Evaluation of Antioxidant and Wound-Healing Properties of EHO-85, a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract
Abstract
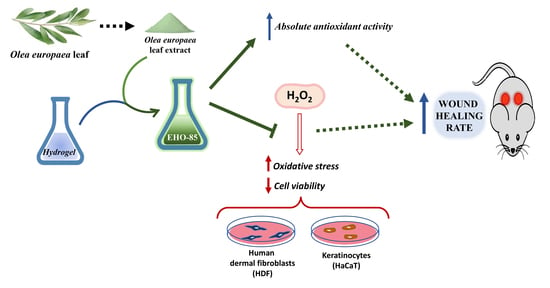
:1. Introduction
2. Materials and Methods
2.1. Amorphous Hydrogel-Containing Olea europaea Leaf Extract (EHO-85)
2.2. Characterization of Phenolic Compounds Contained in the Olea europaea Leaf Extract
2.3. Absolute Antioxidant Activity of Olea europaea Leaf Extract and EHO-85 Hydrogel
2.4. Protection against Oxidative Damage of Olea europaea Leaf Extract on Cell Cultures
2.5. Cell Viability
2.6. Wound-Healing Activity of the EHO-85 Hydrogel In Vivo
2.7. Statistical Analysis
3. Results
3.1. Characterization of Olea europaea Leaf Extract by LC-MS/MS Analysis
3.2. Antioxidant Activity of Olea europaea Leaf Extract and EHO-85
3.3. Oxidative Damage Protection of Olea europaea Leaf Extract
3.4. Olea europaea Leaf Extract Increases the Viability of HDF and HaCaT Cultures Exposed to Oxidative Stress
3.5. Wound-Healing Potential of the Amorphous Hydrogel In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, J.B.; Broszczak, D.A.; Mani, J.S.; Anesi, J.; Naiker, M. A cut above the rest: Oxidative stress in chronic wounds and the potential role of polyphenols as therapeutics. J. Pharm. Pharmacol. 2021, rgab038. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Stratton, R.D. Oxidative Stress and Antioxidant Protection; Armstrong, D., Stratton, R.D., Eds.; Wiley: Hoboken, NJ, USA, 2016; ISBN 9781118832486. [Google Scholar]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.C.; Gougerot-Pocidalo, M.A.; Dang, P.M.C. Priming of the neutrophil respiratory burst: Role in host defense and inflammation. Immunol. Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef]
- Jaganjac, M.; Cipak, A.; Schaur, R.J.; Zarkovic, N. Pathophysiology of neutrophil-mediated extracellular redox reactions. Front. Biosci.-Landmark 2016, 21, 839–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Rojkind, M.; Domínguez-Rosales, J.A.; Nieto, N.; Greenwel, P. Role of hydrogen peroxide and oxidative stress in healing responses. Cell. Mol. Life Sci. 2002, 59, 1872–1891. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.; Ashraf, M.A.B.; Malik, A.; Waquar, S.; Khan, S.A.; Qazi, M.H.; Ahmad, W.; Asif, M.; Khan, S.U.; Zaheer, A.; et al. Comparative study of extrapolative factors linked with oxidative injury and antiinflammatory status in chronic kidney disease patients experiencing cardiovascular distress. PLoS ONE 2017, 12, e0171561. [Google Scholar] [CrossRef]
- Koutakis, P.; Ismaeel, A.; Farmer, P.; Purcell, S.; Smith, R.S.; Eidson, J.L.; Bohannon, W.T. Oxidative stress and antioxidant treatment in patients with peripheral artery disease. Physiol. Rep. 2018, 6, e13650. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Vermeij, W.P.; Backendorf, C. Skin cornification proteins provide global link between ROS detoxification and cell migration during wound healing. PLoS ONE 2010, 5, e11957. [Google Scholar] [CrossRef] [Green Version]
- Wagener, F.A.D.T.G.; Carels, C.E.; Lundvig, D.M.S. Targeting the redox balance in inflammatory skin conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Wound healing essentials: Let there be oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Dhall, S.; Do, D.C.; Garcia, M.; Kim, J.; Mirebrahim, S.H.; Lyubovitsky, J.; Lonardi, S.; Nothnagel, E.A.; Schiller, N.; Martins-Green, M. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J. Diabetes Res. 2014, 2014, 562625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, e1901502. [Google Scholar] [CrossRef] [PubMed]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The role of antioxidants on wound healing: A review of the current evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The role of oxygen in wound healing: A review of the literature. Dermatologic Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Shetty, B.S. Wound Healing and Indigenous Drugs: Role as Antioxidants: A Review. Res. Rev. J. Med. Heal. Sci. 2013, 2, 5–16. [Google Scholar]
- Süntar, I.; Akkol, E.K.; Nahar, L.; Sarker, S.D. Wound healing and antioxidant properties: Do they coexist in plants? Free Radicals Antioxidants 2012, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Nyanhongo, G.S.; Sygmund, C.; Ludwig, R.; Prasetyo, E.N.; Guebitz, G.M. An antioxidant regenerating system for continuous quenching of free radicals in chronic wounds. Eur. J. Pharm. Biopharm. 2013, 83, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Bektas, N.; Şenel, B.; Yenilmez, E.; Özatik, O.; Arslan, R. Evaluation of wound healing effect of chitosan-based gel formulation containing vitexin. Saudi Pharm. J. 2020, 28, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Grzelak, A.; Baumann, M.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Anti-inflammatory and anti-oxidant properties of laccase-synthesized phenolic-O-carboxymethyl chitosan hydrogels. New Biotechnol. 2018, 40, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Tonks, A.J.; Cooper, R.A.; Jones, K.P.; Blair, S.; Parton, J.; Tonks, A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003, 21, 242–247. [Google Scholar] [CrossRef]
- Gabr, S.A.; Alghadir, A.H. Evaluation of the Biological Effects of Lyophilized Hydrophilic Extract of Rhus coriaria on Myeloperoxidase (MPO) Activity, Wound Healing, and Microbial Infections of Skin Wound Tissues. Evid.-Based Complement. Altern. Med. 2019, 2019, 5861537. [Google Scholar] [CrossRef] [Green Version]
- Castro, B.; Bastida, F.D.; Segovia, T.; Casanova, P.L.; Soldevilla, J.J.; Verdú-Soriano, J. The use of an antioxidant dressing on hard-To-heal wounds: A multicentre, prospective case series. J. Wound Care 2017, 26, 742–750. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, Á.; Rosal, A.; Carrasco, E. Valorisation of olea europaea l. Olive leaves through the evaluation of their extracts: Antioxidant and antimicrobial activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Gerothanassis, I.P. Phenolic compounds and antioxidant activity of olive leaf extracts. Nat. Prod. Res. 2012, 26, 186–189. [Google Scholar] [CrossRef]
- Fresno Contreras, M.J.; Ramírez Diéguez, A.; Jiménez Soriano, M.M. Viscosity and temperature relationship in ethanol/water mixtures gelified with Carbopol® UltrezTM 10. Farmaco 2001, 56, 443–445. [Google Scholar] [CrossRef]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological characterization of topical carbomer gels neutralized to different pH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Anjum, A.; Sim, C.H.; Ng, S.F. Hydrogels Containing Antibiofilm and Antimicrobial Agents Beneficial for Biofilm-Associated Wound Infection: Formulation Characterizations and In vitro Study. AAPS PharmSciTech 2018, 19, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.R. Physical Properties of Glycerine. In Glycerine; Jungermann, E., Sonntag, N.O.V., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 113–156. ISBN 9780203753071. [Google Scholar]
- Albèr, C.; Buraczewska-Norin, I.; Kocherbitov, V.; Saleem, S.; Lodén, M.; Engblom, J. Effects of water activity and low molecular weight humectants on skin permeability and hydration dynamics—A double-blind, randomized and controlled study. Int. J. Cosmet. Sci. 2014, 36, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Péterszegi, G.; Isnard, N.; Robert, A.M.; Robert, L. Studies on skin aging. Preparation and properties of fucose-rich oligo- and polysaccharides. Effect on fibroblast proliferation and survival. Biomed. Pharmacother. 2003, 57, 187–194. [Google Scholar] [CrossRef]
- del Mar Delgado-Povedano, M.; Priego-Capote, F.; de Castro, M.D.L. Selective ultrasound-enhanced enzymatic hydrolysis of oleuropein to its aglycon in olive (Olea europaea L.) leaf extracts. Food Chem. 2017, 220, 282–288. [Google Scholar] [CrossRef]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ordóñez-Díaz, J.L.; Hervalejo, A.; Pereira-Caro, G.; Muñoz-Redondo, J.M.; Romero-Rodríguez, E.; Arenas-Arenas, F.J.; Moreno-Rojas, J.M. Effect of rootstock and harvesting period on the bioactive compounds and antioxidant activity of two orange cultivars (‘salustiana’ and ‘sanguinelli’) widely used in juice industry. Processes 2020, 8, 1212. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; Luque De Castro, M.D. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. A 2006, 1108, 76–82. [Google Scholar] [CrossRef]
- Umeno, A.; Takashima, M.; Murotomi, K.; Nakajima, Y.; Koike, T.; Matsuo, T.; Yoshida, Y. Radical-scavenging activity and antioxidative effects of olive leaf components oleuropein and hydroxytyrosol in comparison with homovanillic alcohol. J. Oleo Sci. 2015, 64, 793–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lins, P.G.; Marina Piccoli Pugine, S.; Scatolini, A.M.; de Melo, M.P. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 2018, 4, e00805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, J.E.; Allen, P.; Brunton, N.; O’Grady, M.N.; Kerry, J.P. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: Olive leaf extract (Olea europaea L.), lutein, sesamol and ellagic acid. Food Chem. 2011, 126, 948–955. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Gordon, M.H.; Paiva-Martins, F.; Almeida, M. Antioxidant activity of hydroxytyrosol acetate compared with that of other olive oil polyphenols. J. Agric. Food Chem. 2001, 49, 2480–2485. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Darby, I.A.; Hewitson, T.D. Fibroblast Differentiation in Wound Healing and Fibrosis. Int. Rev. Cytol. 2007, 257, 143–179. [Google Scholar]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Scharffetter-Kochanek, K. Oxidative stress in chronic venous leg ulcers. Wound Repair Regen. 2005, 13, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Wall, I.B.; Moseley, R.; Baird, D.M.; Kipling, D.; Giles, P.; Laffafian, I.; Price, P.E.; Thomas, D.W.; Stephens, P. Fibroblast dysfunction is a key factor in the non-healing of chronic venous leg ulcers. J. Investig. Dermatol. 2008, 128, 2526–2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamers, M.L.; Almeida, M.E.S.; Vicente-Manzanares, M.; Horwitz, A.F.; Santos, M.F. High glucose-mediated oxidative stress impairs cell migration. PLoS ONE 2011, 6, e22865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, C.; Alston, M.; Bickenbach, J.R.; Aykin-Burns, N. Oxygen tension changes the rate of migration of human skin keratinocytes in an age-related manner. Exp. Dermatol. 2011, 20, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Kaviani, M.; Sepasi, S.; Azima, S.; Emamghoreishi, M.; Asadi, N.; Haghpanah, S. The effects of olive leaf extract ointment on pain intensity and early maternal complications in primiparous women. Int. J. Pharm. Pharm. Sci. 2017, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Mehraein, F.; Sarbishegi, M.; Aslani, A. Evaluation of effect of oleuropein on skin wound healing in aged male BALB/c mice. Cell J. 2014, 16, 25–30. [Google Scholar]
- Samancıoğlu, S.; Esen, A.; Ercan, G.; Mansoub, N.H.; Vatansever, S.; Ince, İ. A new dressing material in diabetic wounds: Wound healing activity of oleuropein-rich olive leaf extract in diabetic rats. Eurjther.Comgaziantep Med. J. 2016, 22, 14–21. [Google Scholar] [CrossRef]
- Dasari, N.; Jiang, A.; Skochdopole, A.; Chung, J.; Reece, E.M.; Vorstenbosch, J.; Winocour, S.; Winocour, S. Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin. Plast. Surg. 2021, 35, 153–158. [Google Scholar] [CrossRef]





| COMPOUND | Retention Time (tr)* (min) | Relative Area | Relative Content (%) |
|---|---|---|---|
| Hydroxytyrosol glucoside | 11.3 | 2.66 | 0.12 |
| Oleoside | 11.8 | 57.17 | 2.68 |
| Hydroxytyrosol | 12.5 | 14.27 | 0.67 |
| p-Dihydroxyphenilacetic acid | 16.8 | 3.02 | 0.14 |
| Oleoside-11-methyl ester (isomers 1 & 2) | 18.5/20.7 | 70.06/42.63 | 5.41 |
| Demethyloleuropei | 23.2 | 54.49 | 2.56 |
| Verbascoside | 24.3 | 124.43 | 5.84 |
| Rutin | 24.4 | 12.12 | 0.56 |
| Astilbin | 24.8 | 3.06 | 0.14 |
| α-Taxifolin | 26.5 | 2.31 | 0.10 |
| Apigenin-7-glucoside | 28.2 | 8.65 | 0.40 |
| Oleuropein (isomers 1, 2, 3 & 4) | 28.3/29.4/30.0/30.5 | 81.24/1054.83/103.25/422.66 | 78.07 |
| Luteolin-7-glucoside | 28.5 | 53.16 | 2.49 |
| p-HPEA-EA (tyrosol derivative) | 32.5 | 6.13 | 0.28 |
| 3,4-HPEA-EA (hydroxytyrosol derivatives) | 34.9/35.4 | 6.79/1.87 | 0.39 |
| Luteolin | 35.7 | 3.34 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado-Diaz, A.; Moreno-Rojas, J.M.; Verdú-Soriano, J.; Lázaro-Martínez, J.L.; Rodríguez-Mañas, L.; Tunez, I.; La Torre, M.; Berenguer Pérez, M.; Priego-Capote, F.; Pereira-Caro, G. Evaluation of Antioxidant and Wound-Healing Properties of EHO-85, a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract. Pharmaceutics 2022, 14, 349. https://doi.org/10.3390/pharmaceutics14020349
Casado-Diaz A, Moreno-Rojas JM, Verdú-Soriano J, Lázaro-Martínez JL, Rodríguez-Mañas L, Tunez I, La Torre M, Berenguer Pérez M, Priego-Capote F, Pereira-Caro G. Evaluation of Antioxidant and Wound-Healing Properties of EHO-85, a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract. Pharmaceutics. 2022; 14(2):349. https://doi.org/10.3390/pharmaceutics14020349
Chicago/Turabian StyleCasado-Diaz, Antonio, José Manuel Moreno-Rojas, José Verdú-Soriano, José Luis Lázaro-Martínez, Leocadio Rodríguez-Mañas, Isaac Tunez, Manuel La Torre, Miriam Berenguer Pérez, Feliciano Priego-Capote, and Gema Pereira-Caro. 2022. "Evaluation of Antioxidant and Wound-Healing Properties of EHO-85, a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract" Pharmaceutics 14, no. 2: 349. https://doi.org/10.3390/pharmaceutics14020349