Injectable Nano Drug Delivery Systems for the Treatment of Breast Cancer
Abstract
:1. Introduction
2. Breast Cancer Biology
2.1. Risk Factors
- a.
- Age: With growing age, the risk of developing breast cancer increases. According to the surveillance, epidemiology, and end results databases, a woman in the United States has a 1 in 8 chance of being diagnosed with breast cancer in her lifetime: 1 in 202 from birth to age 39, 1 in 26 from age 40 to 59, and 1 in 28 from age 60 to 69 [26].
- b.
- Breast Pathology: Proliferative breast disease increases the probability of developing breast cancer. Proliferative breast lesions devoid of atypia are just marginally more likely to progress to breast cancer [27]. When atypical hyperplasia is discovered by mammography screening, it suggests a considerable risk for the occurrence of breast cancer. Women with atypia are 4.3 times more likely to be diagnosed with breast cancer than the general population [27,28].
- c.
- Family History: There is a correlation between breast cancer in a woman’s family and her own risk of developing the disease. The possibility is highest if the first-degree relatives of the women have been recognized with breast cancer at an early age. Family history is useful for identifying individuals who may be carriers of a genetic mutation predisposing to breast cancer, such as breast cancer gene-1 or gene-2 [29].
- d.
- Early Menarche: Menarche is the first occurrence of menstruation. Early-age menarche increases the chances of developing breast cancer in both pre- and post-menopausal women compared to women with delayed menarche [30].
- e.
- Bone Density: Bone contains estrogen receptors, so increasing bone density may function as an alternative indicator for circulating estrogen and is linked with a higher risk of breast cancer [31]. According to a meta-analysis, women with a high hip bone density were more likely to be diagnosed with breast cancer [32].
- f.
- Breast Density: Breast density measures the proportion of fibro glandular tissue. If the breasts have a fibro glandular tissue content higher than 75%, then they are considered mammographically dense. Women with dense breasts are more likely to be diagnosed with breast cancer. The chances are even higher if a woman is under the age of 56 [33,34,35].
- g.
- Lifestyle: An increase in weight during the perimenopausal period is associated with an increased risk of breast cancer [36,37,38]. Physical activity demonstrates a protective impact by lowering hormone levels [34]. Similarly, increased alcohol use and smoking habits are linked with an increased breast cancer risk [39,40,41].
- h.
- Breastfeeding: Women who breastfeed have a lower possibility of developing breast cancer. Lactation slows normal ovulatory cycles and reduces endogenous sex hormone levels.
- i.
- Age of Menopause: Delayed menopause is related with an increased risk of being diagnosed with breast cancer.
2.2. Stages of Breast Cancer
- a.
- Stage 0: In Situ
- b.
- Stage I and Stage II: Early-Stage Invasive
- c.
- Stage III: Locally Advanced
- d.
- Stage IV: Metastatic
- (A).
- T: It refers to the size of the tumor. Tumors can be measured using imaging techniques.
- Tx: No evaluation of a tumor.
- T0: No evidence of a tumor.
- Tis: Cancerous cells were detected prior to tumor development.
- T1: Tumor is less than 2 cm.
- T2: Tumor is between 2 and 5 cm.
- T3: Tumor is bigger than 5 cm.
- T4: Tumor with any size that grows into the chest wall or the skin.
- (B).
- N: It is the proliferation of the tumor to the nearby lymph nodes. Cancer found in the lymph node can be minute, called micrometastasis (0.2 mm to 2 mm), or big, called macrometastasis (bigger than 2 mm).
- Nx: No evaluation of lymph nodes.
- N0: No indication of any spread.
- N1: Cancer has spread in smaller amounts to nodes near the breastbone or underarm lymph nodes.
- N2: Cancer has spread in larger quantities to the underarm lymph nodes than in N1.
- N3: Cancer has spread significantly to the underarm lymph nodes.
- (C).
- M: It is the spread of the tumor to several distant parts of the body.
- Mx: No evaluation of metastasis.
- M0: No evidence of any spread.
- M1: Spread of cancer to distant organs or tissues.
- ER: Breast cancers have a receptor that responds to the estrogen hormone.
- PR: Breast cancers have a receptor that responds to the progesterone hormone.
- HER2: Breast cancer makes an excess amount of the protein HER2.
- G: It is the grade of cancer to differentiate cancerous cells from normal cells.
- ⮚
- Grade 1: Cells appear uniform.
- ⮚
- Grade 2: The rate of cell division increases.
- ⮚
- Grade 3: Cells vary in appearance from normal breast tissue.
3. Treatment Options
3.1. Conventional Approaches for Breast Cancer Treatment
- a.
- Surgical Management
- b.
- Adjuvant Radiotherapy
- c.
- Hormonal Therapy
- d.
- Chemotherapy
- i.
- Adjuvant Chemotherapy (After Surgery)
- Cancer with a triple-negative phenotype;
- Cancer with ER expression (chemotherapy given with hormonal therapy);
- Cancer with HER2 overexpression (anthracycline chemotherapy preferred with monoclonal antibodies).
- ii.
- Neoadjuvant Chemotherapy (Before Surgery)
- Extra time for genetic testing;
- Increased longevity of a patient with early-stage cancer, especially with TNBC or HER2-positive breast cancer.
- iii.
- For Metastatic Breast Cancer
3.2. Challenges of Using Conventional Therapy and Their Remedies
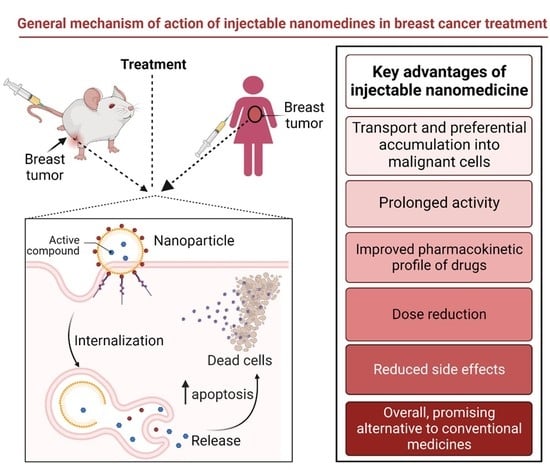
4. Injectable Nano-Drug Delivery Systems
5. Types of Nano-Drug Delivery Systems
5.1. Organic Nanoparticles (NPs)
5.1.1. Micelles
5.1.2. Liposomes
5.1.3. Dendrimers
5.1.4. Polymeric NPs
5.1.5. Drug Nanocrystals
5.1.6. Exosomes
5.2. Inorganic NPs
5.2.1. Gold NPs
5.2.2. Magnetic NPs
5.2.3. Carbon-Based NPs
5.2.4. Quantum Dots
5.2.5. Silica NPs
5.2.6. Ceramic NPs
5.2.7. Upconversion NPs
5.3. Other NPs
5.3.1. Reactive Oxygen Species (ROS)- Responsive NPs
5.3.2. Enzyme-Responsive NPs
5.3.3. Hypoxia-Activated NPs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Dox | Doxorubicin |
| EPR | Enhanced permeation and retention |
| ER | Estrogen receptor |
| FA | Folic acid |
| FDA | Food and Drug Administration |
| HER2 | Human epidermal growth receptor 2 |
| IV | Intravenous |
| miRNA | Micro ribonucleic acid |
| NPs | Nanoparticles |
| PDT | Photodynamic treatment |
| PAMAM | Polyamidoamine |
| PEG | Polyethylene glycol |
| PLA | Polylactic acid |
| PR | Progesterone receptor |
| PS | Photosensitizer |
| QDs | Quantum dots |
| ROS | Reactive oxygen species |
| siRNA | Small interfering ribonucleic acid |
| TNBC | Triple-negative breast cancer |
References
- World Health Organization. Retrieved from World Health Organization Website. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 3 February 2022).
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 2 November 2022).
- Eccles, S.A.; Aboagye, E.O.; Ali, S.; Anderson, A.S.; Armes, J.; Berditchevski, F.; Blaydes, J.P.; Brennan, K.; Brown, N.J.; Bryant, H.E.; et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. BCR 2013, 15, R92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majeed, W.; Aslam, B.; Javed, I.; Khaliq, T.; Muhammad, F.; Ali, A.; Raza, A. Breast cancer: Major risk factors and recent developments in treatment. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 3353–3358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer of the Breast (Female)—Cancer Stat Facts. SEER. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 2 June 2022).
- Greaney, M.L.; Sprunck-Harrild, K.; Ruddy, K.J.; Ligibel, J.; Barry, W.T.; Baker, E.; Meyer, M.; Emmons, K.M.; Partridge, A.H. Study protocol for Young & Strong: A cluster randomized design to increase attention to unique issues faced by young women with newly diagnosed breast cancer. BMC Public Health 2015, 15, 37. [Google Scholar] [CrossRef] [Green Version]
- Villarreal-Garza, C.; Aguila, C.; Magallanes-Hoyos, M.C.; Mohar, A.; Bargalló, E.; Meneses, A.; Cazap, E.; Gomez, H.; López-Carrillo, L.; Chávarri-Guerra, Y.; et al. Breast cancer in young women in Latin America: An unmet, growing burden. Oncologist 2013, 18, 1298–1306. [Google Scholar] [CrossRef] [Green Version]
- Fredholm, H.; Eaker, S.; Frisell, J.; Holmberg, L.; Fredriksson, I.; Lindman, H. Breast cancer in young women: Poor survival despite intensive treatment. PLoS ONE 2009, 4, e7695. [Google Scholar] [CrossRef] [Green Version]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Jain, R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. 2009, 48, 60–103. [Google Scholar] [CrossRef] [Green Version]
- Peer, D.; Karp, J.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotech 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2013, 4, 81–89. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46 Pt 1, 6387–6392. [Google Scholar] [PubMed]
- Huang, K.W.; Hsu, F.F.; Qiu, J.T.; Chern, G.J.; Lee, Y.A.; Chang, C.C.; Huang, Y.T.; Sung, Y.C.; Chiang, C.C.; Huang, R.L.; et al. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci. Adv. 2020, 6, eaax5032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagarajan, D.; McArdle, S. Immune Landscape of Breast Cancers. Biomedicines 2018, 6, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef]
- Agrawal, S.; Dwivedi, M.; Ahmad, H.; Chadchan, S.B.; Arya, A.; Sikandar, R.; Kaushik, S.; Mitra, K.; Jha, R.K.; Dwivedi, A.K. CD44 targeting hyaluronic acid coated lapatinib nanocrystals foster the efficacy against triple-negative breast cancer. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 327–337. [Google Scholar] [CrossRef]
- Most Common Molecular Subtypes of Breast Cancer; Cancer Treatment Centers of America: Boca Raton, FL, USA, 2018.
- Drugs Approved for Breast Cancer. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/breast (accessed on 29 November 2022).
- Chen, X.; Xu, D.; Li, X.; Zhang, J.; Xu, W.; Hou, J.; Zhang, W.; Tang, J. Latest Overview of the Cyclin-Dependent Kinases 4/6 Inhibitors in Breast Cancer: The Past, the Present and the Future. J. Cancer 2019, 10, 6608–6617. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Barrios, C.H. Optimal management of hormone receptor positive metastatic breast cancer in 2016. Ther. Adv. Med. Oncol. 2015, 7, 304–320. [Google Scholar] [CrossRef] [Green Version]
- Wuerstlein, R.; Harbeck, N. Neoadjuvant Therapy for HER2-positive Breast Cancer. Rev. Recent Clin. Trials 2017, 12, 81–92. [Google Scholar] [CrossRef]
- Berrada, N.; Delaloge, S.; André, F. Treatment of triple-negative metastatic breast cancer: Toward individualized targeted treatments or chemosensitization? Ann. Oncol. 2010, 21 (Suppl. 7), vii30–vii35. [Google Scholar] [CrossRef]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, L.C.; Sellers, T.A.; Frost, M.H.; Lingle, W.L.; Degnim, A.C.; Ghosh, K.; Vierkant, R.A.; Maloney, S.D.; Pankratz, V.S.; Hillman, D.W.; et al. Benign breast disease and the risk of breast cancer. N. Engl. J. Med. 2005, 353, 229–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, W.D.; Parl, F.F.; Hartmann, W.H.; Brinton, L.A.; Winfield, A.C.; Worrell, J.A.; Schuyler, P.A.; Plummer, W.D. Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer 1993, 71, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: Collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet 2001, 358, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118,964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012, 13, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kiel, D.P.; Kreger, B.E.; Cupples, L.A.; Ellison, R.C.; Dorgan, J.F.; Schatzkin, A.; Levy, D.; Felson, D.T. Bone mass and the risk of breast cancer among postmenopausal women. N. Engl. J. Med. 1997, 336, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zhang, X.; Qin, A.; Liu, G.; Zhai, Z.; Hao, Y.; Li, H.; Zhu, Z.; Dai, K. Bone mineral density and risk of breast cancer in postmenopausal women. Breast Cancer Res. Treat. 2013, 138, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, J.T.; Kerlikowske, K.; Loh, A.; Cummings, S.R. Personalizing mammography by breast density and other risk factors for breast cancer: Analysis of health benefits and cost-effectiveness. Ann. Intern. Med. 2011, 155, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Nelson, H.D.; Zakher, B.; Cantor, A.; Fu, R.; Griffin, J.; O’Meara, E.S.; Buist, D.S.; Kerlikowske, K.; van Ravesteyn, N.T.; Trentham-Dietz, A.; et al. Risk factors for breast cancer for women aged 40 to 49 years: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 156, 635–648. [Google Scholar] [CrossRef]
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Hislop, G.; Chiarelli, A.; Minkin, S.; et al. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef]
- Lahmann, P.H.; Hoffmann, K.; Allen, N.; van Gils, C.H.; Khaw, K.T.; Tehard, B.; Berrino, F.; Tjønneland, A.; Bigaard, J.; Olsen, A.; et al. Body size and breast cancer risk: Findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Int. J. Cancer 2004, 111, 762–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliassen, A.H.; Colditz, G.A.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Adult weight change and risk of postmenopausal breast cancer. JAMA 2006, 296, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tretli, S. Height and weight in relation to breast cancer morbidity and mortality. A prospective study of 570,000 women in Norway. Int. J. Cancer 1989, 44, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ellison, R.C.; Zhang, Y.; McLennan, C.E.; Rothman, K.J. Exploring the relation of alcohol consumption to risk of breast cancer. Am. J. Epidemiol. 2001, 154, 740–747. [Google Scholar] [CrossRef] [Green Version]
- Gram, I.T.; Park, S.Y.; Kolonel, L.N.; Maskarinec, G.; Wilkens, L.R.; Henderson, B.E.; Le Marchand, L. Smoking and Risk of Breast Cancer in a Racially/Ethnically Diverse Population of Mainly Women Who Do Not Drink Alcohol: The MEC Study. Am. J. Epidemiol. 2015, 182, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Gaudet, M.M.; Gapstur, S.M.; Sun, J.; Diver, W.R.; Hannan, L.M.; Thun, M.J. Active smoking and breast cancer risk: Original cohort data and meta-analysis. J. Natl. Cancer Inst. 2013, 105, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Chuba, P.J.; Hamre, M.R.; Yap, J.; Severson, R.K.; Lucas, D.; Shamsa, F.; Aref, A. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: Analysis of surveillance, epidemiology, and end results data. J. Clin. Oncol. 2005, 23, 5534–5541. [Google Scholar] [CrossRef]
- Markman, M.; Cancer Treatment Center of America. Retrieved from Cancer Treatment Center of America Website. Available online: https://www.cancercenter.com/cancer-types/breast-cancer/stages (accessed on 21 September 2021).
- Radecka, B.; Litwiniuk, M. Breast cancer in young women. Ginekol. Pol. 2016, 87, 659–663. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, C.A.; Domchek, S.M. Breast cancer in young women. Breast Cancer Res. 2010, 12, 212. [Google Scholar] [CrossRef] [Green Version]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Members, P. Tailoring therapies—Improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.H.; Pagani, O.; Abulkhair, O.; Aebi, S.; Amant, F.; Azim, H.A., Jr.; Costa, A.; Delaloge, S.; Freilich, G.; Gentilini, O.D.; et al. First international consensus guidelines for breast cancer in young women (BCY1). Breast 2014, 23, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Medeiros Alencar, V.H.; Badran, A.; Bonfill, X.; et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013, 381, 805–816. [Google Scholar] [CrossRef] [Green Version]
- Chemotherapy for Breast Cancer|Breast Cancer Treatment. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/chemotherapy-for-breast-cancer.html (accessed on 20 August 2022).
- Taxol (Paclitaxel) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/taxol-paclitaxel-342187 (accessed on 27 November 2022).
- (Doxorubicin) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/doxorubicin-342120 (accessed on 27 November 2022).
- Ellence (Epirubicin) dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/epirubicin-342251 (accessed on 27 November 2022).
- Taxotere (Docetaxel) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/taxotere-docetaxel-342192 (accessed on 27 November 2022).
- Adrucil (Fluorouracil) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/adrucil-fluorouracil-342092 (accessed on 27 November 2022).
- Cytoxan (Cyclophosphamide) Dosing, Indications, Interactions, Adverse effects, and More. Available online: https://reference.medscape.com/drug/cytoxan-cyclophosphamide-342214 (accessed on 27 November 2022).
- Abraxane (Paclitaxel Protein Bound) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/abraxane-paclitaxel-protein-bound-999775 (accessed on 27 November 2022).
- Halaven (Eribulin) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/Halaven-eribulin-999614 (accessed on 27 November 2022).
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef]
- Su, S.; M Kang, P. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef] [PubMed]
- Núñez, C.; Capelo, J.L.; Igrejas, G.; Alfonso, A.; Botana, L.M.; Lodeiro, C. An overview of the effective combination therapies for the treatment of breast cancer. Biomaterials 2016, 97, 34–50. [Google Scholar] [CrossRef]
- López-Dávila, V.; Seifalian, A.M.; Loizidou, M. Organic nanocarriers for cancer drug delivery. Curr. Opin. Pharmacol. 2012, 12, 414–419. [Google Scholar] [CrossRef]
- Moreno-Aspitia, A.; Perez, E.A. Treatment options for breast cancer resistant to anthracycline and taxane. Mayo Clin. Proc. 2009, 84, 533–545. [Google Scholar] [CrossRef] [Green Version]
- Hatefi, A.; Amsden, B. Biodegradable injectable in situ forming drug delivery systems. J. Control. Release 2002, 80, 9–28. [Google Scholar] [CrossRef]
- Verma, P.; Thakur, A.S.; Deshmukh, K.; Jha, A.K.; Verma, S. Routes of drug administration. Int. J. Pharm. Stud. Res. 2010, 1, 54–59. [Google Scholar]
- Lehne’s Pharmacology for Nursing Care Elsevier e-Book on VitalSource, 10th Edition—9780323550079. Available online: https://evolve.elsevier.com/cs/product/9780323550079?role=student (accessed on 9 November 2022).
- Biswas, S.; Torchilin, V.P. Nanopreparations for organelle-specific delivery in cancer. Adv. Drug Deliv. Rev. 2014, 66, 26–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos EV, R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Current Nanotechnology Treatments—NCI (NCI Global, NCI Enterprise). [Cgv Article]. Available online: https://www.cancer.gov/nano/cancer-nanotechnology/current-treatments (accessed on 28 November 2022).
- German Breast Group. A Randomized Phase III Trial Comparing Nanoparticle-based Paclitaxel with Solvent-based Paclitaxel as Part of Neoadjuvant Chemotherapy for Patients with Early Breast Cancer (GeparSepto) (Clinical Trial Registration No. NCT01583426). clinicaltrials.gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT01583426 (accessed on 13 November 2022).
- Suelmann, B.B.M. Image-guided Targeted Doxorubicin Delivery with Hyperthermia to Optimize Loco-regional Control in Breast Cancer. Phase I Feasibility Study of Hifu-Induced Hyperthermia, LTLD and Cyclophosphamide for Metastatic Breast Cancer (Clinical Trial Registration No. NCT03749850). clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03749850 (accessed on 11 November 2022).
- Celgene Corporation. A Controlled, Randomized, Phase III, Multicenter, Open Label Study of ABI-007(a Cremophor Free, Protein Stabilized, Nanoparticle Paclitaxel) and Taxol in Patients with Metastatic Breast Cancer (Clinical Trial Registration No. NCT00046527). clinicaltrials.gov. 2006. Available online: https://clinicaltrials.gov/ct2/show/NCT00046527 (accessed on 11 November 2022).
- City of Hope Medical Center. Phase II Prospective Open Label Study of Pertuzumab, Trastuzumab, and Nab-Paclitaxel in Patients with HER-2 Positive Advanced Breast Cancer (Clinical Trial Registration No. NCT01730833). clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT01730833 (accessed on 11 November 2022).
- Fresenius Kabi Oncology Ltd. A Multicentre Phase I Study of Cremophor FreePaclitaxel Nanoparticle in Advanced Breast Cancer (Clinical Trial Registration No. NCT00915369). clinicaltrials.gov. 2010. Available online: https://clinicaltrials.gov/ct2/show/NCT00915369 (accessed on 11 November 2022).
- Mohajer, G.; Lee, E.S.; Bae, Y.H. Enhanced intercellular retention activity of novel pH-sensitive polymeric micelles in wild and multidrug resistant MCF-7 cells. Pharm. Res. 2007, 24, 1618–1627. [Google Scholar] [CrossRef]
- Yuan, Y.; Cai, T.; Xia, X.; Zhang, R.; Chiba, P.; Cai, Y. Nanoparticle delivery of anticancer drugs overcomes multidrug resistance in breast cancer. Drug Deliv. 2016, 23, 3350–3357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Zhu, H.; Ren, Y.; Li, K.; Tian, B.; Han, J.; Feng, D. Preparation of protein-loaded PEG-PLA micelles and the effects of ultrasonication on particle size. Colloid Polym. Sci. 2017, 295, 259–266. [Google Scholar] [CrossRef]
- Lee, K.S.; Chung, H.C.; Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.B.; Rha, S.Y.; Lee, M.Y.; Ro, J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008, 108, 241–250. [Google Scholar] [CrossRef]
- Davaran, S.; Fazeli, H.; Ghamkhari, A.; Rahimi, F.; Molavi, O.; Anzabi, M.; Salehi, R. Synthesis and characterization of novel P(HEMA-LA-MADQUAT) micelles for co-delivery of methotrexate and Chrysin in combination cancer chemotherapy. J. Biomater. Sci. Polym. Ed. 2018, 29, 1265–1286. [Google Scholar] [CrossRef]
- Tagami, T.; Ando, Y.; Ozeki, T. Fabrication of liposomal doxorubicin exhibiting ultrasensitivity against phospholipase A2 for efficient pulmonary drug delivery to lung cancers. Int. J. Pharm. 2017, 517, 35–41. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, S.; Lillard, J.W., Jr.; Singh, R. Drug delivery approaches for breast cancer. Int. J. Nanomed. 2017, 12, 6205–6218. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, J.; Xu, J.; Chen, K.; Zhang, Z.; Duan, J.; Luo, Q.; Du, Y.; Chen, S.; Xie, Y.; et al. Nanostructure of Functional Larotaxel Liposomes Decorated with Guanine-Rich Quadruplex Nucleotide-Lipid Derivative for Treatment of Resistant Breast Cancer. Small 2021, 17, e2007391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Luo, M.; Wei, N.; Chang, A.; Luo, K.Q. Development of a Liposomal Formulation of Acetyltanshinone IIA for Breast Cancer Therapy. Mol. Pharm. 2019, 16, 3873–3886. [Google Scholar] [CrossRef] [PubMed]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.S.; Tekade, R.K.; Chougule, M.B. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: Current progress and advances. J. Control. Release 2014, 194, 238–256. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.-L.; Kang, X.-X.; Wang, Y.-Q.; Zhang, X.-J.; Li, C.-J.; Liu, Y.; Du, L.-B. Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater. 2019, 84, 367–377. [Google Scholar] [CrossRef]
- Jain, A.; Mahira, S.; Majoral, J.-P.; Bryszewska, M.; Khan, W.; Ionov, M. Dendrimer mediated targeting of siRNA against polo-like kinase for the treatment of triple negative breast cancer. J. Biomed. Mater. Res. Part A 2019, 107, 1933–1944. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Labhasetwar, V. Nanotech approaches to drug delivery and imaging. Drug Discov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef]
- Lee, J.H.; Nan, A. Combination Drug Delivery Approaches in Metastatic Breast Cancer. J. Drug Deliv. 2012, 2012, e915375. [Google Scholar] [CrossRef]
- Dashtimoghadam, E.; Mirzadeh, H.; Taromi, F.A.; Nyström, B. Microfluidic self-assembly of polymeric nanoparticles with tunable compactness for controlled drug delivery. Polymer 2013, 54, 4972–4979. [Google Scholar] [CrossRef]
- Jadia, R.; Kydd, J.; Rai, P. Remotely Phototriggered, Transferrin-Targeted Polymeric Nanoparticles for the Treatment of Breast Cancer. Photochem. Photobiol. 2018, 94, 765–774. [Google Scholar] [CrossRef]
- Nabi, P.N.; Vahidfar, N.; Tohidkia, M.R.; Hamidi, A.A.; Omidi, Y.; Aghanejad, A. Mucin-1 conjugated polyamidoamine-based nanoparticles for image-guided delivery of gefitinib to breast cancer. Int. J. Biol. Macromol. 2021, 174, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Ying, K.; Zhu, Y.; Wan, J.; Zhan, C.; Wang, Y.; Xie, B.; Xu, P.; Pan, H.; Wang, H. Macrophage membrane-biomimetic adhesive polycaprolactone nanocamptothecin for improving cancer-targeting efficiency and impairing metastasis. Bioact. Mater. 2022, 20, 449–462. [Google Scholar] [CrossRef]
- Agrawal, S.; Ahmad, H.; Dwivedi, M.; Shukla, M.; Arya, A.; Sharma, K.; Lal, J.; Dwivedi, A.K. PEGylated chitosan nanoparticles potentiate repurposing of ormeloxifene in breast cancer therapy. Nanomedicine 2016, 11, 2147–2169. [Google Scholar] [CrossRef] [PubMed]
- Möschwitzer, J.P. Drug nanocrystals in the commercial pharmaceutical development process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef]
- Miao, X.; Yang, W.; Feng, T.; Lin, J.; Huang, P. Drug nanocrystals for cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1499. [Google Scholar] [CrossRef] [PubMed]
- Rasenack, N.; Müller, B.W. Micron-size drug particles: Common and novel micronization techniques. Pharm. Dev. Technol. 2004, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, H.; Zhang, H.; Dai, W.; Wang, X.; Wang, X.; Zhang, Q. Effects of PEGylated paclitaxel nanocrystals on breast cancer and its lung metastasis. Nanoscale 2015, 7, 10790–10800. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, J. Potential of cancer cell-derived exosomes in clinical application: A review of recent research advances. Clin. Ther. 2014, 36, 863–872. [Google Scholar] [CrossRef]
- Exosome: Function and Role in Cancer Metastasis and Drug Resistance—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5949932/ (accessed on 28 November 2022).
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef]
- Vinik, Y.; Ortega, F.G.; Mills, G.B.; Lu, Y.; Jurkowicz, M.; Halperin, S.; Aharoni, M.; Gutman, M.; Lev, S. Proteomic analysis of circulating extracellular vesicles identifies potential markers of breast cancer progression, recurrence, and response. Sci. Adv. 2020, 6, eaba5714. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, C.; Zhou, T.; Liu, X.; Liu, X.; Li, X.; Chen, D. Role of exosomal proteins in cancer diagnosis. Mol. Cancer 2017, 16, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wee, I.; Syn, N.; Sethi, G.; Goh, B.C.; Wang, L. Role of tumor-derived exosomes in cancer metastasis. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Hench, I.B.; Hench, J.; Tolnay, M. Liquid Biopsy in Clinical Management of Breast, Lung, and Colorectal Cancer. Front. Med. 2018, 5, 9. [Google Scholar] [CrossRef]
- Kourembanas, S. Exosomes: Vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu. Rev. Physiol. 2015, 77, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Wu, Y.; Shen, H.; Lv, M.; Chen, W.; Zhang, X.; Zhong, S.; Tang, J.; Zhao, J. Exosomes in development, metastasis and drug resistance of breast cancer. Cancer Sci. 2015, 106, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Babu, A.; Filant, J.; Moxley, K.M.; Ruskin, R.; Dhanasekaran, D.; Sood, A.K.; McMeekin, S.; Ramesh, R. Exploitation of Exosomes as Nanocarriers for Gene-, Chemo-, and Immune-Therapy of Cancer. J. Biomed. Nanotechnol. 2016, 12, 1159–1173. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Zhao, M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front. Pharmacol. 2017, 7, 533. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control. Release 2020, 318, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Juarranz, A.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res. 1984, 44 (Suppl. 10), 4721s–4730s. [Google Scholar]
- Balivada, S.; Rachakatla, R.S.; Wang, H.; Samarakoon, T.N.; Dani, R.K.; Pyle, M.; Kroh, F.O.; Walker, B.; Leaym, X.; Koper, O.B.; et al. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: A mouse study. BMC Cancer 2010, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Li, C.; Cheng, J.; Yuan, Z. Recent Advances on Inorganic Nanoparticle-Based Cancer Therapeutic Agents. Int. J. Environ. Res. Public Health 2016, 13, 1182. [Google Scholar] [CrossRef] [Green Version]
- Vargas, A.P.; Gámez, F.; Roales, J.; Lopes-Costa, T.; Pedrosa, J.M. A Paper-Based Ultrasensitive Optical Sensor for the Selective Detection of H2S Vapors. Chemosensors 2021, 9, 40. [Google Scholar] [CrossRef]
- Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions|Nature Physical Science. Available online: https://www.nature.com/articles/physci241020a0 (accessed on 23 October 2022).
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Cai, W.; Gao, T.; Hong, H.; Sun, J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol. Sci. Appl. 2008, 1, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Pitsillides, C.M.; Joe, E.K.; Wei, X.; Anderson, R.R.; Lin, C.P. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys. J. 2003, 84, 4023–4032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, E.S.; Morton, J.G.; West, J.L. Nanoparticles for Thermal Cancer Therapy. J. Biomech. Eng. 2009, 131. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Colombeau, L.; Acherar, S.; Baros, F.; Arnoux, P.; Gazzali, A.M.; Zaghdoudi, K.; Toussaint, M.; Vanderesse, R.; Frochot, C. Inorganic Nanoparticles for Photodynamic Therapy. Top. Curr. Chem. 2016, 370, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Penon, O.; Barrios, L.; Amabilino, D.B.; Wurst, K. A New Porphyrin for the Preparation of Functionalized Water-Soluble Gold Nanoparticles with Low Intrinsic Toxicity. ChemistryOpen 2014, 4, 127–136. [Google Scholar] [CrossRef]
- Remote-Controlled Release of Singlet Oxygen by the Plasmonic Heating of Endoperoxide-Modified Gold Nanorods: Towards a Paradigm Change in Photodynamic Therapy—Kolemen—2016—Angewandte Chemie International Edition—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/anie.201510064 (accessed on 23 October 2022).
- Gold Nanoparticles with a Monolayer of Doxorubicin-Conjugated Amphiphilic Block Copolymer for Tumor-Targeted Drug Delivery. Available online: https://read.qxmd.com/read/19674777/gold-nanoparticles-with-a-monolayer-of-doxorubicin-conjugated-amphiphilic-block-copolymer-for-tumor-targeted-drug-delivery (accessed on 23 October 2022).
- Naser Mohammed, S.; Mishaal Mohammed, A.; Al-Rawi, K.F. Novel combination of multi-walled carbon nanotubes and gold nanocomposite for photothermal therapy in human breast cancer model. Steroids 2022, 186, 109091. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Grancharov, S.G.; Zeng, H.; Sun, S.; Wang, S.X.; O’Brien, S.; Murray, C.B.; Kirtley, J.R.; Held, G.A. Bio-Functionalization of Monodisperse Magnetic Nanoparticles and Their Use as Biomolecular Labels in a Magnetic Tunnel Junction Based Sensor. J. Phys. Chem. B 2005, 109, 13030–13035. [Google Scholar] [CrossRef] [Green Version]
- Piao, Y.; Kim, J.; Na, H.B.; Kim, D.; Baek, J.S.; Ko, M.K.; Lee, J.H.; Shokouhimehr, M.; Hyeon, T. Wrap-bake-peel process for nanostructural transformation from beta-FeOOH nanorods to biocompatible iron oxide nanocapsules. Nat. Mater. 2008, 7, 242–247. [Google Scholar] [CrossRef]
- Liu, H.-L.; Hua, M.-Y.; Yang, H.-W.; Huang, C.-Y.; Chu, P.-C.; Wu, J.-S.; Tseng, I.-C.; Wang, J.-J.; Yen, T.-C.; Chen, P.-Y.; et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc. Natl. Acad. Sci. USA 2010, 107, 15205–15210. [Google Scholar] [CrossRef] [Green Version]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy|Chemical Reviews. Available online: https://pubs.acs.org/doi/10.1021/acs.chemrev.5b00008 (accessed on 23 October 2022).
- Huang, P.; Wang, S.; Wang, X.; Shen, G.; Lin, J.; Wang, Z.; Guo, S.; Cui, D.; Yang, M.; Chen, X. Surface Functionalization of Chemically Reduced Graphene Oxide for Targeted Photodynamic Therapy. J. Biomed. Nanotechnol. 2015, 11, 117–125. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, L.; Zhang, X.; Li, N.; Zheng, J.; Guan, M.; Fang, X.; Wang, C.; Shu, C. Enhanced photodynamic efficiency of an aptamer-guided fullerene photosensitizer toward tumor cells. Chem. Asian J. 2013, 8, 2370–2376. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosinger, J.; Lang, K.; Kubát, P. Photoactivatable Nanostructured Surfaces for Biomedical Applications. Top. Curr. Chem. 2016, 370, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, L.; Gao, J.; Liu, Y.; Zhang, J.; Ma, R.; Liu, R.; Zhang, Z. A fullerene-based multi-functional nanoplatform for cancer theranostic applications. Biomaterials 2014, 35, 5771–5784. [Google Scholar] [CrossRef]
- Active Oxygen Species Generated from Photoexcited Fullerene (C60) as Potential Medicines: O2-• versus 1O2|Journal of the American Chemical Society. Available online: https://pubs.acs.org/doi/10.1021/ja0355574 (accessed on 23 October 2022).
- Photodynamic and Photothermal Effects of Semiconducting and Metallic-Enriched Single-Walled Carbon Nanotubes|Journal of the American Chemical Society. Available online: https://pubs.acs.org/doi/10.1021/ja3079972 (accessed on 23 October 2022).
- Zakharian, T.Y.; Seryshev, A.; Sitharaman, B.; Gilbert, B.E.; Knight, V.; Wilson, L.J. A fullerene-paclitaxel chemotherapeutic: Synthesis, characterization, and study of biological activity in tissue culture. J. Am. Chem. Soc. 2005, 127, 12508–12509. [Google Scholar] [CrossRef]
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic therapy with fullerenes in vivo: Reality or a dream? Nanomedicine 2011, 6, 1813–1825. [Google Scholar] [CrossRef] [Green Version]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Kamila, S.; McEwan, C.; Costley, D.; Atchison, J.; Sheng, Y.; Hamilton, G.R.C.; Fowley, C.; Callan, J.F. Diagnostic and Therapeutic Applications of Quantum Dots in Nanomedicine. Top. Curr. Chem. 2016, 370, 203–224. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Kaur, G.; Khurana, R.K.; Kapoor, S.; Singh, B. Quantum Dots and their Potential Role in Cancer Theranostics. Crit. Rev. Ther. Drug Carr. Syst. 2015, 32, 461–502. [Google Scholar] [CrossRef] [PubMed]
- Samia, A.C.S.; Dayal, S.; Burda, C. Quantum dot-based energy transfer: Perspectives and potential for applications in photodynamic therapy. Photochem. Photobiol. 2006, 82, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Monroe, J.D.; Belekov, E.; Er, A.O.; Smith, M.E. Anticancer Photodynamic Therapy Properties of Sulfur-Doped Graphene Quantum Dot and Methylene Blue Preparations in MCF-7 Breast Cancer Cell Culture. Photochem. Photobiol. 2019, 95, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, N.; Salehi, Z.; Khodadadi, A.A.; Shokrgozar, M.A.; Saboury, A.A. Glucosamine-conjugated graphene quantum dots as versatile and pH-sensitive nanocarriers for enhanced delivery of curcumin targeting to breast cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111809. [Google Scholar] [CrossRef]
- Thomas, S.C.; Harshita; Mishra, P.K.; Talegaonkar, S. Ceramic Nanoparticles: Fabrication Methods and Applications in Drug Delivery. Curr. Pharm. Des. 2015, 21, 6165–6188. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Huang, X.-C.; Luo, Y.-L.; Chang, Y.-C.; Hsieh, Y.-Z.; Hsu, H.-Y. Non-metallic nanomaterials in cancer theranostics: A review of silica- and carbon-based drug delivery systems. Sci. Technol. Adv. Mater. 2013, 14, 044407. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Wang, J.; Jiang, X.; Li, X.; Hu, Z.; Ji, Y.; Wu, X.; Chen, C. Mesoporous Silica-Coated Gold Nanorods as a Light-Mediated Multifunctional Theranostic Platform for Cancer Treatment. Adv. Mater. 2022, 34, 2205637. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Kilchrist, K.V.; Li, J.; Duvall, C.L.; Oupický, D. Endosomolytic and Tumor-Penetrating Mesoporous Silica Nanoparticles for siRNA/miRNA Combination Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 4308. [Google Scholar] [CrossRef]
- Moreno-Vega, A.-I.; Gómez-Quintero, T.; Nuñez-Anita, R.-E.; Acosta-Torres, L.-S.; Castaño, V. Polymeric and Ceramic Nanoparticles in Biomedical Applications. J. Nanotechnol. 2012, 2012, e936041. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Barbanente, A.; Nadar, R.A.; Esposti, L.D.; Palazzo, B.; Iafisco, M.; Beucken, J.J.J.P. van den, Leeuwenburgh, S.C.G.; Margiotta, N. Platinum-loaded, selenium-doped hydroxyapatite nanoparticles selectively reduce proliferation of prostate and breast cancer cells co-cultured in the presence of stem cells. J. Mater. Chem. B 2020, 8, 2792–2804. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Banerjee, D.; Liu, Y.; Chen, X.; Liu, X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst 2010, 135, 1839–1854. [Google Scholar] [CrossRef] [PubMed]
- Mader, H.S.; Kele, P.; Saleh, S.M.; Wolfbeis, O.S. Upconverting luminescent nanoparticles for use in bioconjugation and bioimaging. Curr. Opin. Chem. Biol. 2010, 14, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-Y.; Liao, M.-L.; Hong, G.-C.; Chang, W.-W.; Chu, C.-C. Near-Infrared-Triggered Photodynamic Therapy toward Breast Cancer Cells Using Dendrimer-Functionalized Upconversion Nanoparticles. Nanomaterials 2017, 7, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amplifying the Red-Emission of Upconverting Nanoparticles for Biocompatible Clinically Used Prodrug-Induced Photodynamic Therapy|ACS Nano. Available online: https://pubs.acs.org/doi/10.1021/nn505051d (accessed on 25 October 2022).
- Zeng, L.; Pan, Y.; Zou, R.; Zhang, J.; Tian, Y.; Teng, Z.; Wang, S.; Ren, W.; Xiao, X.; Zhang, J.; et al. 808 nm-excited upconversion nanoprobes with low heating effect for targeted magnetic resonance imaging and high-efficacy photodynamic therapy in HER2-overexpressed breast cancer. Biomaterials 2016, 103, 116–127. [Google Scholar] [CrossRef]
- Liu, J.; Bu, W.; Pan, L.; Shi, J. NIR-triggered anticancer drug delivery by upconverting nanoparticles with integrated azobenzene-modified mesoporous silica. Angew. Chem. (Int. Ed. Engl.) 2013, 52, 4375–4379. [Google Scholar] [CrossRef]
- Tian, G.; Gu, Z.; Zhou, L.; Yin, W.; Liu, X.; Yan, L.; Jin, S.; Ren, W.; Xing, G.; Li, S.; et al. Mn2+ dopant-controlled synthesis of NaYF4:Yb/Er upconversion nanoparticles for in vivo imaging and drug delivery. Adv. Mater. 2012, 24, 1226–1231. [Google Scholar] [CrossRef]
- Tian, G.; Zheng, X.; Zhang, X.; Yin, W.; Yu, J.; Wang, D.; Zhang, Z.; Yang, X.; Gu, Z.; Zhao, Y. TPGS-stabilized NaYbF4:Er upconversion nanoparticles for dual-modal fluorescent/CT imaging and anticancer drug delivery to overcome multi-drug resistance. Biomaterials 2015, 40, 107–116. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Z. Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials 2011, 32, 1110–1120. [Google Scholar] [CrossRef]
- Zeng, L.; Luo, L.; Pan, Y.; Luo, S.; Lu, G.; Wu, A. In vivo targeted magnetic resonance imaging and visualized photodynamic therapy in deep-tissue cancers using folic acid-functionalized superparamagnetic-upconversion nanocomposites. Nanoscale 2015, 7, 8946–8954. [Google Scholar] [CrossRef]
- Tong, R.; Langer, R. Nanomedicines Targeting the Tumor Microenvironment. Cancer J. 2015, 21, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Chen, X.; Liu, Z. Natural Products and Nanotechnology against Coronavirus Disease 2019. Front. Chem. 2022, 10, 819969. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Zeng, W.; Wang, Z.; Liu, C.; Zeng, N.; Zhong, K.; Jiang, D.; Wu, Y. A Novel H2O2 Generator for Tumor Chemotherapy-Enhanced CO Gas Therapy. Front. Oncol. 2021, 11, 738567. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, Z.; Chen, M.; Du, L.; Liu, C.; Wang, S.; Zheng, Y.; Liu, W. Tumor-Derived Biomimetic Nanozyme with Immune Evasion Ability for Synergistically Enhanced Low Dose Radiotherapy. J Nanobiotechnol. 2021, 19, 457. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Ning, Z.; Chen, H.; Wu, Y. Nanomaterial Technology and Triple Negative Breast Cancer. Front. Oncol. 2022, 11, 828810. [Google Scholar]
- Zhao, T.; Wu, W.; Sui, L.; Huang, Q.; Nan, Y.; Liu, J.; Ai, K. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact. Mater. 2022, 7, 47–72. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, Q.; Chen, H.; Hou, K.; Zeng, N.; Wu, Y. Smart Nanoparticles for Breast Cancer Treatment Based on the Tumor Microenvironment. Front. Oncol. 2022, 12, 907684. [Google Scholar] [CrossRef]
- Choi, H.W.; Lim, J.H.; Kim, C.W.; Lee, E.; Kim, J.-M.; Chang, K.; Chung, B.G. Near-Infrared Light-Triggered Generation of Reactive Oxygen Species and Induction of Local Hyperthermia from Indocyanine Green Encapsulated Mesoporous Silica-Coated Graphene Oxide for Colorectal Cancer Therapy. Antioxidants 2022, 11, 174. [Google Scholar] [CrossRef]
- Liu, L.; Liu, F.; Liu, D.; Yuan, W.; Zhang, M.; Wei, P.; Yi, T. A Smart Theranostic Prodrug System Activated by Reactive Oxygen Species for Regional Chemotherapy of Metastatic Cancer. Angew. Chem. (Int. Ed. Engl.) 2022, 61, e202116807. [Google Scholar] [CrossRef]
- Raman, D.; Foo, C.H.J.; Clement, M.-V.; Pervaiz, S. Breast Cancer: A Molecular and Redox Snapshot. Antioxid. Redox Signal. 2016, 25, 337–370. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Wan, J.; Zhou, L.; Ma, W.; Yang, Y.; Luo, W.; Yu, Z.; Wang, H. Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Yin, W.; Zhang, X.; Zhu, S.; He, X.; Yu, J.; Xie, J.; Guo, Z.; Yan, L.; Liu, X.; et al. Intelligent MoS2 Nanotheranostic for Targeted and Enzyme-/pH-/NIR-Responsive Drug Delivery to Overcome Cancer Chemotherapy Resistance Guided by PET Imaging. ACS Appl. Mater. Interfaces 2018, 10, 4271–4284. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.; Singh, N.; Surnar, B.; Jayakannan, M. Enzyme and Thermal Dual Responsive Amphiphilic Polymer Core–Shell Nanoparticle for Doxorubicin Delivery to Cancer Cells. Biomacromolecules 2016, 17, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowiak, J.W.; Verduzco, D.; Schramm, K.J.; Gillies, R.J. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol. Pharm. 2011, 8, 2032–2038. [Google Scholar] [CrossRef]
- Mpekris, F.; Panagi, M.; Voutouri, C.; Martin, J.D.; Samuel, R.; Takahashi, S.; Gotohda, N.; Suzuki, T.; Papageorgis, P.; Demetriou, P.; et al. Normalizing the Microenvironment Overcomes Vessel Compression and Resistance to Nano-immunotherapy in Breast Cancer Lung Metastasis. Adv. Sci. 2021, 8, 2001917. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [Green Version]
- Nayak, R.; Meerovich, I.; Dash, A.K. Translational Multi-Disciplinary Approach for the Drug and Gene Delivery Systems for Cancer Treatment. AAPS PharmSciTech 2019, 20, 160. [Google Scholar] [CrossRef]


| Types of Breast Cancer | Receptor | Subtypes | Commonly Used Medications |
|---|---|---|---|
| Hormone positive | ER+ or PR+ | Luminal A and B | Tamoxifen, Exemestane, Anastrozole, Letrozole |
| HER2-positive | HER2+ | - | Trastuzumab, Lapatinib, Neratinib, Pertuzumab |
| Triple-negative | ER-, PR-, and HER2- | Basal-like 1, basal-like 2, immunomodulatory, mesenchymal, mesenchymal stem cell like, and luminal androgen receptor | Paclitaxel, Doxorubicin, 5-fluorouracil, Eribulin, Docetaxel |
| Chemotherapeutic Drugs | Dose | Reference |
|---|---|---|
| Paclitaxel | Node Positive: 175 mg/m2 IV over 3 h q3 weeks 4 times (with doxorubicin (Dox) regimen) Metastatic Disease (failure of initial chemotherapy or relapse within 6 months following adjuvant chemotherapy): 175 mg/m2 IV over 3 h q3 weeks | [51] |
| Dox | 60–75 mg/m2 IV q21 Days | [52] |
| Epirubicin | Day 1: Epirubicin 100 mg/m2 IV, 5-fluorouracil 500 mg/m2 IV, and cyclophosphamide 500 mg/m2 IV followed by repetition up to q21 days × 6 cycles | [53] |
| Docetaxel | Locally Advanced or Metastatic
Initial: 75 mg/m2
| [54] |
| Fluorouracil | 500 or 600 mg/m2 IV on days 1 and 8 q28 days for 6 cycles as a component of a cyclophosphamide-based multidrug regimen | [55] |
| Cyclophosphamide | 600 mg/m2 IV with other antineoplastics | [56] |
| Albumin-bound paclitaxel | 260 mg/m2 IV infused over 30 min q3 weeks | [57] |
| Eribulin | 1.4 mg/m2 IV infused over 2–5 min on days 1 and 8 of 21-day cycle | [58] |
| Year | Product | Nanoparticle (NP) Material | Drug | Indication | Company | Reference |
|---|---|---|---|---|---|---|
| 2007 (S. Korea) | Genexol-PM | PEG-PLA polymeric micelle | Paclitaxel | Breast cancer, lung cancer, ovarian cancer | Samyang/Biopharm | [70] |
| 2005 | Abraxane | NP-bound albumin | Paclitaxel | Breast cancer, pancreatic cancer, non-small cell lung cancer | Abraxis/Celgene | [70] |
| 2000 (EU) | Myocet | Liposome | Dox | Breast cancer | Cephalon | [70] |
| 1998 | Lipo-Dox | Liposome | Dox | Kaposi sarcoma, breast cancer, ovarian cancer | Taiwan Liposome | [70] |
| 1995 1999 2003 2007 (Europe, Canada) | Doxil | Liposome | Dox | Kaposi sarcoma, ovarian cancer, breast cancer, multiple myeloma | Johnson and Johnson | [70] |
| NCT Number | Titles Conditions | Interventions | Phases | References | |
|---|---|---|---|---|---|
| NCT01583426 | Nanoparticle-based paclitaxel vs, solvent-based paclitaxel as part of neoadjuvant chemotherapy for early breast cancer | Tubular breast cancer stage II and III HER2+ breast cancer Invasive ductal breast cancer | Drug: Nab-Paclitaxel Drug: Paclitaxel | Phase 3 | [71] |
| NCT03749850 | Image-guided targeted doxorubicin delivery with hyperthermia to optimize loco-regional control in breast cancer | Metastatic breast cancer Invasive ductal breast cancer Adenocarcinoma breast | Drug: Liposomal Doxorubicin Procedure: Magnetic-resonance-guided high-intensity-focused ultrasound Drug: Cyclophosphamide | Phase 1 | [72] |
| NCT00046527 | Study of ABI-007 and Taxol in patients with metastatic breast cancer | Breast neoplasms Metastases, neoplasm | Drug: ABI-007 | Phase 3 | [73] |
| NCT01730833 | Pertuzumab, Trastuzumab, and Paclitaxel albumin-stabilized NP formulation in treating patients with HER2+ advanced breast cancer | HER2+ breast cancer Breast adenocarcinoma | Biological: Pertuzumab Biological: Trastuzumab Drug: Paclitaxel albumin-stabilized NP formulation Other: Laboratory biomarker analysis | Phase 2 | [74] |
| NCT00915369 | A clinical trial to study the effects of an NP-based paclitaxel drug, which does not contain the solvent cremophor, in advanced breast cancer | Advanced breast cancer | Drug: Nanoxel (Paclitaxel NP formulation) | Phase 1 | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kafle, U.; Agrawal, S.; Dash, A.K. Injectable Nano Drug Delivery Systems for the Treatment of Breast Cancer. Pharmaceutics 2022, 14, 2783. https://doi.org/10.3390/pharmaceutics14122783
Kafle U, Agrawal S, Dash AK. Injectable Nano Drug Delivery Systems for the Treatment of Breast Cancer. Pharmaceutics. 2022; 14(12):2783. https://doi.org/10.3390/pharmaceutics14122783
Chicago/Turabian StyleKafle, Urmila, Satish Agrawal, and Alekha K. Dash. 2022. "Injectable Nano Drug Delivery Systems for the Treatment of Breast Cancer" Pharmaceutics 14, no. 12: 2783. https://doi.org/10.3390/pharmaceutics14122783