georgia Karlioti, Evangelia Balla, Vasileios Daniilidis, Theocharis Kalamas, Myrika Stefanidou, Nikolaos D. Bikiaris, Evi Christodoulou, Ioanna Koumentakou, Evangelos Karavas, and et al. 2022. "Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances" Pharmaceutics 14, no. 2: 359. https://doi.org/10.3390/pharmaceutics14020359