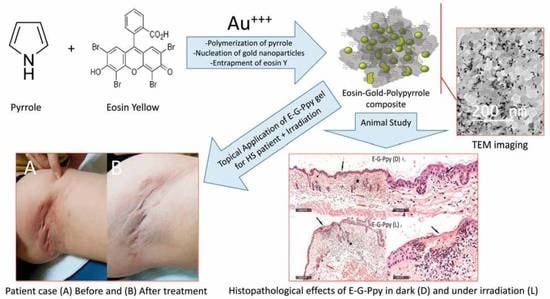
Gold-Polypyrrole-Loaded Eosin in Photo-Mediated Treatment of Hidradenitis Suppurativa: In Vivo Trans-Epidermal Permeation Study and Clinical Case Report
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Free and Eosin-Conjugated Gold-Polypyrrole Nanoparticles (G-Ppy and E-G-Ppy)
2.3. Characterization of G-Ppy NPs and E-G-Ppy NPs
2.4. Topical Hydrogel Preparations of E, G-Ppy NPs, and E-G-Ppy NPs
2.5. In Vivo Evaluation of Trans-Epidermal Permeation Following the Application of G-Ppy NPs and E-G-Ppy NP Hydrogels
2.6. Clinical Case Study
2.7. Treatment Protocol
3. Results
3.1. Preparation and Characterization of G-Ppy NPs and E-G-Ppy NPs
3.2. In Vivo Evaluation of Trans-Epidermal Permeation Following the Application of E, G-Ppy NPs and E-G-Ppy NP Hydrogels
3.3. Clinical Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mordon, S.J. Treating hidradenitis suppurativa with photodynamic therapy. Cosmet. Laser Ther. 2018, 20, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Valladares, M.J.S.; Salvado, N.E.; Prieto, M.A.R. Treatment of hidradenitis suppurativa with intralesional photodynamic therapy with 5-aminolevulinic acid and 630 nm laser beam. J. Dermatol. Sci. 2017, 85, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Paus, L.R.; Kurzen, H.; Kurokawa, I.; Jemec, G.B.E.; Emtestam, L.; Sellheyer, K.; Giamarellos-Bourboulis, E.J.; Nagy, I.; Bechara, F.G.; Sartorius, K.; et al. What causes hidradenitis suppurativa? Exp. Dermatol. 2008, 17, 455–456. [Google Scholar] [CrossRef]
- Fadel, M.A.; Tawfik, A.A. New topical photodynamic therapy for treatment of hidradenitis suppurativa using methylene blue niosomal gel: A single-blind, randomized, comparative study. Clin. Exp. Dermatol. 2015, 40, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-W.; Chen, X.-D.; Wu, X.-Y. 5-ALA PDT successfully treats facial hidradenitis suppurativa-induced severe hypertrophic scar. Photodiagn. Photodyn. Ther. 2019, 28, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Agut-Busquet, E.; Romaní, J.; Gilaberte, Y.; García-Malinis, A.; Ribera-Pibernat, M.; Luelmo, J. Photodynamic therapy with intralesional methylene blue and a 635 nm light-emitting diode lamp in hidradenitis suppurativa: A retrospective follow-up study in 7 patients and a review of the literature. Photochem. Photobiol. Sci. 2016, 15, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Maha, F.; Kawser, K.; Nevien, S.; Doaa, A.; Ghada, Y.; Nasr, M. Nanovesicular photodynamic clinical treatment of resistant plantar warts. Curr. Drug Deliv. 2020, 17, 396–405. [Google Scholar]
- Juarranz, Á.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Fadel, M.; Fadeel, D.A.; Ibrahim, M.; Hathout, R.M.; El-Kholy, A.I. One-step synthesis of polypyrrole-coated gold nanoparticles for use as a photothermally active nano-system. Int. J. Nanomed. 2020, 15, 2605–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kholy, A.I.; Fadeel, D.A.; Nasr, M.; El-Sherbiny, I.; Fadel, M. (Rose Bengal)/(Eosin Yellow)-Gold-Polypyrrole Hybrids: A Design for Dual Photo-Active Nano-System with Ultra-High Loading Capacity. Drug Des. Dev. Ther. 2021, 15, 5011–5023. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.A.; Fadeel, D.A.A.; Sadek, A.; Fadel, M.; Tawfik, A. Intralesional vitamin D3 versus new topical photodynamic therapy in recalcitrant palmoplanter warts Randomized comparative controlled study. Photodiagn. Photodyn. Ther. 2020, 32, 101979. [Google Scholar] [CrossRef] [PubMed]
- Shamali, N.; Preuß, A.; Saltsman, I.; Mahammed, A.; Gross, Z.; Däschlein, G.; Röder, B. In vitro photodynamic inactivation (PDI) of pathogenic germs inducing onychomycosis. Photodiagn. Photodyn. Ther. 2018, 24, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Fadel, M.; Fadeel, D.A.; Tawfik, A.; El-Kholy, A.I.; Mosaad, Y.O. Rose Bengal-gold-polypyrrole nanoparticles as a photothermal/photodynamic dual treatment of recalcitrant plantar warts: Animal and clinical study. J. Drug Deliv. Sci. Technol. 2022, 69, 103095. [Google Scholar] [CrossRef]
- Sartorius, K.; Emtestam, L.; Jemec, G.B.E.; Lapins, J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br. J. Dermatol. 2009, 161, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Fadel, M.; Kassab, K.; Youssef, T.; El-Kholy, A.I. One-step synthesis of phyto-polymer coated gold nanospheres as a delivery system to enhance resveratrol cytotoxicity. Drug Dev. Ind. Pharm. 2019, 45, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Abdulbaqi, I.M.; Darwis, Y.; Assi, R.A.; Khan, N.A.K. Transethosomal gels as carriers for the transdermal delivery of colchicine: Statistical optimization, characterization, and ex vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 795–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, G.; Rouabhia, M.; Wang, Z.; Dao, L.H.; Zhang, Z. A novel electrically conductive and biodegradable composite made of polypyrrole nanoparticles and polylactide. Biomaterials 2004, 25, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Alexiades, M. Randomized, controlled trial of fractional carbon dioxide laser resurfacing followed by ultrashort incubation aminolevulinic acid blue light photodynamic therapy for actinic keratosis. Dermatol. Surg. 2017, 43, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wu, P.; Lin, X.; Chen, H.; Hu, X.; Jin, Y.; Qiu, Y. Fractional carbon dioxide laser–assisted drug delivery of topical timolol solution for the treatment of deep infantile hemangioma: A pilot study. Pediatr. Dermatol. 2014, 31, 286–291. [Google Scholar] [CrossRef] [PubMed]




| Groups (2 Animals per Group) | Topical Treatment * | Light Condition ** |
|---|---|---|
| Control | - | - |
| Group 1 | G-Ppy hydrogel | Dark |
| Light | ||
| Group 2 | Free E hydrogel | Dark |
| Light | ||
| Group 3 | E-G-Ppy hydrogel | Dark |
| Light |
| Group | Epidermal Degenerative Changes | Dermal Ulceration | Inflammatory Cells Infiltrate | Congested Subcutaneous Blood Vessels |
|---|---|---|---|---|
| Control | - | - | - | - |
| Group 1-Dark (G-Ppy) | - | - | - | - |
| Group 1(G-Ppy)-Light | - | - | - | - |
| Group 2-Dark (free E) | + | - | + | - |
| Group 2-Light (Free E) | ++ | - | ++ | - |
| Group 3-Dark (E-G-Ppy) | + | - | + | + |
| Group 3-Light (E-G-Ppy) | + | ++ | +++ | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Kholy, A.I.; Fadel, M.; Nasr, M.; El-Sherbiny, I.; Tawfik, A.; Mosaad, Y.O.; Abdel Fadeel, D. Gold-Polypyrrole-Loaded Eosin in Photo-Mediated Treatment of Hidradenitis Suppurativa: In Vivo Trans-Epidermal Permeation Study and Clinical Case Report. Pharmaceutics 2022, 14, 2197. https://doi.org/10.3390/pharmaceutics14102197
El-Kholy AI, Fadel M, Nasr M, El-Sherbiny I, Tawfik A, Mosaad YO, Abdel Fadeel D. Gold-Polypyrrole-Loaded Eosin in Photo-Mediated Treatment of Hidradenitis Suppurativa: In Vivo Trans-Epidermal Permeation Study and Clinical Case Report. Pharmaceutics. 2022; 14(10):2197. https://doi.org/10.3390/pharmaceutics14102197
Chicago/Turabian StyleEl-Kholy, Abdullah I., Maha Fadel, Maha Nasr, Ibrahim El-Sherbiny, Abeer Tawfik, Yasser O. Mosaad, and Doaa Abdel Fadeel. 2022. "Gold-Polypyrrole-Loaded Eosin in Photo-Mediated Treatment of Hidradenitis Suppurativa: In Vivo Trans-Epidermal Permeation Study and Clinical Case Report" Pharmaceutics 14, no. 10: 2197. https://doi.org/10.3390/pharmaceutics14102197