Enhanced Nasal Deposition and Anti-Coronavirus Effect of Favipiravir-Loaded Mucoadhesive Chitosan–Alginate Nanoparticles
Abstract
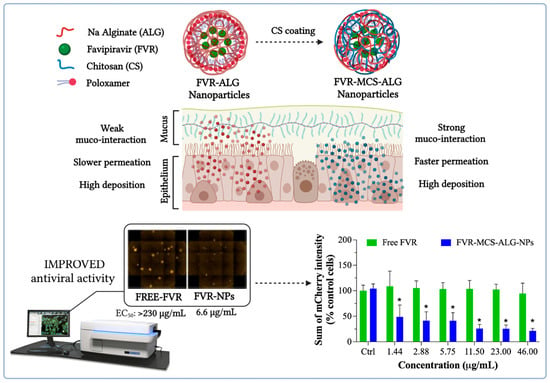
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Fabrication and Optimization by BBD and RSM
2.3. Physicochemical Characterization
2.4. Mucoadhesion Study
In Vitro Mucin Adsorption Studies
2.5. In Vitro FVR Release Study
2.6. Ex Vivo Transmucosal Studies
2.7. Biocompatibility Studies
2.8. Viral Infectivity
2.9. Statistical Analysis
3. Results
3.1. Analysis and Selection of Optimal Mathematical Model
3.2. Optimization and Validation
3.3. Characterization of Optimized FVR-MCS-ALG-NPs
3.3.1. Physicochemical Characterization
3.3.2. Mucoadhesive Study of FVR-MCS-ALG-NPs
3.3.3. In Vitro Release Study of FVR from FVR-MCS-ALG-NPs Using SnakeSkin™ Artificial Membrane
3.4. Nasal Mucosa Permeation and Retention Studies
3.5. Biocompatibility Study in RMPI 2650 Human Nasal Epithelia and Porcine Nasal Mucosa
3.6. Antiviral Activity of FVR against PEDV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yesudhas, D.; Srivastava, A.; Gromiha, M.M. COVID-19 outbreak: History, mechanism, transmission, structural studies and therapeutics. Infection 2021, 49, 199–213. [Google Scholar] [CrossRef]
- Malik, J.A.; Ahmed, S.; Mir, A.; Shinde, M.; Bender, O.; Alshammari, F.; Ansari, M.; Anwar, S. The SARS-CoV-2 mutations versus vaccine effectiveness: New opportunities to new challenges. J. Infect. Public Health 2022, 15, 228–240. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Agoramoorthy, G.; Lee, S.S. The Drug Repurposing for COVID-19 Clinical Trials Provide Very Effective Therapeutic Combinations: Lessons Learned From Major Clinical Studies. Front. Pharmacol. 2021, 12, 704205. [Google Scholar] [CrossRef]
- Hashemian, S.M.R.; Pourhanifeh, M.H.; Hamblin, M.R.; Shahrzad, M.K.; Mirzaei, H. RdRp inhibitors and COVID-19: Is molnupiravir a good option? Biomed. Pharmacother. 2022, 146, 112517. [Google Scholar] [CrossRef]
- Chen, R.; Wang, T.; Song, J.; Pu, D.; He, D.; Li, J.; Yang, J.; Li, K.; Zhong, C.; Zhang, J. Antiviral Drug Delivery System for Enhanced Bioactivity, Better Metabolism and Pharmacokinetic Characteristics. Int. J. Nanomed. 2021, 16, 4959–4984. [Google Scholar] [CrossRef]
- Thakur, V.; Ratho, R.K.; Panda, J.J. Respiratory delivery of favipiravir-tocilizumab combination through mucoadhesive protein-lipidic nanovesicles: Prospective therapeutics against COVID-19. Virusdisease 2021, 32, 132–136. [Google Scholar] [CrossRef]
- Moshikur, R.M.; Ali, M.K.; Wakabayashi, R.; Moniruzzaman, M.; Goto, M. Favipiravir-Based Ionic Liquids as Potent Antiviral Drugs for Oral Delivery: Synthesis, Solubility, and Pharmacokinetic Evaluation. Mol. Pharm. 2021, 18, 3108–3115. [Google Scholar] [CrossRef]
- Abd Elkodous, M.; Olojede, S.O.; Morsi, M.; El-Sayyad, G.S. Nanomaterial-based drug delivery systems as promising carriers for patients with COVID-19. RSC Adv. 2021, 11, 26463–26480. [Google Scholar] [CrossRef]
- Tulbah, A.S.; Lee, W.H. Physicochemical Characteristics and In Vitro Toxicity/Anti-SARS-CoV-2 Activity of Favipiravir Solid Lipid Nanoparticles (SLNs). Pharmaceuticals 2021, 14, 1059. [Google Scholar] [CrossRef]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef]
- Higgins, T.S.; Wu, A.W.; Illing, E.A.; Sokoloski, K.J.; Weaver, B.A.; Anthony, B.P.; Hughes, N.; Ting, J.Y. Intranasal Antiviral Drug Delivery and Coronavirus Disease 2019 (COVID-19): A State of the Art Review. Otolaryngol. Head Neck Surg. 2020, 163, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Domenico, M.B.D.; Collares, K.; Santos, R.B.D.; Lenz, U.; Antunes, V.P.; Godinho, V.W.; Cesca, H.; Ponciano, T.H.J.; Corazza, P.H. Hydrogen peroxide as an auxiliary treatment for COVID-19 in Brazil: A randomized double-blind clinical trial. Epidemiol. Health 2021, 43, e2021051. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.J.; Rumi, S.; Banu, S.S.; Uddin, A.; Islam, M.S.; Arefin, M.K. Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: A structured summary of a study protocol for an open-label randomized clinical trial. Trials 2021, 22, 2. [Google Scholar] [CrossRef]
- Watchorn, J.; Clasky, A.J.; Prakash, G.; Johnston, I.A.E.; Chen, P.Z.; Gu, F.X. Untangling Mucosal Drug Delivery: Engineering, Designing, and Testing Nanoparticles to Overcome the Mucus Barrier. ACS Biomater. Sci. Eng. 2022, 8, 1396–1426. [Google Scholar] [CrossRef]
- Truong, T.H.; Alcantara, K.P.; Bulatao, B.P.I.; Sorasitthiyanukarn, F.N.; Muangnoi, C.; Nalinratana, N.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-coated nanostructured lipid carriers for transdermal delivery of tetrahydrocurcumin for breast cancer therapy. Carbohydr. Polym. 2022, 288, 119401. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Pyrc, K.; Milewska, A.; Duran, E.B.; Botwina, P.; Dabrowska, A.; Jedrysik, M.; Benedyk, M.; Lopes, R.; Arenas-Pinto, A.; Badr, M.; et al. SARS-CoV-2 inhibition using a mucoadhesive, amphiphilic chitosan that may serve as an anti-viral nasal spray. Sci. Rep. 2021, 11, 20012. [Google Scholar] [CrossRef]
- Hussein, N.; Omer, H.; Ismael, A.; Albed Alhnan, M.; Elhissi, A.; Ahmed, W. Spray-dried alginate microparticles for potential intranasal delivery of ropinirole hydrochloride: Development, characterization and histopathological evaluation. Pharm. Dev. Technol. 2020, 25, 290–299. [Google Scholar] [CrossRef]
- Serrano-Aroca, A.; Takayama, K.; Tunon-Molina, A.; Seyran, M.; Hassan, S.S.; Pal Choudhury, P.; Uversky, V.N.; Lundstrom, K.; Adadi, P.; Palu, G.; et al. Carbon-Based Nanomaterials: Promising Antiviral Agents to Combat COVID-19 in the Microbial-Resistant Era. ACS Nano 2021, 15, 8069–8086. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan oligosaccharide/alginate nanoparticles as an effective carrier for astaxanthin with improving stability, in vitro oral bioaccessibility, and bioavailability. Food Hydrocoll. 2022, 124, 107246. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Thaweesest, W.; Rojsitthisak, P.; Rojsitthisak, P. Enhanced cytotoxic, antioxidant and anti-inflammatory activities of curcumin diethyl disuccinate using chitosan-tripolyphosphate nanoparticles. J. Drug Deliv. Sci. Technol. 2019, 53, 101118. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 705–718. [Google Scholar] [CrossRef] [Green Version]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Tummino, M.L.; Magnacca, G.; Cimino, D.; Laurenti, E.; Nistico, R. The Innovation Comes from the Sea: Chitosan and Alginate Hybrid Gels and Films as Sustainable Materials for Wastewater Remediation. Int. J. Mol. Sci. 2020, 21, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, L.; Yao, C.; Xie, G.; Song, S.; Li, H.; Qu, Y.; Tao, X. Novel Formulations of the Antiviral Drug Favipiravir: Improving Permeability and Tabletability. Cryst. Growth Des. 2021, 21, 3807–3817. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Yuksel, N.; Kanık, A.E.; Baykara, T. Comparison of in vitro dissolution profiles by ANOVA-based, model-dependent and -independent methods. Int. J. Pharm. 2000, 209, 57–67. [Google Scholar] [CrossRef]
- Heredia, N.S.; Vizuete, K.; Flores-Calero, M.; Pazmino, V.K.; Pilaquinga, F.; Kumar, B.; Debut, A. Comparative statistical analysis of the release kinetics models for nanoprecipitated drug delivery systems based on poly(lactic-co-glycolic acid). PLoS ONE 2022, 17, e0264825. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising carrier of novel curcumin diethyl diglutarate. Int. J. Biol. Macromol. 2019, 131, 1125–1136. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef]
- He, J.; Zhong, C.; Mi, J. Modeling of drug release from bioerodible polymer matrices. Drug Deliv. 2005, 12, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Thai, H.; Thuy Nguyen, C.; Thi Thach, L.; Thi Tran, M.; Duc Mai, H.; Thi Thu Nguyen, T.; Duc Le, G.; Van Can, M.; Dai Tran, L.; Long Bach, G.; et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef] [Green Version]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.; Hwang, J.; Lee, H.; Hammond, P.T.; Choi, J.; Hong, J. In vitro blood cell viability profiling of polymers used in molecular assembly. Sci. Rep. 2017, 7, 9481. [Google Scholar] [CrossRef]
- Pozzoli, M.; Ong, H.X.; Morgan, L.; Sukkar, M.; Traini, D.; Young, P.M.; Sonvico, F. Application of RPMI 2650 nasal cell model to a 3D printed apparatus for the testing of drug deposition and permeation of nasal products. Eur. J. Pharm. Biopharm. 2016, 107, 223–233. [Google Scholar] [CrossRef]
- Sibinovska, N.; Zakelj, S.; Kristan, K. Suitability of RPMI 2650 cell models for nasal drug permeability prediction. Eur. J. Pharm. Biopharm. 2019, 145, 85–95. [Google Scholar] [CrossRef]
- ISO. ISO 10993-5:2009 Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity; ISO, Vernier: Geneva, Switzerland, 2009. [Google Scholar]
- Singh, G.; Singh, P.; Pillatzki, A.; Nelson, E.; Webb, B.; Dillberger-Lawson, S.; Ramamoorthy, S. A Minimally Replicative Vaccine Protects Vaccinated Piglets Against Challenge With the Porcine Epidemic Diarrhea Virus. Front. Vet. Sci. 2019, 6, 347. [Google Scholar] [CrossRef]
- Sadique, M.A.; Yadav, S.; Ranjan, P.; Verma, S.; Salammal, S.T.; Khan, M.A.; Kaushik, A.; Khan, R. High-performance antiviral nano-systems as a shield to inhibit viral infections: SARS-CoV-2 as a model case study. J. Mater. Chem. B 2021, 9, 4620–4642. [Google Scholar] [CrossRef]
- San, H.H.M.; Alcantara, K.P.; Bulatao, B.P.I.; Chaichompoo, W.; Nalinratana, N.; Suksamrarn, A.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Development of Turmeric Oil-Loaded Chitosan/Alginate Nanocapsules for Cytotoxicity Enhancement against Breast Cancer. Polymers 2022, 14, 1835. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Wang, X.; Zhou, Y.; Gao, X.; Puligundla, P.; Wan, X. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017, 231, 19–24. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2021, 256, 117426. [Google Scholar] [CrossRef]
- Bruinsmann, F.A.; Pigana, S.; Aguirre, T.; Dadalt Souto, G.; Garrastazu Pereira, G.; Bianchera, A.; Tiozzo Fasiolo, L.; Colombo, G.; Marques, M.; Raffin Pohlmann, A.; et al. Chitosan-Coated Nanoparticles: Effect of Chitosan Molecular Weight on Nasal Transmucosal Delivery. Pharmaceutics 2019, 11, 86. [Google Scholar] [CrossRef] [Green Version]
- Gholizadeh, H.; Cheng, S.; Pozzoli, M.; Messerotti, E.; Traini, D.; Young, P.; Kourmatzis, A.; Ong, H.X. Smart thermosensitive chitosan hydrogel for nasal delivery of ibuprofen to treat neurological disorders. Expert Opin. Drug Deliv. 2019, 16, 453–466. [Google Scholar] [CrossRef]
- Jengarn, J.; Wongthida, P.; Wanasen, N.; Frantz, P.N.; Wanitchang, A.; Jongkaewwattana, A. Genetic manipulation of porcine epidemic diarrhoea virus recovered from a full-length infectious cDNA clone. J. Gen. Virol. 2015, 96, 2206–2218. [Google Scholar] [CrossRef]









| Variables | Levels | |
|---|---|---|
| Low | High | |
| Independent (Factors) | ||
| A = ALG:CS mass ratio | 1:0.0250 | 1:0.1000 |
| B = FVR (mg/mL) | 5 | 15 |
| C = Poloxamer-407 (% w/v) | 1 | 2 |
| Dependent (Responses) | Goals | |
| Y1 = size (nm) | Minimize | |
| Y2 = ζ-potential (mV) | ≥ ± 20 | |
| Y3 = LC (%) | Maximize | |
| Y4 = EE (%) | Maximize | |
| Code | A | B | C | Y1 (nm) | Y2 (mV) | Y3 (%) | Y4 (%) |
|---|---|---|---|---|---|---|---|
| F1 | 1: 0.0250 | 5 | 1.5 | 227 ± 6 | −21.9 ± 0.6 | 5.0 ± 0.9 | 33.7 ± 5.6 |
| F2 | 1: 0.1000 | 5 | 1.5 | 289 ± 16 | −27.8 ± 0.4 | 7.4 ± 0.5 | 60.9 ± 5.0 |
| F3 | 1: 0.0250 | 15 | 1.5 | 239 ± 2 | −24.7 ± 0.5 | 12.1 ± 0.9 | 34.4 ± 3.5 |
| F4 | 1: 0.1000 | 15 | 1.5 | 284 ± 5 | −20.8 ± 0.6 | 24.3 ± 0.3 | 84.8 ± 0.7 |
| F5 | 1: 0.0250 | 10 | 1 | 243 ± 7 | −21.6 ± 0.9 | 11.4 ± 0.5 | 33.6 ± 0.3 |
| F6 | 1: 0.1000 | 10 | 1 | 288 ± 5 | −21.3 ± 2.0 | 15.4 ± 0.8 | 58.9 ± 0.8 |
| F7 | 1: 0.0250 | 10 | 2 | 231 ± 8 | −20.3 ± 0.3 | 7.4 ± 0.6 | 37.4 ± 3.8 |
| F8 | 1: 0.1000 | 10 | 2 | 274 ± 14 | −21.2 ± 1.1 | 12.0 ± 0.6 | 69.4 ± 0.9 |
| F9 | 1: 0.0625 | 5 | 1 | 258 ± 16 | −22.5 ± 1.2 | 11.9 ± 0.6 | 63.3 ± 0.3 |
| F10 | 1: 0.0625 | 15 | 1 | 281 ± 8 | −20.4 ± 0.7 | 23.4 ± 1.1 | 82.8 ± 2.9 |
| F11 | 1: 0.0625 | 5 | 2 | 245 ± 16 | −22.6 ± 2.3 | 7.3 ± 0.5 | 73.7 ± 4.3 |
| F12 | 1: 0.0625 | 15 | 2 | 248 ± 12 | −19.9 ± 1.0 | 21.5 ± 1.9 | 84.2 ± 3.3 |
| F13 * | 1: 0.0625 | 10 | 1.5 | 258 ± 5 | −21.4 ± 0.7 | 22.0 ± 0.8 | 90.7 ± 1.0 |
| F14 * | 1: 0.0625 | 10 | 1.5 | 260 ± 7 | −22.0 ± 2.8 | 22.6 ± 2.9 | 87.1 ± 13.3 |
| F15 * | 1: 0.0625 | 10 | 1.5 | 251 ± 14 | −21.2 ± 3.2 | 21.9 ± 0.9 | 89.8 ± 0.8 |
| Optimal Conditions | Responses | Predicted Responses | Observed Responses | % Error |
|---|---|---|---|---|
| A: 1: 0.057 (ALG:CS mass ratio) | Y1 (nm) | 261.8 | 233.5 ± 7.7 | –10.7 |
| B: 12.871 (mg/mL) | Y2 (mV) | –21.2 | –21.6 ± 0.8 | 1.8 |
| C: 1.24 (% w/v) | Y3 (%) | 24.0 | 26.0 ± 0.7 | 8.5 |
| Y4 (%) | 84.1 | 84.6 ± 0.7 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcantara, K.P.; Nalinratana, N.; Chutiwitoonchai, N.; Castillo, A.L.; Banlunara, W.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Enhanced Nasal Deposition and Anti-Coronavirus Effect of Favipiravir-Loaded Mucoadhesive Chitosan–Alginate Nanoparticles. Pharmaceutics 2022, 14, 2680. https://doi.org/10.3390/pharmaceutics14122680
Alcantara KP, Nalinratana N, Chutiwitoonchai N, Castillo AL, Banlunara W, Vajragupta O, Rojsitthisak P, Rojsitthisak P. Enhanced Nasal Deposition and Anti-Coronavirus Effect of Favipiravir-Loaded Mucoadhesive Chitosan–Alginate Nanoparticles. Pharmaceutics. 2022; 14(12):2680. https://doi.org/10.3390/pharmaceutics14122680
Chicago/Turabian StyleAlcantara, Khent Primo, Nonthaneth Nalinratana, Nopporn Chutiwitoonchai, Agnes L. Castillo, Wijit Banlunara, Opa Vajragupta, Pornchai Rojsitthisak, and Pranee Rojsitthisak. 2022. "Enhanced Nasal Deposition and Anti-Coronavirus Effect of Favipiravir-Loaded Mucoadhesive Chitosan–Alginate Nanoparticles" Pharmaceutics 14, no. 12: 2680. https://doi.org/10.3390/pharmaceutics14122680