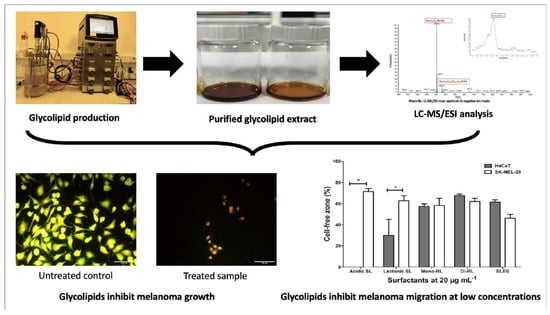
Biosurfactants as Anticancer Agents: Glycolipids Affect Skin Cells in a Differential Manner Dependent on Chemical Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Purification of Glycolipids
2.2. Characterization of Purified Glycolipids Preparations
2.3. Cell Culture
2.4. Cell Viability Assays
2.5. Cell Morphology Assessment
2.6. Acridine Orange and Propidium Iodine Staining
2.7. Scratch Assay
2.8. Statistical Analysis
3. Results
3.1. Characterization of Glycolipid Preparations
3.2. Glycolipids Affect the Viability of HaCaT and SK-Mel-28 Cell Differentially According to Molecular Structure
3.3. Glycolipids Affect Cell Morphology of HaCaT and SK-Mel-28 Cell Lines
3.4. Glycolipids Mediate Cell Death of HaCaT and SK-Mel-28 Cell Lines via Necrosis
3.5. Glycolipids Inhibit Cellular Migration in SK-MEL-28 Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institution Cancer Stat Facts: Melanoma of the Skin. Available online: https://seer.cancer.gov/statfacts/html/melan.html (accessed on 3 December 2021).
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 3 December 2021).
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Domingues, B.; Lopes, J.; Soares, P.; Populo, H. Melanoma treatment in review. ImmunoTargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkwood, J.M.; Ibrahim, J.G.; Sosman, J.A.; Sondak, V.K.; Agarwala, S.S.; Ernstoff, M.S.; Rao, U. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: Results of intergroup trial E1694/S9512/C509801. J. Clin. Oncol. 2001, 19, 2370. [Google Scholar] [CrossRef] [PubMed]
- Bright, R.; Coventry, B.J.; Eardley-Harris, N.; Briggs, N. Clinical Response Rates from Interleukin-2 Therapy for Metastatic Melanoma over 30 Years’ Experience: A Meta-Analysis of 3312 Patients. J. Immunother. 2017, 40, 21. [Google Scholar] [CrossRef]
- Weide, B.; Martens, A.; Wistuba-Hamprecht, K.; Zelba, H.; Maier, L.; Lipp, H.-P.; Klumpp, B.D.; Soffel, D.; Eigentler, T.K.; Garbe, C. Combined treatment with ipilimumab and intratumoral interleukin-2 in pretreated patients with stage IV melanoma—Safety and efficacy in a phase II study. Cancer Immunol. Immunother. 2017, 66, 441–449. [Google Scholar] [CrossRef]
- Specenier, P. Nivolumab in melanoma. Expert Rev. Anticancer Ther. 2016, 16, 1247–1261. [Google Scholar] [CrossRef]
- Twigg, M.S.; Baccile, N.; Banat, I.M.; Déziel, E.; Marchant, R.; Roelants, S.; Van Bogaert, I.N.A. Microbial biosurfactant research: Time to improve the rigour in the reporting of synthesis, functional characterization and process development. Microb. Biotechnol. 2021, 14, 147–170. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef]
- Mnif, I.; Ellouz-Chaabouni, S.; Ghribi, D. Glycolipid Biosurfactants, Main Classes, Functional Properties and Related Potential Applications in Environmental Biotechnology. J. Polym. Environ. 2018, 26, 2192–2206. [Google Scholar] [CrossRef]
- Rudden, M.; Tsauosi, K.; Marchant, R.; Banat, I.M.; Smyth, T.J. Development and validation of an ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method for the quantitative determination of rhamnolipid congeners. Appl. Microbiol. Biotechnol. 2015, 99, 9177–9187. [Google Scholar] [CrossRef]
- Dubeau, D.; Déziel, E.; Woods, D.E.; Lépine, F. Burkholderia thailandensis harbors two identical rhl gene clusters responsible for the biosynthesis of rhamnolipids. BMC Microbiol. 2009, 9, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtzman, C.P.; Price, N.P.J.; Ray, K.J.; Kuo, T.M. Production of sophorolipid biosurfactants by multiple species of the Starmerella (Candida) bombicola yeast clade. FEMS Microbiol. Lett. 2010, 311, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naughton, P.J.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharmaceutics 2020, 12, 1099. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactants Produced by Marine Microorganisms with Therapeutic Applications. Mar. Drugs 2016, 14, 38. [Google Scholar] [CrossRef] [Green Version]
- Gudiña, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Song, X.; Zhang, H.; Qu, Y.B.; Miao, J.Y. Sophorolipid produced from the new yeast strain Wickerhamiella domercqiae induces apoptosis in H7402 human liver cancer cells. Appl. Microbiol. Biotechnol. 2006, 72, 52–59. [Google Scholar] [CrossRef]
- Chen, J.; Song, X.; Zhang, H.; Qu, Y. Production, structure elucidation and anticancer properties of sophorolipid from Wickerhamiella domercqiae. Enzyme Microb. Technol. 2006, 39, 501–506. [Google Scholar] [CrossRef]
- Fu, S.L.; Wallner, S.R.; Bowne, W.B.; Hagler, M.D.; Zenilman, M.E.; Gross, R.; Bluth, M.H. Sophorolipids and Their Derivatives Are Lethal against Human Pancreatic Cancer Cells. J. Surg. Res. 2008, 148, 77. [Google Scholar] [CrossRef]
- Dhar, S.; Reddy, E.M.; Prabhune, A.; Pokharkar, V.; Shiras, A.; Prasad, B.L. V Cytotoxicity of sophorolipid-gellan gum-gold nanoparticle conjugates and their doxorubicin loaded derivatives towards human glioma and human glioma stem cell lines. Nanoscale 2011, 3, 575–580. [Google Scholar] [CrossRef]
- Shao, L.; Song, X.; Ma, X.; Li, H.; Qu, Y. Bioactivities of Sophorolipid with Different Structures against Human Esophageal Cancer Cells. J. Surg. Res. 2012, 173, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.A.C.; Faustino, C.M.C.; Guerreiro, P.S.; Frade, R.F.M.; Bronze, M.R.; Castro, M.F.; Ribeiro, M.H.L. Development of novel sophorolipids with improved cytotoxic activity toward MDA-MB-231 breast cancer cells. J. Mol. Recognit. 2015, 28, 155. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, W.; Ma, X.; Li, J.; Song, X. In Vitro and in Vivo Anticancer Activity of Sophorolipids to Human Cervical Cancer. Appl. Biochem. Biotechnol. 2017, 181, 1372–1387. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.; Lydon, H.; Roelants, S.L.K.W.; Van Bogaert, I.N.A.; Marchant, R.; Banat, I.M.; Mitchell, C.A. Lactonic Sophorolipids Increase Tumor Burden in Apcmin+/− Mice. PLoS ONE 2016, 11, e0156845. [Google Scholar] [CrossRef] [PubMed]
- Thanomsub, B.; Pumeechockchai, W.; Limtrakul, A.; Arunrattiyakorn, P.; Petchleelaha, W.; Nitoda, T.; Kanzaki, H. Chemical structures and biological activities of rhamnolipids produced by Pseudomonas aeruginosa B189 isolated from milk factory waste. Bioresour. Technol. 2006, 97, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Christova, N.; Tuleva, B.; Kril, A.; Georgieva, M.; Konstantinov, S.; Terziyski, I.; Nikolova, B.; Stoineva, I. Chemical Structure and In Vitro Antitumor Activity of Rhamnolipids from Pseudomonas aeruginosa BN10. Appl. Biochem. Biotechnol. 2013, 170, 676–689. [Google Scholar] [CrossRef]
- Haque, F.; Khan, M.S.A.; AlQurashi, N. ROS-Mediated Necrosis by Glycolipid Biosurfactants on Lung, Breast, and Skin Melanoma Cells. Front. Oncol. 2021, 11, 253. [Google Scholar] [CrossRef]
- Smyth Thomas, J.P.; Perfumo, A.; Marchant, R.; Banat, I.M. Isolation and analysis of low molecular weight microbial glycolipids. In Handbook of Hydrocarbon and Lipid Microbiology, 1st ed.; Timmis, K.N., Ed.; Springer: Berlin, Germany, 2010; pp. 3705–3723. [Google Scholar]
- Ribble, D.; Goldstein, N.B.; Norris, D.A.; Shellman, Y.G. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ammann, K.R.; DeCook, K.J.; Tran, P.L.; Merkle, V.M.; Wong, P.K.; Slepian, M.J. Collective cell migration of smooth muscle and endothelial cells: Impact of injury versus non-injury stimuli. J. Biol. Eng. 2015, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Atale, N.; Gupta, S.; Yadav, U.C.S.; Rani, V. Cell-death assessment by fluorescent and nonfluorescent cytosolic and nuclear staining techniques. J. Microsc. 2014, 255, 7. [Google Scholar] [CrossRef] [PubMed]
- Sample, A.; He, Y.Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Tykodi, S.S.; Thompson, J.A. Treatment of metastatic melanoma: An overview. Oncology 2009, 23, 488. [Google Scholar] [PubMed]
- Guy, G.P.; Ekwueme, D.U.; Tangka, F.K.; Richardson, L.C. Melanoma treatment costs: A systematic review of the literature, 1990-2011. Am. J. Prev. Med. 2012, 43, 537. [Google Scholar] [CrossRef] [Green Version]
- Xavier, M.H.S.B.; Drummond-Lage, A.P.; Baeta, C.; Rocha, L.; Almeida, A.M.; Wainstein, A.J.A. Delay in cutaneous melanoma diagnosis: Sequence analyses from suspicion to diagnosis in 211 patients. Medicine 2016, 95, e4396. [Google Scholar] [CrossRef]
- Rigel, D.S. The effect of sunscreen on melanoma risk. Dermatol. Clin. 2002, 20, 601–606. [Google Scholar] [CrossRef]
- American Cancer Society Skin Cancer Prevention and Early Detection: How Do I Protect Myself from UV Rays? Available online: https://www.cancer.org/healthy/be-safe-in-sun/uv-protection.html (accessed on 27 December 2021).
- Seweryn, A. Interactions between surfactants and the skin—Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- Ceresa, C.; Fracchia, L.; Williams, M.; Banat, I.M.; Díaz De Rienzo, M.A. The effect of sophorolipids against microbial biofilms on medical-grade silicone. J. Biotechnol. 2020, 309, 34–43. [Google Scholar] [CrossRef]
- Das, P.; Yang, X.P.; Ma, L.Z. Analysis of biosurfactants from industrially viable Pseudomonas strain isolated from crude oil suggests how rhamnolipids congeners affect emulsification property and antimicrobial activity. Front. Microbiol. 2014, 5, 696. [Google Scholar] [CrossRef]
- Christova, N.; Tuleva, B.; Cohen, R.; Ivanova, G.; Stoev, G.; Stoilova-Disheva, M.; Stoineva, I. Chemical Characterization and Physical and Biological Activities of Rhamnolipids Produced by Pseudomonas aeruginosa BN10. Zeitschrift Naturforsch. C 2011, 66, 394–402. [Google Scholar] [CrossRef]
- Mishra, N.; Rana, K.; Seelam, S.D.; Kumar, R.; Pandey, V.; Salimath, B.P.; Agsar, D. Characterization and Cytotoxicity of Pseudomonas Mediated Rhamnolipids Against Breast Cancer MDA-MB-231 Cell Line. Front. Bioeng. Biotechnol. 2021, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Pontes, C.; Alves, M.; Santos, C.; Ribeiro, M.H.; Gonçalves, L.; Bettencourt, A.F.; Ribeiro, I.A.C. Can Sophorolipids prevent biofilm formation on silicone catheter tubes? Int. J. Pharm. 2016, 513, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Díaz De Rienzo, M.A.; Banat, I.M.; Dolman, B.; Winterburn, J.; Martin, P.J. Sophorolipid biosurfactants: Possible uses as antibacterial and antibiofilm agent. New Biotechnol. 2015, 32, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.I.; Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Sodium chloride effect on the aggregation behaviour of rhamnolipids and their antifungal activity. Sci. Rep. 2017, 7, 12907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semkova, S.; Antov, G.; Iliev, I.; Tsoneva, I.; Lefterov, P.; Christova, N.; Nacheva, L.; Stoineva, I.; Kabaivanova, L.; Staneva, G.; et al. Rhamnolipid Biosurfactants—Possible Natural Anticancer Agents and Autophagy Inhibitors. Seperations 2021, 8, 92. [Google Scholar] [CrossRef]
- Lydon, H.L.; Baccile, N.; Callaghan, B.; Marchant, R.; Mitchell, C.A.; Banat, I.M. Adjuvant Antibiotic Activity of Acidic Sophorolipids with Potential for Facilitating Wound Healing. Antimicrob. Agents Chemother. 2022, 61, e02547-16. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, B.V.G.; Nagakubo, T.; Toyofuku, M.; Nomura, N.; Utada, A.S. Synergy between Sophorolipid Biosurfactant and SDS Increases the Efficiency of P. aeruginosa Biofilm Disruption. Langmuir 2020, 36, 6411–6420. [Google Scholar] [CrossRef]
- Rahimi, K.; Lotfabad, T.B.; Jabeen, F.; Mohammad Ganji, S. Cytotoxic effects of mono- and di-rhamnolipids from Pseudomonas aeruginosa MR01 on MCF-7 human breast cancer cells. Colloids Surf. B Biointerfaces 2019, 181, 943–952. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Y.; Alfred, A.T.; Xin, X.; Yang, S. Chemical structures and biological activities of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa M14808. J. Chem. Pharm. Res. 2013, 5, 177. [Google Scholar]
- Zbytek, B.; Carlson, J.A.; Granese, J.; Ross, J.; Mihm, M.; Slominski, A. Current concepts of metastasis in melanoma. Expert Rev. Dermatol. 2008, 3, 569. [Google Scholar] [CrossRef] [Green Version]
- Novikov, N.M.; Zolotaryova, S.Y.; Gautreau, A.M.; Denisov, E.V. Mutational drivers of cancer cell migration and invasion. Br. J. Cancer 2021, 124, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Lu, S.-H.; Chang, C.-C.; Thomas, P.A.; Jayakumar, T.; Sheu, J.-R. Hinokitiol, a tropolone derivative, inhibits mouse melanoma (B16-F10) cell migration and in vivo tumor formation. Eur. J. Pharmacol. 2015, 746, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Wyckoff, J.; Condeelis, J. Cell migration in tumors. Curr. Opin. Cell Biol. 2005, 17, 559–564. [Google Scholar] [CrossRef] [PubMed]




| RT (min) | m/z Value | Compound | Mw (Da) | Relative (%) | |
|---|---|---|---|---|---|
| Acidic-SL Preparation | 13.03 | 595.3 | Acidic, C16:0 | 596 | 6.31 |
| 13.64 | 619.1 | Acidic, C18:2 | 620 | 0.72 | |
| 13.88 | 621.3 | Acidic, C18:1 | 622 | 65.53 | |
| 14.46 | 623.3 | Acidic, C18:0 | 624 | 14.93 | |
| 7.28 | 637.3 | Acidic, R1 = Ac, C16:0 | 638 | 3.58 | |
| 15.21 | 665.3 | Acidic, 1Ac, C18:0 | 666 | 1.14 | |
| 15.01 | 663.2 | Acidic, R1 = Ac, C18:1 | 664 | 2.55 | |
| 15.62 | 679.2 | Acidic, R1 + R2 = Ac, C16:0 | 680 | 0.56 | |
| 16.19 | 705.2 | Acidic, R1 + R2 = Ac, C18:1 | 706 | 2.90 | |
| 16.95 | 707.3 | Acidic, R1 + R2 = Ac, C18:0 | 708 | 0.85 | |
| 13.03 | 721.2 | Acidic, R1 = Ac, C22:0 | 722 | 0.65 | |
| 15.57 | 791.3 | Acidic, R1 + R2 = Ac, C24:0 | 792 | 0.29 | |
| Lactonic-SL Preparation | 12.86 | 705.1 | Acidic, R1 + R2 = Ac, C18:1 * | 706 | 10.14 |
| 14.21 | 645.1 | Lactonic, R1 = Ac, C18:1 | 646 | 3.24 | |
| 16.36 | 685.1 | Lactonic, R1 + R2 = Ac, C18:2 | 686 | 15.95 | |
| 17.36 | 687.1 | Lactonic, R1 + R2 = Ac, C18:1 | 688 | 63.40 | |
| 18.93 | 689.1 | Lactonic, R1 + R2 = Ac, C18:0 | 690 | 7.27 | |
| Mono-RL Preparation | 19.62 | 332.9 | Rha-C10 | 334 | 1.35 |
| 19.65 | 502.9 | Rha-C10-C10 | 504 | 84.40 | |
| 19.60 | 505.0 | Rha-Rha-C12:1 * | 506 | 2.97 | |
| 21.77 | 528.9 | Rha-C10-C12:1/ Rha-C12:1-C10 | 530 | 6.63 | |
| 23.12 | 530.9 | Rha-C10-C12/ Rha-C12-C10 | 532 | 4.65 | |
| Di-RL Preparation | 11.03 | 332.9 | Rha-C10 * | 334 | 0.19 |
| 9.78 | 479.0 | Rha-Rha-C10 | 480 | 23.84 | |
| 29.81 | 502.9 | Rha-C10-C10 * | 504 | 0.59 | |
| 29.79 | 505.0 | Rha-Rha-C12:1 | 506 | 0.15 | |
| 12.96 | 507.0 | Rha-Rha-C12 | 508 | 1.15 | |
| 31.36 | 528.0 | Rha-C10-C12:1/Rha-C12:1-C10 * | 530 | 1.04 | |
| 31.33 | 530.9 | Rha-C10-C12/Rha-C12-C10 * | 532 | 0.27 | |
| 15.36 | 621.0 | Rha-Rha-C10-C8/Rha-Rha-C8-C10 | 662 | 0.95 | |
| 16.29 | 647.1 | Rha-Rha-C10-C10:1/Rha-Rha-C8-C12:1 | 648 | 0.26 | |
| 17.30 | 649.1 | Rha-Rha-C10-C10 | 650 | 57.99 | |
| 17.88 | 663.0 | Rha-Rha-C10-C10-CH3 | 664 | 0.27 | |
| 18.15 | 675.1 | Rha-Rha-C10-C12:1/Rha-Rha-C12:1-C10 | 676 | 4.18 | |
| 19.83 | 677.1 | Rha-Rha-C10-C12/Rha-Rha-C12-C10 | 678 | 8.72 | |
| 21.48 | 703.1 | Rha-Rha-C10-C14:1/Rha-Rha-C12:1-C12 | 704 | 0.18 | |
| 23.00 | 705.0 | Rha-Rha-C12-C12 | 706 | 0.13 | |
| 31.18 | 988.0 | Rha-Rha-C14-C14-C14 | 989 | 0.07 |
| Surfactant | IC50 (± SEM) (μg mL−1) | Significant | p Value | |
|---|---|---|---|---|
| HaCaT | SK-Mel-28 | |||
| Lactonic sophorolipid (0–100 μg mL−1) | 62.62 (1.34) | 53.83 (1.64) | Yes | 0.0142 |
| Acidic sophorolipid (0–100 μg mL−1) | ND | ND | - | - |
| Di–rhamnolipid (0–100 μg mL−1) | 47.57 (2.76) | 40.79 (0.85) | No | 0.0789 |
| Mono–rhamnolipid (0–100 μg mL−1) | ND | ND | - | - |
| SLES (0–100 μg mL−1) | 65.50 (1.26) | 65.06 (0.46) | No | 0.7571 |
| Acidic sophorolipid (0–500 μg mL−1) | ND | ND | - | - |
| Mono-rhamnolipid (0–500 μg mL−1) | 628.3 (47.61) | 570.4 (44.11) | No | 0.4228 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Biosurfactants as Anticancer Agents: Glycolipids Affect Skin Cells in a Differential Manner Dependent on Chemical Structure. Pharmaceutics 2022, 14, 360. https://doi.org/10.3390/pharmaceutics14020360
Adu SA, Twigg MS, Naughton PJ, Marchant R, Banat IM. Biosurfactants as Anticancer Agents: Glycolipids Affect Skin Cells in a Differential Manner Dependent on Chemical Structure. Pharmaceutics. 2022; 14(2):360. https://doi.org/10.3390/pharmaceutics14020360
Chicago/Turabian StyleAdu, Simms A., Matthew S. Twigg, Patrick J. Naughton, Roger Marchant, and Ibrahim M. Banat. 2022. "Biosurfactants as Anticancer Agents: Glycolipids Affect Skin Cells in a Differential Manner Dependent on Chemical Structure" Pharmaceutics 14, no. 2: 360. https://doi.org/10.3390/pharmaceutics14020360