The Effect of Voriconazole on Tacrolimus in Kidney Transplantation Recipients: A Real-World Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Tacrolimus and Voriconazole Plasma Concentration Measurement
2.3. Genotype and Phenotype Assignment of CYP3A5 and CPY2C19
2.4. Statistical Analysis
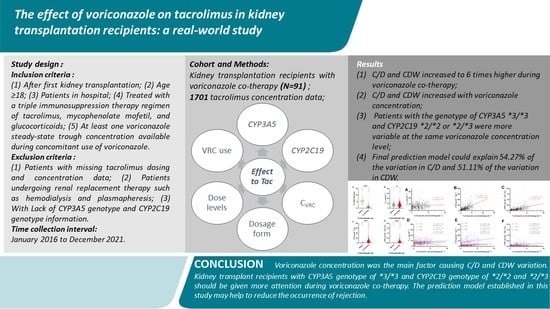
3. Results
3.1. Study Population, TDM Results, and Laboratory Tests
3.2. Effect of Voriconazole Co-Therapy on Daily Dose, CTac, C/D, and CDW of Tacrolimus
3.3. Effect of Voriconazole Dose on Daily Dose, CTac, C/D, and CDW of Tacrolimus
3.4. Effect of Voriconazole Dosage Form on Daily Dose, CTac, C/D, and CDW of Tacrolimus
3.5. Effect of Voriconazole Concentration on Daily Dose, CTac, C/D, and CDW of Tacrolimus
3.6. Effect of CYP3A5 Genotypes on Daily Dose, CTac, C/D, and CDW of Tacrolimus
3.7. Effect of CYP3A5 Genotypes on Daily Dose, CTac, C/D, and CDW of Tacrolimus
3.8. Determinants of Tacrolimus CTac, C/D, and CDW
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yoshimura, N.; Oka, T. Medical and surgical complications of renal transplantation: Diagnosis and management. Med. Clin. N. Am. 1990, 74, 1025–1037. [Google Scholar] [CrossRef]
- Azarfar, A.; Ravanshad, Y.; Mehrad-Majd, H.; Esmaeeli, M.; Aval, S.B.; Emadzadeh, M.; Salehi, M.; Moradi, A.; Golsorkhi, M.; Khazaei, M.R. Comparison of tacrolimus and cyclosporine for immunosuppression after renal transplantation: An updated systematic review and meta-analysis. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2018, 29, 1376–1385. [Google Scholar] [CrossRef]
- Ramanathan, V.; Goral, S.; Helderman, J.H. Renal transplantation. Semin. Nephrol. 2001, 21, 213–219. [Google Scholar] [CrossRef]
- Shrestha, B.M. Two Decades of Tacrolimus in Renal Transplant: Basic Science and Clinical Evidences. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2017, 15, 1–9. [Google Scholar] [CrossRef]
- Gooptu, M.; Antin, J.H. GVHD Prophylaxis 2020. Front. Immunol. 2021, 12, 605726. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, M.; Zhang, W.; Ming, Y. Pharmacokinetics, Pharmacodynamics and Pharmacogenetics of Tacrolimus in Kidney Transplantation. Curr. Drug Metab. 2018, 19, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Woillard, J.B.; Debord, J.; Monchaud, C.; Saint-Marcoux, F.; Marquet, P. Population Pharmacokinetics and Bayesian Estimators for Refined Dose Adjustment of a New Tacrolimus Formulation in Kidney and Liver Transplant Patients. Clin. Pharmacokinet. 2017, 56, 1491–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamashiro, E.Y.; Felipe, C.R.; Genvigir, F.D.V.; Rodrigues, A.C.; Campos, A.B.; Hirata, R.D.C.; Tedesco-Silva, H.; Medina-Pestana, J.O. Influence of CYP3A4 and CYP3A5 polymorphisms on tacrolimus and sirolimus exposure in stable kidney transplant recipients. Drug Metab. Pers. Ther. 2017, 32, 89–95. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Schladt, D.P.; Guan, W.; Wu, B.; van Setten, J.; Keating, B.J.; Iklé, D.; Remmel, R.P.; Dorr, C.R.; Mannon, R.B.; et al. Tacrolimus troughs and genetic determinants of metabolism in kidney transplant recipients: A comparison of four ancestry groups. Am. J. Transplant. 2019, 19, 2795–2804. [Google Scholar] [CrossRef]
- Jouve, T.; Noble, J.; Rostaing, L.; Malvezzi, P. An update on the safety of tacrolimus in kidney transplant recipients, with a focus on tacrolimus minimization. Expert Opin. Drug Saf. 2019, 18, 285–294. [Google Scholar] [CrossRef]
- Krämer, B.K.; Montagnino, G.; Krüger, B.; Margreiter, R.; Olbricht, C.J.; Marcen, R.; Sester, U.; Kunzendorf, U.; Dietl, K.H.; Rigotti, P.; et al. Efficacy and safety of tacrolimus compared with ciclosporin-A in renal transplantation: 7-year observational results. Transpl. Int. 2016, 29, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-q.; Zhang, L.; Zhang, Y.; Sun, L.; Chan, W.; Huang, J.; Wang, Z.; Wang, X.-x.; Li, M.; Liu, F.; et al. Expert consensus on tacrolimus individualized therapy for Solid organ transplantation in China. Eval. Anal. Drug Use Chin. Hosp. 2021, 21, 1409–1424. [Google Scholar] [CrossRef]
- Gavaldà, J.; Meije, Y.; Fortún, J.; Roilides, E.; Saliba, F.; Lortholary, O.; Muñoz, P.; Grossi, P.; Cuenca-Estrella, M. Invasive fungal infections in solid organ transplant recipients. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20 (Suppl. 7), 27–48. [Google Scholar] [CrossRef] [Green Version]
- Shoham, S.; Marr, K.A. Invasive fungal infections in solid organ transplant recipients. Future Microbiol. 2012, 7, 639–655. [Google Scholar] [CrossRef] [Green Version]
- Balcan, B.; Ozcelik, U.; Ugurlu, A.O.; Aydin, M.; Nalcaci, S.; Yarbug Karakayali, F. Increased Mortality Among Renal Transplant Patients With Invasive Pulmonary Aspergillus Infection. Prog. Transplant. 2018, 28, 349–353. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Hou, K.; Liu, F.; Luo, X.; He, S.; Hu, L.; Yang, C.; Huang, L.; Feng, Y. The influence of CYP2C19 polymorphisms on voriconazole trough concentrations: Systematic review and meta-analysis. Mycoses 2021, 64, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Mohamud, M.A.; Smith, A.R.; Jacobson, P.A.; Jaber, M.M.; Alharbi, A.F.; Fisher, J.; Kirstein, M.N. CYP2C19 Phenotype and Body Weight-Guided Voriconazole Initial Dose in Infants and Children after Hematopoietic Cell Transplantation. Antimicrob. Agents Chemother. 2021, 65, e0062321. [Google Scholar] [CrossRef]
- Kirbs, C.; Kluwe, F.; Drescher, F.; Lackner, E.; Matzneller, P.; Weiss, J.; Zeitlinger, M.; Kloft, C. High voriconazole target-site exposure after approved sequence dosing due to nonlinear pharmacokinetics assessed by long-term microdialysis. Eur. J. Pharm. Sci. 2019, 131, 218–229. [Google Scholar] [CrossRef]
- Lamoureux, F.; Duflot, T.; Woillard, J.B.; Metsu, D.; Pereira, T.; Compagnon, P.; Morisse-Pradier, H.; El Kholy, M.; Thiberville, L.; Stojanova, J.; et al. Impact of CYP2C19 genetic polymorphisms on voriconazole dosing and exposure in adult patients with invasive fungal infections. Int. J. Antimicrob. Agents 2016, 47, 124–131. [Google Scholar] [CrossRef]
- Chang, H.H.; Lee, N.Y.; Ko, W.C.; Lee, H.C.; Yang, Y.H.; Wu, C.J.; Chang, C.M. Voriconazole inhibition of tacrolimus metabolism in a kidney transplant recipient with fluconazole-resistant cryptococcal meningitis. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2010, 14, e348–e350. [Google Scholar] [CrossRef] [Green Version]
- Suetsugu, K.; Mori, Y.; Yamamoto, N.; Shigematsu, T.; Miyamoto, T.; Egashira, N.; Akashi, K.; Masuda, S. Impact of CYP3A5, POR, and CYP2C19 Polymorphisms on Trough Concentration to Dose Ratio of Tacrolimus in Allogeneic Hematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2019, 20, 2413. [Google Scholar] [CrossRef] [Green Version]
- VFEND® (Voriconazole) Tablets, for Oral Use and for Intravenous Use. 2021. Available online: https://www.drugfuture.com/fda/drugview/021266 (accessed on 14 January 2021).
- Vanhove, T.; Bouwsma, H.; Hilbrands, L.; Swen, J.J.; Spriet, I.; Annaert, P.; Vanaudenaerde, B.; Verleden, G.; Vos, R.; Kuypers, D.R.J. Determinants of the Magnitude of Interaction Between Tacrolimus and Voriconazole/Posaconazole in Solid Organ Recipients. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2017, 17, 2372–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, T.; Kato, J.; Yamane, A.; Sakurai, M.; Kohashi, S.; Kikuchi, T.; Ono, Y.; Okamoto, S. Drug interaction between voriconazole and tacrolimus and its association with the bioavailability of oral voriconazole in recipients of allogeneic hematopoietic stem cell transplantation. Int. J. Hematol. 2012, 95, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Venkataramanan, R.; Zang, S.; Gayowski, T.; Singh, N. Voriconazole inhibition of the metabolism of tacrolimus in a liver transplant recipient and in human liver microsomes. Antimicrob. Agents Chemother. 2002, 46, 3091–3093. [Google Scholar] [CrossRef] [Green Version]
- Capone, D.; Tarantino, G.; Gentile, A.; Sabbatini, M.; Polichetti, G.; Santangelo, M.; Nappi, R.; Ciotola, A.; D’Alessandro, V.; Renda, A.; et al. Effects of voriconazole on tacrolimus metabolism in a kidney transplant recipient. J. Clin. Pharm. Ther. 2010, 35, 121–124. [Google Scholar] [CrossRef]
- Laboratories, A. ARCHITECT Tacrolimus Reagent Kit Instructions for IL77-35. 2009. Available online: https://www.ilexmedical.com/files/PDF/Tacrolimus_ARC.pdf (accessed on 14 April 2009).
- Lamba, J.K.; Lin, Y.S.; Schuetz, E.G.; Thummel, K.E. Genetic contribution to variable human CYP3A-mediated metabolism. Adv. Drug Deliv. Rev. 2002, 54, 1271–1294. [Google Scholar] [CrossRef]
- Shahbaz, H.; Gupta, M. Creatinine Clearance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ota, R.; Hirata, A.; Noto, K.; Yokoyama, S.; Hosomi, K.; Takada, M.; Matsuoka, H. Relationship between the blood concentrations of tacrolimus and voriconazole in hematopoietic stem cell transplant recipients. Int. J. Clin. Pharmacol. Ther. 2019, 57, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.R.; Amital, A.; Fuks, L.; Shitrit, D. Voriconazole and itraconazole in lung transplant recipients receiving tacrolimus (FK 506): Efficacy and drug interaction. Clin. Transplant. 2011, 25, E163–E167. [Google Scholar] [CrossRef]
- Mimura, A.; Yamaori, S.; Ikemura, N.; Katsuyama, Y.; Matsuzawa, N.; Ohmori, S. Influence of azole antifungal drugs on blood tacrolimus levels after switching from intravenous tacrolimus to once-daily modified release tacrolimus in patients receiving allogeneic hematopoietic stem cell transplantation. J. Clin. Pharm. Ther. 2019, 44, 565–571. [Google Scholar] [CrossRef]
- Imamura, C.K.; Furihata, K.; Okamoto, S.; Tanigawara, Y. Impact of cytochrome P450 2C19 polymorphisms on the pharmacokinetics of tacrolimus when coadministered with voriconazole. J. Clin. Pharmacol. 2016, 56, 408–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everton, J.B.F.; Patrício, F.J.B.; Faria, M.S.; Ferreira, T.C.A.; Romao, E.A.; Silva, G.E.B.; Magalhães, M. CYP3A5 and PPARA genetic variants are associated with low trough concentration to dose ratio of tacrolimus in kidney transplant recipients. Eur. J. Clin. Pharmacol. 2021, 77, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Pinon, M.; De Nicolò, A.; Pizzol, A.; Antonucci, M.; D’Avolio, A.; Serpe, L.; Dell’Olio, D.; Catalano, S.; Tandoi, F.; Romagnoli, R.; et al. Early impact of donor CYP3A5 genotype and Graft-to-Recipient Weight Ratio on tacrolimus pharmacokinetics in pediatric liver transplant patients. Sci. Rep. 2021, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Y.; Wang, X.; Wang, C.; Lin, W.; Jiao, Z.; Wang, Z. Wuzhi Capsule Dosage Affects Tacrolimus Elimination in Adult Kidney Transplant Recipients, as Determined by a Population Pharmacokinetics Analysis. Pharm. Pers. Med. 2021, 14, 1093–1106. [Google Scholar] [CrossRef]
- Li, C.; Lu, J.; Zhou, S.; Wei, Y.; Lv, C.; Liu, T.; Wu, Y.; Wu, D.; Qi, J.; Cai, R. Influential Factors and Efficacy Analysis of Tacrolimus Concentration After Allogeneic Hematopoietic Stem Cell Transplantation in Children with beta-Thalassemia Major. Pharm. Pers. Med. 2021, 14, 1221–1237. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, F.; Wang, Z.; Wen, P.; Fan, J. The influence of recipient SLCO1B1 rs2291075 polymorphism on tacrolimus dose–corrected trough concentration in the early period after liver transplantationPharmacology. Eur. J. Clin. 2021, 77, 859–867. [Google Scholar] [CrossRef]
- Rangel, E.B. Tacrolimus in pancreas transplant: A focus on toxicity, diabetogenic effect and drug-drug interactions. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1585–1605. [Google Scholar] [CrossRef]
- Scalea, J.R.; Levi, S.T.; Ally, W.; Brayman, K.L. Tacrolimus for the prevention and treatment of rejection of solid organ transplants. Expert Rev. Clin. Immunol. 2016, 12, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Jindal, R.M.; Sidner, R.A.; Milgrom, M.L. Post-transplant diabetes mellitus. The role of immunosuppression. Drug Saf. 1997, 16, 242–257. [Google Scholar] [CrossRef]
- Montero, N.; Pascual, J. Immunosuppression and Post-transplant Hyperglycemia. Curr. Diabetes Rev. 2015, 11, 144–154. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Radek, M.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. Diabetes and Cardiovascular Risk in Renal Transplant Patients. Int. J. Mol. Sci. 2021, 22, 3422. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Flechner, S.M.; Ismail, H.R.; Flechner, L.M.; Zhou, L.; Derweesh, I.H.; Goldfarb, D.; Modlin, C.; Novick, A.C.; Faiman, C. Posttransplant diabetes mellitus in kidney transplant recipients receiving calcineurin or mTOR inhibitor drugs. Transplantation 2006, 81, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Castillo, S.; Plaza, A.; Stojanova, J.; Navarro, G.; Carmona, R.; Corvalán, F.; Cerpa, L.; Sandoval, C.; Muñoz, D.; Leiva, M.; et al. Effect of CYP3A4, CYP3A5, MDR1 and POR Genetic Polymorphisms in Immunosuppressive Treatment in Chilean Kidney Transplanted Patients. Front. Pharmacol. 2021, 12, 674117. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Teng, R.C.; Zhu, H.J.; Fang, Y. CYP3A4/5 polymorphisms affect the blood level of cyclosporine and tacrolimus in Chinese renal transplant recipients. Int. J. Clin. Pharmacol. Ther. 2013, 51, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Lunde, I.; Bremer, S.; Midtvedt, K.; Mohebi, B.; Dahl, M.; Bergan, S.; Åsberg, A.; Christensen, H. The influence of CYP3A, PPARA, and POR genetic variants on the pharmacokinetics of tacrolimus and cyclosporine in renal transplant recipients. Eur. J. Clin. Pharmacol. 2014, 70, 685–693. [Google Scholar] [CrossRef]






| Characteristics (N = 91) | Value |
|---|---|
| Male, n (%) | 70 (76.90) |
| Age (years) a | 40.00 [32.00, 49.00] |
| Wt (kg) b | 60.02 (13.14) |
| Etiology of chronic nephritis | |
| Unknown cause | 68 (74.43) |
| Glomerulonephritis | 11 (12.09) |
| Polycystic disease | 5(5.49) |
| Hypertension | 3 (3.30) |
| Diabetes | 3 (3.30) |
| Lupus nephritis | 1 (1.10) |
| Kidney source, n (%) | |
| DBD | 73 (80.2) |
| DCD | 7 (7.70) |
| RD | 11 (12.10) |
| Genotype of CYP3A5, n (%) | |
| *1/*1 | 6 (6.60) |
| *1/*3 | 46 (50.50) |
| *3/*3 | 39 (42.90) |
| Genotype of CYP2C19, n (%) | |
| *1/*1 | 35 (38.50) |
| *1/*2 | 40 (44.00) |
| *1/*3 | 12 (13.20) |
| *2/*2 | 2 (2.20) |
| *2/*3 | 2 (2.20) |
| Parameter | Value |
|---|---|
| Number of CTac | 1701 |
| Tac daily dose (mg) b | 3.33 ± 2.68 |
| CTac (ng·mL−1) b | 7.16 ± 3.77 |
| C/D (ng·mL−1/mg) a | 6.50 [4.60, 9.10] |
| CDW (ng·mL−1·mg−1/kg) a | 0.04 [0.02, 0.12] |
| No. of CVRC | 1455(85.54%) |
| CVRC (μg·mL−1) a | 0.00 [0.00, 1.98] |
| VRC daily dose (mg), n (%) | |
| 0 | 845 (50.17) |
| 100 | 9 (0.53) |
| 150 | 8 (0.47) |
| 200 | 72 (4.23) |
| 250 | 3 (0.18) |
| 300 | 140 (8.23) |
| 350 | 27 (1.59) |
| 400 | 558 (32.69) |
| 450 | 11 (0.65) |
| 500 | 1 (0.06) |
| 600 | 9 (0.53) |
| 800 | 3 (0.18) |
| Parameter | Value |
|---|---|
| White blood cell count (109/L) a | 7.07 [5.16, 9.45] |
| Red blood cell count (1012/L) a | 2.98 [2.57, 3.68] |
| Lymphocyte count (%) b | 14.07 ± 11.48 |
| Neutrophilic granulocyte (%) b | 78.41 ± 14.68 |
| Hematocrit (%) a | 26.70 [23.50, 32.00] |
| Hemoglobin (g/L) a | 87.00 [77.00, 105.00] |
| Blood platelet count (109/L) | 176.36 ± 70.69 |
| Alanine aminotransferase (U/L) a | 12.90 [8.05, 21.70] |
| Aspartate aminotransferase (U/L) a | 13.90 [10.50, 20.30] |
| Total bilirubin (μmol/L) a | 6.70 [5.00, 8.80] |
| Direct bilirubin (μmol/L) a | 2.70 [1.95, 3.70] |
| Total bile acid (μmol/L) a | 3.50 [2.30, 5.69] |
| Albumin (g/L) b | 34.42 ± 4.42 |
| Blood urea nitrogen (mmol/L) a | 16.20 [10.73, 26.64] |
| Serum creatinine (μmol/L) a | 170.00 [120.20, 306.05] |
| CCR * (mL/min) a | 40.72 [22.97, 60.01] |
| Prothrombin time (s) a | 12.80 [11.80, 13.70] |
| International normalized ratio a | 1.04 [0.93, 1.11] |
| Prothrombin activity (%) a | 97.50 [89.00, 110.80] |
| Procalcitonin (ng/mL) a | 0.17 [0.09, 0.39] |
| C reactive protein (mg/L) a | 5.56 [1.75, 28.05] |
| Parameter | Sample (N, %) a | CTac b (ng·mL−1) | Daily Dose b (mg) | C/D b (ng mL−1/mg) | CDW b (ng·mL−1·mg−1/kg) | |
|---|---|---|---|---|---|---|
| Voriconazole use | Yes | 84 (49.67) | 7.05 ± 4.09 | 1.00 (0.5–2.00) | 6.96 (3.62–12.00) | 0.12 [0.06, 0.21] |
| No | 856 (50.33) | 7.27 ± 3.41 | 5.50 (4.00–7.00) | 1.27 (0.90–1.83) | 0.02 [0.01, 0.03] | |
| p | / | 0.23 | <0.0001 | <0.0001 | <0.001 | |
| Voriconazole Dosage form | Injection | 66 (7.96) | 1.00 [0.50, 3.00] | 6.75 [4.70, 10.00] | 5.00 [2.60, 7.35] | 0.09 [0.05, 0.15] |
| Oral | 763 (92.04) | 1.00 [0.50, 1.50] | 6.20 [4.10, 9.25] | 7.20 [3.98, 12.60] | 0.12 [0.07, 0.22] | |
| P | / | <0.001 | 0.278 | <0.001 | 0.007 | |
| Voriconazole daily dose (mg) | 0 | 860 (50.56) | 6.60 [4.90, 8.90] | 5.50 [4.00, 7.00] | 1.27 [0.91, 1.84] | 0.02 [0.01, 0.03] |
| 100–250 | 92 (5.41) | 5.50 [3.40, 8.50] | 1.00 [0.50, 2.50] | 5.31 [1.75, 11.50] | 0.10 [0.03, 0.22] | |
| 300–350 | 167 (9.82) | 5.90 [4.10, 8.75] | 0.50 [0.40, 1.25] | 8.20 [5.27, 15.10] | 0.15 [0.08, 0.29] | |
| 400 | 558 (32.80) | 6.55 [4.40, 9.80] | 1.00 [0.50, 2.00] | 7.00 [3.63, 11.80] | 0.12 [0.07, 0.20] | |
| >450 | 24 (1.41) | 5.75 [4.15, 9.72] | 1.00 [0.88, 1.62] | 5.60 [3.00, 7.85] | 0.09 [0.04, 0.14] | |
| p | <0.001 | 0.004 | <0.001 | <0.001 | <0.001 | |
| Parameter | Sample (N, %) a | CTac b (ng·mL−1) | Daily Dose b (mg) | C/D b (ng mL−1/mg) | CDW b (ng·mL−1·mg−1/kg) | |
|---|---|---|---|---|---|---|
| CYP3A5 | EM (*1/*1) | 6 (6.60) | 5.50 [4.57, 8.62] | 3.25 [2.00, 6.00] | 1.88 [1.28, 3.60] | 0.03 [0.02, 0.06] |
| IM (*1/*3) | 46 (50.50) | 6.60 [4.70, 8.90] | 3.50 [1.50, 6.00] | 1.80 [1.09, 4.70] | 0.03 [0.02, 0.08] | |
| PM (*3/*3) | 39 (42.90) | 6.55 [4.60, 9.70] | 1.25 [0.50, 4.00] | 5.18 [1.50, 12.29] | 0.09 [0.03, 0.22] | |
| p | <0.001 | 0.237 | <0.001 | <0.001 | <0.001 | |
| CYP2C19 | *1/*1 | 35 (38.50) | 6.80 [5.00, 9.50] | 3.00 [1.00, 6.00] | 2.20 [1.20, 5.40] | 0.04 [0.02, 0.09] |
| *1/*2 | 40 (44.00) | 6.50 [4.70, 8.90] | 2.50 [0.50, 5.00] | 2.92 [1.24, 8.00] | 0.05 [0.02, 0.14] | |
| *1/*3 | 12 (13.20) | 5.80 [3.70, 8.80] | 3.00 [0.75, 5.50] | 2.03 [1.00, 9.00] | 0.04 [0.02, 0.19] | |
| *2/*2 | 2 (2.20) | 7.65 [6.08, 10.10] | 0.25 [0.25, 0.50] | 22.60 [11.10, 27.80] | 0.35 [0.19, 0.47] | |
| *2/*3 | 2 (2.20) | 3.47 [2.90, 5.08] | 0.50 [0.50, 0.75] | 7.25 [3.55, 13.80] | 0.15 [0.08, 0.29] | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Parameters | Estimate Coefficients | Std. Error | t | VIF | p |
|---|---|---|---|---|---|
| (Intercept) | 5.893 | 0.729 | 8.080 | <0.001 | |
| Male | 1.491 | 0.384 | 3.881 | 1.147 | <0.001 |
| CYP3A5*3*3 | 1.239 | 0.337 | 3.676 | <0.001 | |
| Tac Daily Dose | −0.911 | 0.085 | −10.671 | 2.449 | <0.001 |
| Postoperative time | −0.041 | 0.017 | −2.405 | 1.281 | 0.016 |
| VRC Daily Dose | −0.006 | 0.001 | −4.719- | 3.031 | <0.001 |
| CVRC | 2.830 | 0.156 | 18.127 | 2.336 | <0.001 |
| Lymphocyte count | −0.043 | 0.015 | −2.870 | 1.312 | 0.004 |
| Blood urea nitrogen | 0.044 | 0.014 | 3.046 | 2.016 | 0.002 |
| Serum creatinine | −0.002 | 0.001 | −2.167 | 1.990 | 0.030 |
| CYP2C19 substrate a | −0.854 | 0.297 | 2.879 | 1.071 | 0.004 |
| CYP2C19 inducer b | −1.314 | 0.587 | −2.238 | 1.063 | 0.025 |
| F R2 Adjusted R2 | 106.3 | ||||
| 0.5479 | |||||
| 0.5427 | |||||
| p | <0.001 | ||||
| Reference | Transplantation Type | No. | DV a | Predictors | Method | R2 |
|---|---|---|---|---|---|---|
| Our study | Kidney | 91 | C/D | CYP3A5 genotype, POT, Tac Daily dose, VRC Daily dose, CVRC, LYM, BUN CREA, CYP2C19 substrate, and CYP2C19 inducer | Multiple linear regression | 0.548 |
| Vanhove T. et al. [24] | Kidney and lung | 126 | C/D | HCT, age, CYP3A5 genotype, and CYP3A4 substrate | Multiple linear regression | 0.22 |
| Janaína F. et al. [35] | Kidney | 127 | C/D | Age, POT, CYP3A5, and PPARA genotype | Multiple linear regression | 0.123 |
| Pinon M. et al. [36] | Liver | 49 | CDW | CYP3A5 genotype and GRWR | Multiple linear regression | 0.173 |
| Chengxin L. et al. [37] | Hematopoietic stem cell | 46 | C/D | Sex, weight, POT, HGB, PLT, CREA, and VRC | Multiple linear regression | 0.33 |
| Lizhi C. et al. [38] | Kidney | 142 | C | CREA, HCT, Wu-zhi capsule, CYP3A5 genotype, and Tac daily dose | Correlation analysis | NA |
| Suetsugu. K. et al. [22] | Hematopoietic stem cell | 36 | C/D a | CYP3A4 genotype, POR*1/*1 CYP2C19 genotype, and VRC | Multiple logistical regression | NA |
| Yi W. et al. [39] | Liver | 210 | C/D | Tac daily dose, POT, TBIL, and SLCO1B1 rs2291075 | Multiple factor analysis | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.-C.; Xiao, C.-L.; Hou, J.-J.; Li, J.-K.; Zhang, B.-K.; Xie, X.-B.; Fang, C.-H.; Peng, F.-H.; Sandaradura, I.; Yan, M. The Effect of Voriconazole on Tacrolimus in Kidney Transplantation Recipients: A Real-World Study. Pharmaceutics 2022, 14, 2739. https://doi.org/10.3390/pharmaceutics14122739
Zhao Y-C, Xiao C-L, Hou J-J, Li J-K, Zhang B-K, Xie X-B, Fang C-H, Peng F-H, Sandaradura I, Yan M. The Effect of Voriconazole on Tacrolimus in Kidney Transplantation Recipients: A Real-World Study. Pharmaceutics. 2022; 14(12):2739. https://doi.org/10.3390/pharmaceutics14122739
Chicago/Turabian StyleZhao, Yi-Chang, Chen-Lin Xiao, Jing-Jing Hou, Jia-Kai Li, Bi-Kui Zhang, Xu-Biao Xie, Chun-Hua Fang, Feng-Hua Peng, Indy Sandaradura, and Miao Yan. 2022. "The Effect of Voriconazole on Tacrolimus in Kidney Transplantation Recipients: A Real-World Study" Pharmaceutics 14, no. 12: 2739. https://doi.org/10.3390/pharmaceutics14122739