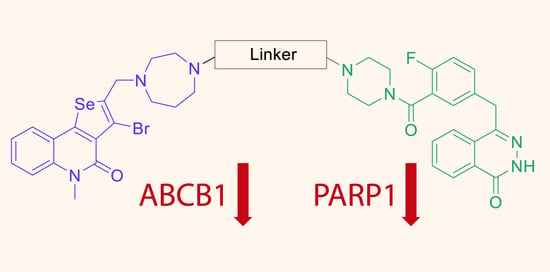
Olaparib Conjugates with Selenopheno[3,2-c]quinolinone Inhibit PARP1 and Reverse ABCB1-Related Multidrug Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental
2.2. General Procedure for the Preparation of Selenopheno[3,2-c]quinolinones 2–4
2.2.1. 4-(4-((3-Bromo-5-methyl-4-oxo-4,5-dihydroselenopheno[3,2-c]quinolin-2-yl) methyl)-1,4-diazepan-1-yl)-4-oxobutanoic Acid (2)
2.2.2. 10-(4-((3-Bromo-5-methyl-4-oxo-4,5-dihydroselenopheno[3,2-c]quinolin-2-yl) methyl)-1,4-diazepan-1-yl)-10-oxodecanoic Acid (3)
2.2.3. 12-(4-((3-Bromo-5-methyl-4-oxo-4,5-dihydroselenopheno[3,2-c]quinolin-2-yl) methyl)-1,4-diazepan-1-yl)-12-oxododecanoic Acid (4)
2.3. General Procedure for the Preparation of Selenopheno[3,2-c]quinolinones 5a–c
2.3.1. 1-(4-((3-Bromo-5-methyl-4-oxo-4,5-dihydroselenopheno[3,2-c]quinolin-2-yl) methyl)-1,4-diazepan-1-yl)-4-(4-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoyl)piperazin-1-yl)butane-1,4-dione (5a)
2.3.2. 1-(4-((3-Bromo-5-methyl-4-oxo-4,5-dihydroselenopheno[3,2-c]quinolin-2-yl) methyl)-1,4-diazepan-1-yl)-10-(4-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoyl)piperazin-1-yl)decane-1,10-dione (5b)
2.3.3. 1-(4-((3-Bromo-5-methyl-4-oxo-4,5-dihydroselenopheno[3,2-c]quinolin-2-yl) methyl)-1,4-diazepan-1-yl)-12-(4-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoyl)piperazin-1-yl)dodecane-1,12-dione (5c)
2.4. Cytotoxicity Assay
2.5. Rhodamine-123 Accumulation Assay
2.6. Statistical Analysis
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tung, N.; Garber, J.E. PARP Inhibition in Breast Cancer: Progress Made and Future Hopes. npj Breast Cancer 2022, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Lord, C.J.; Serra, V.; Tutt, A.; Balmaña, J.; Castroviejo-Bermejo, M.; Cruz, C.; Oaknin, A.; Kaye, S.B.; De Bono, J.S. A Decade of Clinical Development of PARP Inhibitors in Perspective. Ann. Oncol. 2019, 30, 1437–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noordermeer, S.M.; van Attikum, H. PARP Inhibitor Resistance: A Tug-of-War in BRCA-Mutated Cells. Trends Cell Biol. 2019, 29, 820–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flippot, R.; Patrikidou, A.; Aldea, M.; Colomba, E.; Lavaud, P.; Albigès, L.; Naoun, N.; Blanchard, P.; Terlizzi, M.; Garcia, C.; et al. PARP Inhibition, a New Therapeutic Avenue in Patients with Prostate Cancer. Drugs 2022, 82, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Haddad, G.; Saade, M.C.; Eid, R.; Haddad, F.G.; Kourie, H.R. PARP Inhibitors: A Tsunami of Indications in Different Malignancies. Pharmacogenomics 2020, 21, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Karihtala, P.; Moschetta, M.; Karathanasi, A.; Sadauskaite, A.; Rassy, E.; Pavlidis, N. Combined Strategies with Poly (ADP-Ribose) Polymerase (PARP) Inhibitors for the Treatment of Ovarian Cancer: A Literature Review. Diagnostics 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidyanathan, A.; Sawers, L.; Gannon, A.L.; Chakravarty, P.; Scott, A.L.; Bray, S.E.; Ferguson, M.J.; Smith, G. ABCB1 (MDR1) Induction Defines a Common Resistance Mechanism in Paclitaxel- and Olaparib-Resistant Ovarian Cancer Cells. Br. J. Cancer 2016, 115, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.P.; Moser, S.C.; Ganesan, S.; Jonkers, J. Understanding and Overcoming Resistance to PARP Inhibitors in Cancer Therapy. Nat. Rev. Clin. Oncol. 2021, 18, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Němcová-Fürstová, V.; Kopperová, D.; Balušíková, K.; Ehrlichová, M.; Brynychová, V.; Václavíková, R.; Daniel, P.; Souček, P.; Kovář, J. Characterization of Acquired Paclitaxel Resistance of Breast Cancer Cells and Involvement of ABC Transporters. Toxicol. Appl. Pharmacol. 2016, 310, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the Role of ABC Transporters in Multidrug-Resistant Cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Lombard, A.P.; Liu, C.; Armstrong, C.M.; D’Abronzo, L.S.; Lou, W.; Chen, H.; Dall’Era, M.; Ghosh, P.M.; Evans, C.P.; Gao, A.C. Overexpressed ABCB1 Induces Olaparib-Taxane Cross-Resistance in Advanced Prostate Cancer. Transl. Oncol. 2019, 12, 871–878. [Google Scholar] [CrossRef]
- Lheureux, S.; Oaknin, A.; Garg, S.; Bruce, J.P.; Madariaga, A.; Dhani, N.C.; Bowering, V.; White, J.; Accardi, S.; Tan, Q.; et al. EVOLVE: A Multicenter Open-Label Single-Arm Clinical and Translational Phase II Trial of Cediranib plus Olaparib for Ovarian Cancer after PARP Inhibition Progression. Clin. Cancer Res. 2020, 26, 4206–4215. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.I.; Tseng, Y.J.; Chen, M.H.; Huang, C.Y.F.; Chang, P.M.H. Clinical Perspective of FDA Approved Drugs With P-Glycoprotein Inhibition Activities for Potential Cancer Therapeutics. Front. Oncol. 2020, 10, 561936. [Google Scholar] [CrossRef] [PubMed]
- Dalton, W.S.; Crowley, J.J.; Salmon, S.S.; Grogan, T.M.; Laufman, L.R.; Weiss, G.R.; Bonnet, J.D. A Phase III Randomized Study of Oral Verapamil as a Chemosensitizer to Reverse Drug Resistance in Patients with Refractory Myeloma. A Southwest Oncology Group Study. Cancer 1995, 75, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Cripe, L.D.; Uno, H.; Paietta, E.M.; Litzow, M.R.; Ketterling, R.P.; Bennett, J.M.; Rowe, J.M.; Lazarus, H.M.; Luger, S.; Tallman, M.S. Zosuquidar, a Novel Modulator of P-Glycoprotein, Does Not Improve the Outcome of Older Patients with Newly Diagnosed Acute Myeloid Leukemia: A Randomized, Placebo-Controlled Trial of the Eastern Cooperative Oncology Group 3999. Blood 2010, 116, 4077–4085. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Martins-Teixeira, M.B.; Carvalho, I. Antitumour Anthracyclines: Progress and Perspectives. ChemMedChem 2020, 15, 933–948. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.H.; Chen, Z.S. Overcoming ABC Transporter-Mediated Multidrug Resistance: Molecular Mechanisms and Novel Therapeutic Drug Strategies. Drug Resist. Updat. 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Park, H.J.; Bae, J.S.; Kim, K.M.; Moon, Y.J.; Park, S.H.; Ha, S.H.; Hussein, U.K.; Zhang, Z.; Park, H.S.; Park, B.H.; et al. The PARP Inhibitor Olaparib Potentiates the Effect of the DNA Damaging Agent Doxorubicin in Osteosarcoma. J. Exp. Clin. Cancer Res. 2018, 37, 1–15. [Google Scholar] [CrossRef]
- Perez-Fidalgo, J.A.; Cortés, A.; Guerra, E.; García, Y.; Iglesias, M.; Bohn Sarmiento, U.; Calvo García, E.; Manso Sánchez, L.; Santaballa, A.; Oaknin, A.; et al. Olaparib in Combination with Pegylated Liposomal Doxorubicin for Platinum-Resistant Ovarian Cancer Regardless of BRCA Status: A GEICO Phase II Trial (ROLANDO Study). ESMO Open 2021, 6, 100212. [Google Scholar] [CrossRef]
- Spengler, G.; Gajdács, M.; Marć, M.A.; Domínguez-Álvarez, E.; Sanmartín, C. Organoselenium Compounds as Novel Adjuvants of Chemotherapy Drugs—A Promising Approach to Fight Cancer Drug Resistance. Molecules 2019, 24, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo, L.B.; Nogueira-Librelotto, D.R.; Mathes, D.; de Vargas, J.M.; da Rosa, R.M.; Rodrigues, O.E.D.; Vinardell, M.P.; Mitjans, M.; Rolim, C.M.B. Overcoming MDR by Associating Doxorubicin and PH-Sensitive PLGA Nanoparticles Containing a Novel Organoselenium Compound—An In Vitro Study. Pharmaceutics 2022, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Szemer, N.; Dobiasov, S.; Salard, N.; Habibullah, G.; Sevilla-hern, C.; Benito-lama, M.; Alonso-mart, F.; Viktorov, J.; Spengler, G. Cyano- and Ketone-Containing Selenoesters as Multi-Target Compounds against Resistant Cancers. Cancers 2021, 13, 4563. [Google Scholar] [CrossRef] [PubMed]
- Marć, M.A.; Domínguez-álvarez, E.; Latacz, G.; Doroz-Płonka, A.; Sanmartín, C.; Spengler, G.; Handzlik, J. Pharmaceutical and Safety Profile Evaluation of Novel Selenocompounds with Noteworthy Anticancer Activity. Pharmaceutics 2022, 14, 367. [Google Scholar] [CrossRef] [PubMed]
- Arsenyan, P.; Vasiljeva, J.; Shestakova, I.; Domracheva, I.; Belyakov, S. The Synthesis and Cytotoxic Properties of Selenopheno[3,2-c]- and Selenopheno-[2,3-c]Quinolones. Chem. Heterocycl. Compd. 2014, 49, 1674–1680. [Google Scholar] [CrossRef]
- Arsenyan, P.; Vasiljeva, J.; Domracheva, I.; Kanepe-Lapsa, I.; Gulbe, A. Selenopheno[2,3-:f] Coumarins: Novel Scaffolds with Antimetastatic Activity against Melanoma and Breast Cancer. New J. Chem. 2019, 43, 11851–11864. [Google Scholar] [CrossRef]
- Vasiljeva, J.; Makrecka-Kuka, M.; Domracheva, I.; Vilks, K.; Dimitrijevs, P.; Arsenyan, P. Development of Prospective Non-Toxic Inhibitors of ABCB1 Activity and Expression in a Series of Selenophenoquinolinones. New J. Chem 2022, 46, 7424–7432. [Google Scholar] [CrossRef]
- Reilly, S.W.; Puentes, L.N.; Wilson, K.; Hsieh, C.J.; Weng, C.C.; Makvandi, M.; Mach, R.H. Examination of Diazaspiro Cores as Piperazine Bioisosteres in the Olaparib Framework Shows Reduced DNA Damage and Cytotoxicity. J. Med. Chem. 2018, 61, 5367–5379. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Chen, S.; Sun, Q.; Wang, N.; Li, D.; Miao, S.; Gao, C.; Chen, Y.; Tan, C.; Jiang, Y. Olaparib Hydroxamic Acid Derivatives as Dual PARP and HDAC Inhibitors for Cancer Therapy. Bioorganic Med. Chem. 2017, 25, 4100–4109. [Google Scholar] [CrossRef]
- Ofori, S.; Awuah, S.G. Small-Molecule Poly(ADP-Ribose) Polymerase and PD-L1 Inhibitor Conjugates as Dual-Action Anticancer Agents. ACS Omega 2019, 4, 12584–12597. [Google Scholar] [CrossRef] [PubMed]



| Compounds | PARP1, µM | H9C2, IC50, µM | MES-SA, IC50, µM | MCF-7, IC50, µM | HCC1937, IC50, µM |
|---|---|---|---|---|---|
| Doxorubicin | - | 3.90 ± 0.60 | 0.084 ± 0.011 | 0.47 ± 0.14 | 1.08 ± 0.10 |
| Olaparib | 0.0035 ± 0.0001 | >300 | >100 | >100 | >100 |
| 1 | >1 | >300 | 21.0 ± 3.9 | 17.1 ± 1.9 | 19.9 ± 4.8 |
| 5a | 0.0046 ± 0.0005 | >300 | 7.1 ± 2.7 | >100 | 3.1 ± 1.0 |
| 5b | 0.0130 ± 0.0018 | >300 | 32.5 ± 1.6 | >100 | >100 |
| 5c | 0.1468 ± 0.0022 | >300 | >100 | >100 | >100 |
| Compounds | MES-SA/Dx5 | Doxorubicin | RF | |
|---|---|---|---|---|
| IC50, µM | IC20, µM | IC50, µM [a] | ||
| vehicle | - | - | 2.40 ± 0.41 | - |
| Olaparib | >100 | - | - | - |
| 1 [27] | 21.13 ± 2.49 | 7.96 ± 3.03 | 0.466 ± 0.077 | 4.72 ± 0.63 |
| 5a | 21.05 ± 3.94 | 0.480 ± 0.094 | 2.855 ± 0.286 | 0.84 ± 0.16 |
| 5b | 1.667 ± 0.030 | 0.860 ± 0.096 | 0.702 ± 0.162 | 3.42 ± 0.12 |
| 5c | 26.20 ± 0.03 | 0.549 ± 0.074 | 0.715 ± 0.175 | 3.36 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makrecka-Kuka, M.; Vasiljeva, J.; Dimitrijevs, P.; Arsenyan, P. Olaparib Conjugates with Selenopheno[3,2-c]quinolinone Inhibit PARP1 and Reverse ABCB1-Related Multidrug Resistance. Pharmaceutics 2022, 14, 2571. https://doi.org/10.3390/pharmaceutics14122571
Makrecka-Kuka M, Vasiljeva J, Dimitrijevs P, Arsenyan P. Olaparib Conjugates with Selenopheno[3,2-c]quinolinone Inhibit PARP1 and Reverse ABCB1-Related Multidrug Resistance. Pharmaceutics. 2022; 14(12):2571. https://doi.org/10.3390/pharmaceutics14122571
Chicago/Turabian StyleMakrecka-Kuka, Marina, Jelena Vasiljeva, Pavels Dimitrijevs, and Pavel Arsenyan. 2022. "Olaparib Conjugates with Selenopheno[3,2-c]quinolinone Inhibit PARP1 and Reverse ABCB1-Related Multidrug Resistance" Pharmaceutics 14, no. 12: 2571. https://doi.org/10.3390/pharmaceutics14122571