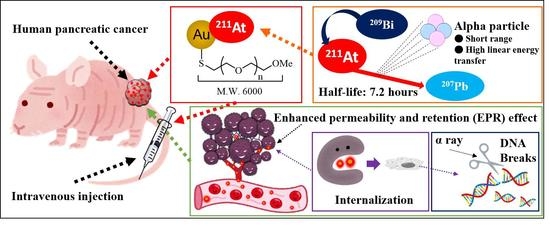
Astatine-211-Labeled Gold Nanoparticles for Targeted Alpha-Particle Therapy via Intravenous Injection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Astatine-211 Production
2.2. AuNPs Modification and Characterization
2.3. 211At-Labeling of AuNPs
2.4. In Vivo Biodistribution and Therapy Efficacy
2.5. Internalization Evaluation
2.6. Statistical Analysis
3. Results
3.1. 211At-Labeled AuNPs Synthesis
3.2. Biodistribution Study
3.3. In Vivo Therapeutic Effect
3.4. Cellular Uptake of AuNPs and DNA DSBs In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerra Liberal, F.D.C.; O’Sullivan, J.M.; McMahon, S.J.; Prise, K.M. Targeted Alpha Therapy: Current Clinical Applications. Cancer Biother. Radiopharm. 2020, 35, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.J.; Raja, C.; Rizvi, S.; Li, Y.; Tsui, W.; Zhang, D.; Song, E.; Qu, C.F.; Kearsley, J.; Graham, P.; et al. Targeted Alpha Therapy for Cancer. Phys. Med. Biol. 2004, 49, 3703–3712. [Google Scholar] [CrossRef] [PubMed]
- Norum, J.; Traasdahl, E.R.; Totth, A.; Nieder, C.; Olsen, J.A. Health Economics and Radium-223 (Xofigo®) in the Treatment of Metastatic Castration-Resistant Prostate Cancer (MCRPC): A Case History and a Systematic Review of the Literature. Glob. J. Health Sci. 2015, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225 Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]
- Watabe, T.; Liu, Y.; Kaneda-Nakashima, K.; Shirakami, Y.; Lindner, T.; Ooe, K.; Toyoshima, A.; Nagata, K.; Shimosegawa, E.; Haberkorn, U.; et al. Theranostics Targeting Fibroblast Activation Protein in the Tumor Stroma: 64 Cu- and 225 Ac-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J. Nucl. Med. 2020, 61, 563–569. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Zalutsky, M. Astatine Radiopharmaceuticals: Prospects and Problems. Curr. Radiopharm. 2008, 1, 177–196. [Google Scholar] [CrossRef] [Green Version]
- Zalutsky, M.; Vaidyanathan, G. Astatine-211-Labeled Radiotherapeutics An Emerging Approach to Targeted Alpha-Particle Radiotherapy. Curr. Pharm. Des. 2000, 6, 1433–1455. [Google Scholar] [CrossRef]
- Carlin, S.; Mairs, R.J.; Welsh, P.; Zalutsky, M.R. Sodium-Iodide Symporter (NIS)-Mediated Accumulation of [211At]Astatide in NIS-Transfected Human Cancer Cells. Nucl. Med. Biol. 2002, 29, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Kaneda-Nakashima, K.; Liu, Y.; Shirakami, Y.; Ooe, K.; Toyoshima, A.; Shimosegawa, E.; Fukuda, M.; Shinohara, A.; Hatazawa, J. Enhancement of 211 At Uptake via the Sodium Iodide Symporter by the Addition of Ascorbic Acid in Targeted α-Therapy of Thyroid Cancer. J. Nucl. Med. 2019, 60, 1301–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watabe, T.; Kaneda-Nakashima, K.; Ooe, K.; Liu, Y.; Kurimoto, K.; Murai, T.; Shidahara, Y.; Okuma, K.; Takeuchi, M.; Nishide, M.; et al. Extended Single-Dose Toxicity Study of [211At]NaAt in Mice for the First-in-Human Clinical Trial of Targeted Alpha Therapy for Differentiated Thyroid Cancer. Ann. Nucl. Med. 2021, 35, 702–718. [Google Scholar] [CrossRef]
- Yanagida, O.; Kanai, Y.; Chairoungdua, A.; Kim, D.K.; Segawa, H.; Nii, T.; Cha, S.H.; Matsuo, H.; Fukushima, J.; Fukasawa, Y.; et al. Human L-Type Amino Acid Transporter 1 (LAT1): Characterization of Function and Expression in Tumor Cell Lines. Biochim. Biophys. Acta BBA Biomembr. 2001, 1514, 291–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneda-Nakashima, K.; Zhang, Z.; Manabe, Y.; Shimoyama, A.; Kabayama, K.; Watabe, T.; Kanai, Y.; Ooe, K.; Toyoshima, A.; Shirakami, Y.; et al. α-Emitting Cancer Therapy Using 211At-AAMT Targeting LAT1. Cancer Sci. 2021, 112, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Huang, X.; Kadonaga, Y.; Katayama, D.; Ooe, K.; Shimoyama, A.; Kabayama, K.; Toyoshima, A.; Shinohara, A.; Hatazawa, J.; et al. Intratumoral Administration of Astatine-211-Labeled Gold Nanoparticle for Alpha Therapy. J. Nanobiotechnol. 2021, 19, 223. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold Nanoparticle-Based Colorimetric Biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef] [Green Version]
- Haume, K.; Rosa, S.; Grellet, S.; Śmiałek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold Nanoparticles for Cancer Radiotherapy: A Review. Cancer Nanotechnol. 2016, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold Nanoparticles for In Vitro Diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef] [Green Version]
- Dziawer, L.; Koźmiński, P.; Męczyńska-Wielgosz, S.; Pruszyński, M.; Łyczko, M.; Wąs, B.; Celichowski, G.; Grobelny, J.; Jastrzębski, J.; Bilewicz, A. Gold Nanoparticle Bioconjugates Labelled with 211 At for Targeted Alpha Therapy. RSC Adv. 2017, 7, 41024–41032. [Google Scholar] [CrossRef] [Green Version]
- Sporer, E.; Poulie, C.B.M.; Lindegren, S.; Aneheim, E.; Jensen, H.; Bäck, T.; Kempen, P.J.; Kjaer, A.; Herth, M.M.; Jensen, A.I. Surface Adsorption of the Alpha-Emitter Astatine-211 to Gold Nanoparticles Is Stable In Vivo and Potentially Useful in Radionuclide Therapy. J. Nanotheranostics 2021, 2, 196–207. [Google Scholar] [CrossRef]
- Iwasaki, T.; Tokuda, Y.; Kotake, A.; Okada, H.; Takeda, S.; Kawano, T.; Nakayama, Y. Cellular Uptake and in Vivo Distribution of Polyhistidine Peptides. J. Control. Release 2015, 210, 115–124. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Jeon, J.; Hong, S.H.; Rhim, W.-K.; Lee, Y.-S.; Youn, H.; Chung, J.-K.; Lee, M.C.; Lee, D.S.; Kang, K.W.; et al. Tumor Targeting and Imaging Using Cyclic RGD-PEGylated Gold Nanoparticle Probes with Directly Conjugated Iodine-125. Small 2011, 7, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Vilchis-Juárez, A.; Ferro-Flores, G.; Santos-Cuevas, C.; Morales-Avila, E.; Ocampo-García, B.; Díaz-Nieto, L.; Luna-Gutiérrez, M.; Jiménez-Mancilla, N.; Pedraza-López, M.; Gómez-Oliván, L. Molecular Targeting Radiotherapy with Cyclo-RGDfK(C) Peptides Conjugated to 177 Lu-Labeled Gold Nanoparticles in Tumor-Bearing Mice. J. Biomed. Nanotechnol. 2014, 10, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Aung, W.; Jin, Z.-H.; Furukawa, T.; Claron, M.; Boturyn, D.; Sogawa, C.; Tsuji, A.B.; Wakizaka, H.; Fukumura, T.; Fujibayashi, Y.; et al. Micro–Positron Emission Tomography/Contrast-Enhanced Computed Tomography Imaging of Orthotopic Pancreatic Tumor–Bearing Mice Using the α v β 3 Integrin Tracer 64 Cu-Labeled Cyclam-RAFT-c(-RGDfK-) 4. Mol. Imaging 2013, 12, 376–387. [Google Scholar] [CrossRef]
- Toyoshima, A.; Zhang, Z.; Kanda, A.; Ikeda, T.; Ichimura, S.; Ooe, K.; Nagata, K.; Yoshimura, T.; Shinohara, A. Isolation of At-211 by Dry-Distillation under Oxidative Conditions for Targeted Alpha Therapy in Osaka University. J. Med. Imaging Radiat. Sci. 2019, 50, S76–S77. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Feng, Y.; Zhao, X.-G.; Vaidyanathan, G.; Zalutsky, M.R.; Vo-Dinh, T. Gold Nanostars: A Novel Platform for Developing 211At-Labeled Agents for Targeted Alpha-Particle Therapy. Int. J. Nanomed. 2021, 16, 7297–7305. [Google Scholar] [CrossRef]
- Dziawer, Ł.; Majkowska-Pilip, A.; Gaweł, D.; Godlewska, M.; Pruszyński, M.; Jastrzębski, J.; Wąs, B.; Bilewicz, A. Trastuzumab-Modified Gold Nanoparticles Labeled with 211At as a Prospective Tool for Local Treatment of HER2-Positive Breast Cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef] [Green Version]
- Stylianopoulos, T. EPR-Effect: Utilizing Size-Dependent Nanoparticle Delivery to Solid Tumors. Ther. Deliv. 2013, 4, 421–423. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The Entry of Nanoparticles into Solid Tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A Review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR Effect: Unique Features of Tumor Blood Vessels for Drug Delivery, Factors Involved, and Limitations and Augmentation of the Effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of Enhanced Permeability and Retention Effect (EPR): Nanoparticle-Based Precision Tools for Targeting of Therapeutic and Diagnostic Agent in Cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Sykes, E.A.; Dai, Q.; Sarsons, C.D.; Chen, J.; Rocheleau, J.V.; Hwang, D.M.; Zheng, G.; Cramb, D.T.; Rinker, K.D.; Chan, W.C.W. Tailoring Nanoparticle Designs to Target Cancer Based on Tumor Pathophysiology. Proc. Natl. Acad. Sci. USA 2016, 113, E1142–E1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chongsathidkiet, P.; Crawford, B.M.; Odion, R.; Dechant, C.A.; Kemeny, H.R.; Cui, X.; Maccarini, P.F.; Lascola, C.D.; Fecci, P.E.; et al. Plasmonic Gold Nanostar-Mediated Photothermal Immunotherapy for Brain Tumor Ablation and Immunologic Memory. Immunotherapy 2019, 11, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomedicine 2018, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aldahhan, B.; Almohazey, D.; Khan, F.A. Emerging trends in the application of gold nanoformulations in colon cancer diagnosis and treatment. Semin. Cancer Biol. 2022, 86, 1056–1065. [Google Scholar] [CrossRef]
- Krikas, A.; Neofytou, P.; Gakis, G.P.; Xiarchos, I.; Charitidis, C.; Tran, L. Modeling of clearance, retention, and translocation of inhaled gold nanoparticles in rats. Inhal. Toxicol. 2022, 34, 361–379. [Google Scholar] [CrossRef]
- Liu, X.; Huang, N.; Li, H.; Jin, Q.; Ji, J. Surface and Size Effects on Cell Interaction of Gold Nanoparticles with Both Phagocytic and Nonphagocytic Cells. Langmuir 2013, 29, 9138–9148. [Google Scholar] [CrossRef]
- Lee, E.; Jeon, H.; Lee, M.; Ryu, J.; Kang, C.; Kim, S.; Jung, J.; Kwon, Y. Molecular Origin of AuNPs-Induced Cytotoxicity and Mechanistic Study. Sci. Rep. 2019, 9, 2494. [Google Scholar] [CrossRef] [Green Version]
- Miao, Z.; Gao, Z.; Chen, R.; Yu, X.; Su, Z.; Wei, G. Surface-bioengineered Gold Nanoparticles for Biomedical Applications. Curr. Med. Chem. 2018, 25, 1920–1944. [Google Scholar] [CrossRef]
- Escudero-Francos, M.A.; Cepas, V.; González-Menédez, P.; Badía-Laíño, R.; Díaz-García, M.E.; Sainz, R.M.; Mayo, J.C.; Hevia, D. Cellular Uptake and Tissue Biodistribution of Functionalized Gold Nanoparticles and Nanoclusters. J. Biomed. Nanotechnol. 2017, 13, 167–179. [Google Scholar] [CrossRef]
- Barani, M.; Sabir, F.; Rahdar, A.; Arshad, R.; Kyzas, G.Z. Nanotreatment and Nanodiagnosis of Prostate Cancer: Recent Updates. Nanomaterials 2020, 10, 1696. [Google Scholar] [CrossRef]
- Kaur, H.; Pujari, G.; Semwal, M.K.; Sarma, A.; Avasthi, D.K. In vitro studies on radiosensitization effect of glucose capped gold nanoparticles in photon and ion irradiation of HeLa cells. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2013, 301, 7–11. [Google Scholar] [CrossRef]
- Chen, J.; Hu, C.; Niestroj, M.; Yuan, D.; Chang, S. Treating cancer stem cells and cancer metastasis using glucose-coated gold nanoparticles. Int. J. Nanomed. 2015, 10, 2065–2077. [Google Scholar] [CrossRef] [Green Version]
- Bogdanov, A.A., Jr.; Gupta, S.; Koshkin, N.; Corr, S.J.; Zhang, S.; Curley, S.A.; Han, G. Gold Nanoparticles Stabilized with MPEG-Grafted Poly(l-lysine): In Vitro and in Vivo Evaluation of a Potential Theranostic Agent. Bioconjug. Chem. 2015, 26, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Zhang, F.; Qi, X.; Yang, Y.; Yan, C.; Jiang, J.; Deng, J. Chiral polymer modified nanoparticles selectively induce autophagy of cancer cells for tumor ablation. J. Nanobiotechnol. 2018, 16, 55. [Google Scholar] [CrossRef] [Green Version]
- El-Safoury, D.M.; Ibrahim, A.B.; El-Setouhy, D.A.; Khowessah, O.M.; Motaleb, M.A.; Sakr, T.M. Amelioration of Tumor Targeting and In Vivo Biodistribution of 99mTc-Methotrexate-Gold Nanoparticles (99mTc-Mex-AuNPs). J. Pharm. Sci. 2021, 110, 2955–2965. [Google Scholar] [CrossRef]
- Gupta, A.; Mathur, R.; Singh, S.; Bag, N.; Khan, U.A.; Ahmad, F.J.; Gabr, G.A.; Kesharwani, P.; Jain, G.K. 99mTc-Methionine Gold Nanoparticles as a Promising Biomaterial for Enhanced Tumor Imaging. J. Pharm. Sci. 2021, 110, 888–897. [Google Scholar] [CrossRef]
- Jang, C.; Lee, J.H.; Sahu, A.; Tae, G. The Synergistic Effect of Folate and RGD Dual Ligand of Nanographene Oxide on Tumor Targeting and Photothermal Therapy in Vivo. Nanoscale 2015, 7, 18584–18594. [Google Scholar] [CrossRef]
- Goodman, T.T.; Ng, C.P.; Pun, S.H. 3-D Tissue Culture Systems for the Evaluation and Optimization of Nanoparticle-Based Drug Carriers. Bioconjug. Chem. 2008, 19, 1951–1959. [Google Scholar] [CrossRef]


| 3 h | 24 h | |||
|---|---|---|---|---|
| 5 nm 211At-AuNPs@mPEG | 30 nm 211At-AuNPs@mPEG | 5 nm 211At-AuNPs@mPEG | 30 nm 211At-AuNPs@mPEG | |
| Thyroid | 12.12 ± 5.69 | 2.61 ± 0.39 | 4.560 ± 1.05 | 6.98 ± 2.33 |
| Liver | 2.88 ± 0.82 | 5.87 ± 3.79 | 2.18 ± 0.37 | 2.08 ± 1.16 |
| Stomach | 11.73 ± 5.22 | 2.03 ± 0.56 | 2.45 ± 0.60 | 4.71 ± 2.19 |
| Small intestine | 2.10 ± 0.76 | 0.85 ± 0.13 | 0.81 ± 0.09 | 0.74 ± 0.23 |
| Colon | 1.36 ± 0.41 # | 0.34 ± 0.02 | 0.88 ± 0.18 | 0.67 ± 0.24 |
| Kidney | 2.80 ± 0.58 # | 1.27 ± 0.21 | 1.90 ± 0.26 | 1.17 ± 0.38 |
| Blood | 7.06 ± 0.88 *### | 0.59 ± 0.04 | 2.60 ± 0.54 # | 0.57 ± 0.20 |
| Urine | 8.99 ± 3.23 | 3.73 ± 1.67 | 14.68 ± 1.97 # | 14.90 ± 3.72 |
| Tumor | 2.25 ± 0.67 | 1.29 ± 0.17 | 1.80 ± 0.20 # | 0.85 ± 0.21 |
| 3 h | 24 h | |||
|---|---|---|---|---|
| 5 nm 211At-AuNPs@mPEG | 30 nm 211At-AuNPs@mPEG | 5 nm 211At-AuNPs@mPEG | 30 nm 211At-AuNPs@mPEG | |
| Thyroid | 0.67 ± 0.26 | 0.19 ± 0.02 | 0.31 ± 0.05 | 0.42 ± 0.12 |
| Liver | 3.97 ± 1.17 | 7.61 ± 5.22 | 2.68 ± 0.41 | 2.70 ± 1.42 |
| Stomach | 3.96 ± 2.00 | 0.52 ± 0.17 | 0.64 ± 0.19 | 1.23 ± 0.51 |
| Small intestine | 1.93 ± 0.73 | 0.82 ± 0.12 | 0.86 ± 0.10 | 0.81 ± 0.18 |
| Colon | 0.70 ± 0.39 | 0.08 ± 0.02 | 0.23 ± 0.06 | 0.14 ± 0.03 |
| Kidney | 1.23 ± 0.25 # | 0.55 ± 0.08 | 0.81 ± 0.10 | 0.48 ± 0.14 |
| Blood | 1.79 ± 0.43 # | 0.30 ± 0.09 | 1.26 ± 0.20 | 0.29 ± 0.07 ## |
| Urine | 0.57 ± 0.22 | 0.63 ± 0.36 | 2.24 ± 0.58 * | 0.14 ± 0.11 # |
| Tumor | 2.03 ± 0.62 | 1.45 ± 0.21 | 1.92 ± 0.77 | 0.91 ± 0.44 |
| 3 h | 24 h | |||
|---|---|---|---|---|
| 5 nm 211At-AuNPs@H16 | 5 nm 211At-AuNPs@H16/RGD | 5 nm 211At-AuNPs@H16 | 5 nm 211At-AuNPs@H16/RGD | |
| Thyroid | 3.64 ± 1.38 | 3.29 ± 1.29 | 3.34 ± 0.47 | 2.60 ± 0.51 |
| Liver | 17.83 ± 8.54 | 16.02 ± 7.08 * | 16.91 ± 6.48 | 41.59 ± 2.20 # |
| Stomach | 3.74 ± 1.19 | 3.89 ± 1.23 | 1.73 ± 0.20 | 2.45 ± 0.56 |
| Small intestine | 0.85 ± 0.28 | 1.11 ± 0.31 | 0.46 ± 0.06 | 1.01 ± 0.07 ### |
| Colon | 0.68 ± 0.19 | 0.67 ± 0.15 | 0.43 ± 0.11 | 0.72 ± 0.10 |
| Kidney | 1.74 ± 0.46 | 1.64 ± 0.56 | 0.85 ± 0.12 | 2.09 ± 0.28 ### |
| Blood | 6.91 ± 1.48 ** | 7.44 ± 1.59 ** | 0.64 ± 0.11 | 0.44 ± 0.05 |
| Urine | 12.98 ± 5.97 | 8.04 ± 1.79 ** | 4.17 ± 1.29 | 0.44 ± 0.43 # |
| Tumor | 1.36 ± 0.44 | 2.34 ± 0.94 | 1.31 ± 0.27 | 2.07 ± 0.47 |
| 3 h | 24 h | |||
|---|---|---|---|---|
| 5 nm 211At-AuNPs@H16 | 5 nm 211At-AuNPs@H16/RGD | 5 nm 211At-AuNPs@H16 | 5 nm 211At-AuNPs@H16/RGD | |
| Thyroid | 0.23 ± 0.09 | 0.17 ± 0.06 | 0.17 ± 0.02 | 0.15 ± 0.05 |
| Liver | 23.32 ± 10.85 | 20.73 ± 8.52 | 22.09 ± 7.91 | 44.26 ± 1.78 *# |
| Stomach | 1.45 ± 0.41 | 1.54 ± 0.46 | 0.66 ± 0.10 | 0.83 ± 0.15 |
| Small intestine | 1.18 ± 0.39 | 1.34 ± 0.39 | 0.53 ± 0.08 | 1.08 ± 0.11 ## |
| Colon | 0.13 ± 0.04 | 0.19 ± 0.07 | 0.14 ± 0.06 | 0.17 ± 0.01 |
| Kidney | 0.69 ± 0.18 | 0.67 ± 0.26 | 0.34 ± 0.04 | 0.69 ± 0.10 # |
| Blood | 2.91 ± 0.41 | 3.79 ± 0.64 | 0.25 ± 0.03 *** | 0.23 ± 0.03 ** |
| Urine | 1.27 ± 0.85 | 0.42 ± 0.16 | 0.09 ± 0.03 | 0.53 ± 0.17 # |
| Tumor | 0.28 ± 0.14 | 0.45 ± 0.19 | 0.23 ± 0.09 | 0.15 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Kaneda-Nakashima, K.; Kadonaga, Y.; Kabayama, K.; Shimoyama, A.; Ooe, K.; Kato, H.; Toyoshima, A.; Shinohara, A.; Haba, H.; et al. Astatine-211-Labeled Gold Nanoparticles for Targeted Alpha-Particle Therapy via Intravenous Injection. Pharmaceutics 2022, 14, 2705. https://doi.org/10.3390/pharmaceutics14122705
Huang X, Kaneda-Nakashima K, Kadonaga Y, Kabayama K, Shimoyama A, Ooe K, Kato H, Toyoshima A, Shinohara A, Haba H, et al. Astatine-211-Labeled Gold Nanoparticles for Targeted Alpha-Particle Therapy via Intravenous Injection. Pharmaceutics. 2022; 14(12):2705. https://doi.org/10.3390/pharmaceutics14122705
Chicago/Turabian StyleHuang, Xuhao, Kazuko Kaneda-Nakashima, Yuichiro Kadonaga, Kazuya Kabayama, Atsushi Shimoyama, Kazuhiro Ooe, Hiroki Kato, Atsushi Toyoshima, Atsushi Shinohara, Hiromitsu Haba, and et al. 2022. "Astatine-211-Labeled Gold Nanoparticles for Targeted Alpha-Particle Therapy via Intravenous Injection" Pharmaceutics 14, no. 12: 2705. https://doi.org/10.3390/pharmaceutics14122705