Light-Switchable Membrane Permeability in Giant Unilamellar Vesicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Synthesis of Photoswitchable Compound 1
2.2. Optical Spectroscopy
2.3. Preparation of Phospholipid Giant Unilamellar Vesicles
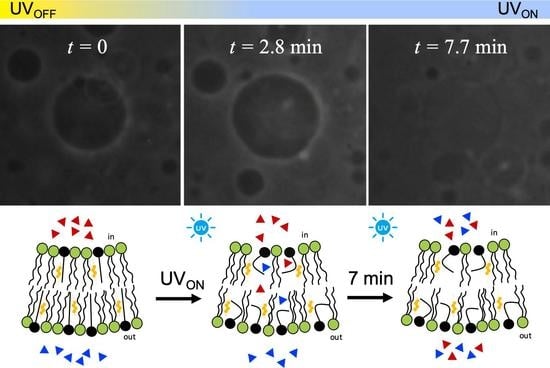
2.4. Observations under Microscopy of Light-Triggered GUV Shape Transitions
2.5. Cargo-Release Experiments
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ludlow, R.F.; Otto, S. Systems Chemistry. Chem. Soc. Rev. 2008, 37, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Stano, P.; Rampioni, G.; D’Angelo, F.; Altamura, E.; Mavelli, F.; Marangoni, R.; Rossi, F.; Damiano, L. Current Directions in Synthetic Cell Research. In Advances in Bionanomaterials; Lecture Notes in Bioengineering; Springer: Cham, Switzerland, 2018; pp. 141–154. ISBN 978-3-319-62026-8. [Google Scholar]
- Ashkenasy, G.; Hermans, T.M.; Otto, S.; Taylor, A.F. Systems Chemistry. Chem. Soc. Rev. 2017, 46, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Altamura, E.; Albanese, P.; Mavelli, F.; Stano, P. The Rise of the Nested Multicompartment Model in Synthetic Cell Research. Front. Mol. Biosci. 2021, 8, 750576. [Google Scholar] [CrossRef] [PubMed]
- Miele, Y.; Holló, G.; Lagzi, I.; Rossi, F. Shape Deformation, Budding and Division of Giant Vesicles and Artificial Cells: A Review. Life 2022, 12, 841. [Google Scholar] [CrossRef]
- Guindani, C.; da Silva, L.C.; Cao, S.; Ivanov, T.; Landfester, K. Synthetic Cells: From Simple Bio-Inspired Modules to Sophisticated Integrated Systems. Angew. Chem. 2022, 134, e202110855. [Google Scholar] [CrossRef]
- Meng, F.; Zhong, Z.; Feijen, J. Stimuli-Responsive Polymersomes for Programmed Drug Delivery. Biomacromolecules 2009, 10, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Giuseppone, N. Toward Self-Constructing Materials: A Systems Chemistry Approach. Acc. Chem. Res. 2012, 45, 2178–2188. [Google Scholar] [CrossRef]
- Urban, M.W. Stimuli-Responsive Materials: From Molecules to Nature Mimicking Materials Design; Royal Society of Chemistry: London, UK, 2019; ISBN 978-1-78801-809-8. [Google Scholar]
- Sato, W.; Zajkowski, T.; Moser, F.; Adamala, K.P. Synthetic Cells in Biomedical Applications. WIREs Nanomed. Nanobiotechnol. 2021, 14, e1761. [Google Scholar] [CrossRef]
- Imai, M.; Sakuma, Y.; Kurisu, M.; Walde, P. From Vesicles toward Protocells and Minimal Cells. Soft Matter 2022, 18, 4823–4849. [Google Scholar] [CrossRef]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future Perspectives and Recent Advances in Stimuli-Responsive Materials. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Lorenzo, R.A.; Carro, A.M.; Concheiro, A.; Alvarez-Lorenzo, C. Stimuli-Responsive Materials in Analytical Separation. Anal. Bioanal. Chem. 2015, 407, 4927–4948. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, M.; Li, X.; Xu, W.; Ahiabu, A.; Perdiz, J.; Liu, Z.; Serpe, M.J. Stimuli-Responsive Polymers: Fundamental Considerations and Applications. Macromol. Res. 2017, 25, 513–527. [Google Scholar] [CrossRef]
- Altamura, E.; Albanese, P.; Milano, F.; Giotta, L.; Trotta, M.; Ferretta, A.; Cocco, T.; Mavelli, F. Optimizing Enzymatic Photo-Redox Cycles by a Hybrid Protein Complex Chain. ChemPhotoChem 2021, 5, 26–31. [Google Scholar] [CrossRef]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-Responsive Polymers and Their Applications in Nanomedicine. Biointerphases 2012, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Kowal, J.; Wu, D.; Mikhalevich, V.; Palivan, C.G.; Meier, W. Hybrid Polymer–Lipid Films as Platforms for Directed Membrane Protein Insertion. Langmuir 2015, 31, 4868–4877. [Google Scholar] [CrossRef]
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.; Car, A.; Meier, W. Bioinspired Polymer Vesicles and Membranes for Biological and Medical Applications. Chem. Soc. Rev. 2016, 45, 377–411. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhou, W.; Qi, C.; Kong, T. Interface Engineering in Multiphase Systems toward Synthetic Cells and Organelles: From Soft Matter Fundamentals to Biomedical Applications. Adv. Mater. 2020, 32, 2002932. [Google Scholar] [CrossRef]
- Miele, Y.; Mingotaud, A.-F.; Caruso, E.; Malacarne, M.C.; Izzo, L.; Lonetti, B.; Rossi, F. Hybrid Giant Lipid Vesicles Incorporating a PMMA-Based Copolymer. Biochim. Biophys. Acta BBA-Gen. Subj. 2021, 1865, 129611. [Google Scholar] [CrossRef]
- Beharry, A.A.; Woolley, G.A. Azobenzene Photoswitches for Biomolecules. Chem. Soc. Rev. 2011, 40, 4422. [Google Scholar] [CrossRef]
- Szymański, W.; Beierle, J.M.; Kistemaker, H.A.V.; Velema, W.A.; Feringa, B.L. Reversible Photocontrol of Biological Systems by the Incorporation of Molecular Photoswitches. Chem. Rev. 2013, 113, 6114–6178. [Google Scholar] [CrossRef]
- Leippe, P.; Frank, J.A. Designing Azobenzene-Based Tools for Controlling Neurotransmission. Curr. Opin. Struct. Biol. 2019, 57, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Berizzi, A.E.; Goudet, C. Strategies and Considerations of G-Protein-Coupled Receptor Photopharmacology. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 88, pp. 143–172. ISBN 978-0-12-820187-9. [Google Scholar]
- Hüll, K.; Morstein, J.; Trauner, D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Liang, X. Modification of Nucleic Acids by Azobenzene Derivatives and Their Applications in Biotechnology and Nanotechnology. Chem.-Asian J. 2014, 9, 3344–3358. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; Sessa, L.; di Matteo, M.; Panunzi, B.; Piotto, S.; Concilio, S. Azobenzene as Antimicrobial Molecules. Molecules 2022, 27, 5643. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Guo, T.; Wang, Z.; Zhao, Y. Triggered Azobenzene-Based Prodrugs and Drug Delivery Systems. J. Control. Release 2022, 345, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Diguet, A.; Yanagisawa, M.; Liu, Y.-J.; Brun, E.; Abadie, S.; Rudiuk, S.; Baigl, D. UV-Induced Bursting of Cell-Sized Multicomponent Lipid Vesicles in a Photosensitive Surfactant Solution. J. Am. Chem. Soc. 2012, 134, 4898–4904. [Google Scholar] [CrossRef] [PubMed]
- Morstein, J.; Impastato, A.C.; Trauner, D. Photoswitchable Lipids. ChemBioChem 2021, 22, 73–83. [Google Scholar] [CrossRef]
- Liu, D.; Wang, S.; Xu, S.; Liu, H. Photocontrollable Intermittent Release of Doxorubicin Hydrochloride from Liposomes Embedded by Azobenzene-Contained Glycolipid. Langmuir 2017, 33, 1004–1012. [Google Scholar] [CrossRef]
- Pernpeintner, C.; Frank, J.A.; Urban, P.; Roeske, C.R.; Pritzl, S.D.; Trauner, D.; Lohmüller, T. Light-Controlled Membrane Mechanics and Shape Transitions of Photoswitchable Lipid Vesicles. Langmuir 2017, 33, 4083–4089. [Google Scholar] [CrossRef]
- Pritzl, S.D.; Urban, P.; Prasselsperger, A.; Konrad, D.B.; Frank, J.A.; Trauner, D.; Lohmüller, T. Photolipid Bilayer Permeability Is Controlled by Transient Pore Formation. Langmuir 2020, 36, 13509–13515. [Google Scholar] [CrossRef]
- Doroudgar, M.; Morstein, J.; Becker-Baldus, J.; Trauner, D.; Glaubitz, C. How Photoswitchable Lipids Affect the Order and Dynamics of Lipid Bilayers and Embedded Proteins. J. Am. Chem. Soc. 2021, 143, 25. [Google Scholar] [CrossRef] [PubMed]
- Pritzl, S.D.; Konrad, D.B.; Ober, M.F.; Richter, A.F.; Frank, J.A.; Nickel, B.; Trauner, D.; Lohmüller, T. Optical Membrane Control with Red Light Enabled by Red-Shifted Photolipids. Langmuir 2021, 3, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.Z.-J.; Muñana, P.S.; Dupont, J.; Zhou, S.S.; Chen, J.L.-Y. Reversible Formation of a Light-Responsive Catalyst by Utilizing Intermolecular Cooperative Effects. Angew. Chem. Int. Ed. 2019, 58, 15254–15258. [Google Scholar] [CrossRef] [PubMed]
- Zinser, E.; Sperka-Gottlieb, C.D.; Fasch, E.V.; Kohlwein, S.D.; Paltauf, F.; Daum, G. Phospholipid Synthesis and Lipid Composition of Subcellular Membranes in the Unicellular Eukaryote Saccharomyces Cerevisiae. J. Bacteriol. 1991, 173, 2026–2034. [Google Scholar] [CrossRef] [Green Version]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Vail, D.M.; MacEwen, E.G.; Kurzman, I.D.; Dubielzig, R.R.; Helfand, S.C.; Kisseberth, W.C.; London, C.A.; Obradovich, J.E.; Madewell, B.R.; Rodriguez, C.O., Jr. Liposome-Encapsulated Muramyl Tripeptide Phosphatidylethanolamine Adjuvant Immunotherapy for Splenic Hemangiosarcoma in the Dog: A Randomized Multi-Institutional Clinical Trial. Clin. Cancer Res. 1995, 1, 1165–1170. [Google Scholar]
- Pabst, G.; Katsaras, J. Liposomes, Lipid Bilayers and Model Membranes: From Basic Research to Application; Taylor & Francis Group: Abingdon, UK, 2016; ISBN 978-1-138-19875-3. [Google Scholar]
- Cho, E.; Lu, Y. Compartmentalizing Cell-Free Systems: Toward Creating Life-like Artificial Cells and Beyond. ACS Synth. Biol. 2020, 9, 2881–2901. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Paús, A.; Pérez-Pomeda, I.; Calpena, A.; Haro, I.; Gómara, M.J. Lipid Vesicles Loaded with an HIV-1 Fusion Inhibitor Peptide as a Potential Microbicide. Pharmaceutics 2020, 12, 502. [Google Scholar] [CrossRef]
- Petroni, D.; Riccardi, C.; Cavasso, D.; Russo Krauss, I.; Paduano, L.; Montesarchio, D.; Menichetti, L. Synthesis and Characterization of Multifunctional Nanovesicles Composed of POPC Lipid Molecules for Nuclear Imaging. Molecules 2021, 26, 6591. [Google Scholar] [CrossRef]
- Filipová, L.; Kohagen, M.; Štacko, P.; Muchová, E.; Slavíček, P.; Klán, P. Photoswitching of Azobenzene-Based Reverse Micelles above and at Subzero Temperatures as Studied by NMR and Molecular Dynamics Simulations. Langmuir 2017, 33, 2306–2317. [Google Scholar] [CrossRef]
- Pautot, S.; Frisken, B.J.; Weitz, D.A. Production of Unilamellar Vesicles Using an Inverted Emulsion. Langmuir 2003, 19, 2870–2879. [Google Scholar] [CrossRef]
- Chiba, M.; Miyazaki, M.; Ishiwata, S. Quantitative Analysis of the Lamellarity of Giant Liposomes Prepared by the Inverted Emulsion Method. Biophys. J. 2014, 107, 346–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stano, P.; de Souza, T.P.; Carrara, P.; Altamura, E.; D’Aguanno, E.; Caputo, M.; Luisi, P.L.; Mavelli, F. Recent Biophysical Issues about the Preparation of Solute-Filled Lipid Vesicles. Mech. Adv. Mater. Struct. 2015, 22, 748–759. [Google Scholar] [CrossRef]
- Miele, Y.; Bánsági, T.; Taylor, A.; Stano, P.; Rossi, F. Engineering Enzyme-Driven Dynamic Behaviour in Lipid Vesicles. In Advances in Artificial Life, Evolutionary Computation and Systems Chemistry; Rossi, F., Mavelli, F., Stano, P., Caivano, D., Eds.; Communications in Computer and Information Science; Springer International Publishing: Cham, Switzerland, 2016; pp. 197–208. ISBN 978-3-319-32694-8. [Google Scholar]
- Altamura, E.; Albanese, P.; Marotta, R.; Milano, F.; Fiore, M.; Trotta, M.; Stano, P.; Mavelli, F. Chromatophores Efficiently Promote Light-Driven ATP Synthesis and DNA Transcription inside Hybrid Multicompartment Artificial Cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2012170118. [Google Scholar] [CrossRef] [PubMed]
- Shimane, Y.; Kuruma, Y. Rapid and Facile Preparation of Giant Vesicles by the Droplet Transfer Method for Artificial Cell Construction. Front. Bioeng. Biotechnol. 2022, 10, 873854. [Google Scholar] [CrossRef] [PubMed]
- Sessa, L.; Concilio, S.; di Martino, M.; Nardiello, A.M.; Miele, Y.; Rossi, F.; Brunetti, J.; Panunzi, B.; Piotto, S. A Selective Nile Red Based Solvatochromic Probe: A Study of Fluorescence in LUVs and GUVs Model Membranes. Dyes Pigment. 2021, 196, 109759. [Google Scholar] [CrossRef]
- Svetina, S.; Žekš, B. Shape Behavior of Lipid Vesicles as the Basis of Some Cellular Processes. Anat. Rec. Off. Publ. Am. Assoc. Anat. 2002, 268, 215–225. [Google Scholar] [CrossRef]
- Bian, X.; Litvinov, S.; Koumoutsakos, P. Bending Models of Lipid Bilayer Membranes: Spontaneous Curvature and Area-Difference Elasticity. Comput. Methods Appl. Mech. Eng. 2020, 359, 112758. [Google Scholar] [CrossRef] [Green Version]
- Seifert, U.; Berndl, K.; Lipowsky, R. Shape Transformations of Vesicles: Phase Diagram for Spontaneous-Curvature and Bilayer-Coupling Models. Phys. Rev. A 1991, 44, 1182–1202. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; Seifert, U.; Wortis, M.; Döbereiner, H.-G. Budding Transitions of Fluid-Bilayer Vesicles: The Effect of Area-Difference Elasticity. Phys. Rev. E 1994, 49, 5389–5407. [Google Scholar] [CrossRef] [Green Version]
- Sackmann, E.; Duwe, H.-P.; Engelhardt, H. Membrane Bending Elasticity and Its Role for Shape Fluctuations and Shape Transformations of Cells and Vesicles. Faraday Discuss. Chem. Soc. 1986, 81, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, T.; Sakuma, Y.; Urakami, N.; Ziherl, P.; Imai, M. Role of Inverse-Cone-Shape Lipids in Temperature-Controlled Self-Reproduction of Binary Vesicles. Biophys. J. 2016, 110, 1551–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikari, K.; Sakuma, Y.; Jimbo, T.; Kodama, A.; Imai, M.; Monnard, P.-A.; Rasmussen, S. Dynamics of Fatty Acid Vesicles in Response to PH Stimuli. Soft Matter 2015, 11, 6327–6334. [Google Scholar] [CrossRef] [PubMed]
- Miele, Y.; Medveczky, Z.; Hollo, G.; Tegze, B.; Derenyi, I.; Horvolgyi, Z.; Altamura, E.; Lagzi, I.; Rossi, F. Self-Division of Giant Vesicles Driven by an Internal Enzymatic Reaction. Chem. Sci. 2020, 11, 3228–3235. [Google Scholar] [CrossRef] [Green Version]
- Holló, G.; Miele, Y.; Rossi, F.; Lagzi, I. Shape Changes and Budding of Giant Vesicles Induced by an Internal Chemical Trigger: An Interplay between Osmosis and PH Change. Phys. Chem. Chem. Phys. 2021, 23, 4262–4270. [Google Scholar] [CrossRef]
- Miele, Y.; Holló, G.; Lagzi, I.; Rossi, F. Effect of the Membrane Composition of Giant Unilamellar Vesicles on Their Budding Probability: A Trade-Off between Elasticity and Preferred Area Difference. Life 2021, 11, 634. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.K.; Morstein, J.; Trauner, D. Photopharmacological Control of Cell Signaling with Photoswitchable Lipids. Curr. Opin. Pharmacol. 2022, 63, 102202. [Google Scholar] [CrossRef]
- Wood, R.E.; Wirth, F.P.; Morgan, H.E. Glucose Permeability of Lipid Bilayer Membranes. Biochim. Biophys. Acta 1968, 163, 171–178. [Google Scholar] [CrossRef]
- Emami, S.; Su, W.-C.; Purushothaman, S.; Ngassam, V.N.; Parikh, A.N. Permeability and Line-Tension-Dependent Response of Polyunsaturated Membranes to Osmotic Stresses. Biophys. J. 2018, 115, 1942–1955. [Google Scholar] [CrossRef] [Green Version]
- Cama, J.; Chimerel, C.; Pagliara, S.; Javer, A.; Keyser, U.F. A Label-Free Microfluidic Assay to Quantitatively Study Antibiotic Diffusion through Lipid Membranes. Lab Chip 2014, 14, 2303–2308. [Google Scholar] [CrossRef] [Green Version]
- Purushothaman, S.; Cama, J.; Keyser, U.F. Dependence of Norfloxacin Diffusion across Bilayers on Lipid Composition. Soft Matter 2016, 12, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Le Ny, A.-L.M.; Schmidt, J.; Talmon, Y.; Chmelka, B.F.; Lee, C.T. Photo-Assisted Gene Delivery Using Light-Responsive Catanionic Vesicles. Langmuir 2009, 25, 5713–5724. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Wang, Y.; Wang, L.; Kouyama, T.; Gotoh, T.; Wada, S.; Wang, J.-Y. A Light-Responsive Self-Assembly Formed by a Cationic Azobenzene Derivative and SDS as a Drug Delivery System. Sci. Rep. 2017, 7, 39202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Londoño-Berrío, M.; Pérez-Buitrago, S.; Ortiz-Trujillo, I.C.; Hoyos-Palacio, L.M.; Orozco, L.Y.; López, L.; Zárate-Triviño, D.G.; Capobianco, J.A.; Mena-Giraldo, P. Cytotoxicity and Genotoxicity of Azobenzene-Based Polymeric Nanocarriers for Phototriggered Drug Release and Biomedical Applications. Polymers 2022, 14, 3119. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jeong, D.; Shinde, V.V.; Hu, Y.; Kim, C.; Jung, S. Azobenzene-Grafted Carboxymethyl Cellulose Hydrogels with Photo-Switchable, Reduction-Responsive and Self-Healing Properties for a Controlled Drug Release System. Int. J. Biol. Macromol. 2020, 163, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Deka, S.R.; Yadav, S.; Mahato, M.; Sharma, A.K. Azobenzene-Aminoglycoside: Self-Assembled Smart Amphiphilic Nanostructures for Drug Delivery. Colloids Surf. B Biointerfaces 2015, 135, 150–157. [Google Scholar] [CrossRef]
- Xiong, H.; Alberto, K.A.; Youn, J.; Taura, J.; Morstein, J.; Li, X.; Wang, Y.; Trauner, D.; Slesinger, P.A.; Nielsen, S.O.; et al. Optical Control of Neuronal Activities with Photoswitchable Nanovesicles. Nano Res. 2022, in press. [Google Scholar] [CrossRef]
- Mcoyi, S.; Amoako, D.G.; Somboro, A.M.; Khumalo, H.M.; Khan, R.B. The Molecular Effect of 1,4,7-triazacyclononane on Oxidative Stress Parameters in Human Hepatocellular Carcinoma (HepG2) Cells. J. Biochem. Mol. Toxicol. 2020, 34, e22607. [Google Scholar] [CrossRef]
- Li, Y.-J.; Wu, J.-Y.; Liu, J.; Xu, W.; Qiu, X.; Huang, S.; Hu, X.-B.; Xiang, D.-X. Artificial Exosomes for Translational Nanomedicine. J. Nanobiotechnology 2021, 19, 242. [Google Scholar] [CrossRef]
- Jhan, Y.-Y.; Prasca-Chamorro, D.; Palou Zuniga, G.; Moore, D.M.; Arun Kumar, S.; Gaharwar, A.K.; Bishop, C.J. Engineered Extracellular Vesicles with Synthetic Lipids via Membrane Fusion to Establish Efficient Gene Delivery. Int. J. Pharm. 2020, 573, 118802. [Google Scholar] [CrossRef]
- Piffoux, M.; Silva, A.K.A.; Wilhelm, C.; Gazeau, F.; Tareste, D. Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems. ACS Nano 2018, 12, 6830–6842. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albanese, P.; Cataldini, S.; Ren, C.Z.-J.; Valletti, N.; Brunetti, J.; Chen, J.L.-Y.; Rossi, F. Light-Switchable Membrane Permeability in Giant Unilamellar Vesicles. Pharmaceutics 2022, 14, 2777. https://doi.org/10.3390/pharmaceutics14122777
Albanese P, Cataldini S, Ren CZ-J, Valletti N, Brunetti J, Chen JL-Y, Rossi F. Light-Switchable Membrane Permeability in Giant Unilamellar Vesicles. Pharmaceutics. 2022; 14(12):2777. https://doi.org/10.3390/pharmaceutics14122777
Chicago/Turabian StyleAlbanese, Paola, Simone Cataldini, Chloe Z.-J. Ren, Nadia Valletti, Jlenia Brunetti, Jack L.-Y. Chen, and Federico Rossi. 2022. "Light-Switchable Membrane Permeability in Giant Unilamellar Vesicles" Pharmaceutics 14, no. 12: 2777. https://doi.org/10.3390/pharmaceutics14122777