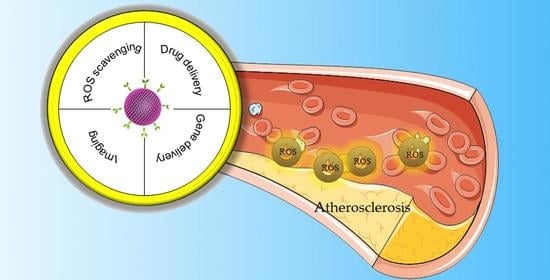
Recent Advances in ROS-Sensitive Nano-Formulations for Atherosclerosis Applications
Abstract
:1. Introduction
2. ROS-Sensitive Functional Molecular Structures
2.1. Water Solubility Switch
2.1.1. Polypropylene Sulfides (PPS)
2.1.2. Selenium-Containing Block Copolymer
2.1.3. Polythioether Ketal
2.2. Structural Degradation
2.2.1. Boronic Esters
2.2.2. Silicon
2.2.3. Proline Oligomers
2.2.4. Polythioketal
2.3. Other Types
3. ROS-Sensitive Nano-Formulations for Atherosclerosis Applications
3.1. Nano-Formulations for Scavenging Excessive ROS
3.2. ROS-Sensitive Nano-Formulations for Controlling Cargo Release
3.3. ROS-Sensitive Nano-Formulations for Imaging
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OH | hydroxyl radicals |
| andro | andrographolide |
| AIE | aggregation-induced emission |
| ASOs | antisense oligonucleotides |
| BSA | bovine serum albumin |
| CeO2 | cerium oxide |
| CVDs | cardiovascular diseases |
| H2O2 | hydrogen peroxide |
| HA | hyaluronic acid |
| HASF | oligomeric hyaluronic acid-2′-[propane-2,2-diyllbls(thio)] diacetic acl-hydroxymethylferrocene |
| LDL | low-density lipoprotein |
| Lp | liposomes |
| MacTNP | macrophage-targeted theranostic nanoparticles |
| MMP | matrix metalloproteinase |
| MnO2 | manganese dioxide |
| mTOR | mammalian target of rapamycin |
| NIRF | near-infrared fluorescence |
| O2- | superoxide |
| ONOO− | peroxide nitrate |
| Ox-LDL | low-density lipoprotein |
| PBAPs | phenylboronic acid pinacol esters |
| PEG | polyethylene glycol |
| PEG–PPS | poly(ethylene glycol)–poly(propylene sulfide) |
| PGED–PPS | poly(glycidyl methacrylate)–polypropylene sulfide |
| PPS | polypropylene sulfides |
| RNAi | RNA interference |
| ROS | reactive oxygen species |
| S2P | stabilin-2-specific peptide ligand |
| siRNA | short interfering RNA |
| TEMPOL | 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl |
| TP | two-photon fluorophore |
| TPCD | superoxide dismutase mimetic agent TEMPO and PBAP in β-cyclodextrin |
| VSMCs | vascular smooth muscle cells |
References
- Tørris, C.; Småstuen, M.C.; Molin, M. Nutrients in fish and possible associations with cardiovascular disease risk factors in metabolic syndrome. Nutrients 2018, 10, 952. [Google Scholar] [CrossRef] [Green Version]
- Narasimhan, S.D. Beyond statins: New therapeutic frontiers for cardiovascular disease. Cell 2017, 169, 971–973. [Google Scholar] [CrossRef] [Green Version]
- Lobatto, M.E.; Calcagno, C.; Millon, A.; Senders, M.L.; Fay, F.; Robson, P.M.; Ramachandran, S.; Binderup, T.; Paridaans, M.P.M.; Sensarn, S.; et al. Atherosclerotic Plaque targeting mechanism of long-circulating nanoparticles established by multimodal imaging. ACS Nano 2015, 9, 1837–1847. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Phillips, E.; Riggins, T.; Sangha, G.; Chakraborty, S.; Lee, J.; Lycke, R.; Hernandez, C.; Soepriatna, A.; Thorne, B.; et al. Imaging of Small animal peripheral artery disease models: Recent advancements and translational potential. Int. J. Mol. Sci. 2015, 16, 11131–11177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malekmohammad, K.; Sewell, R.D.E.; Rafieian-Kopaei, M. Antioxidants and Atherosclerosis: Mechanistic Aspects. Biomolecules 2019, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Kwak, L.; Ballew, S.H.; Grams, M.E.; Selvin, E.; Folsom, A.R.; Coresh, J.; Tang, W. Chronic Kidney disease measures and the risk of abdominal aortic aneurysm. Atherosclerosis 2018, 279, 107–113. [Google Scholar] [CrossRef]
- Hess, C.N.; Norgren, L.; Ansel, G.M.; Capell, W.H.; Fletcher, J.P.; Fowkes, F.G.R.; Gottsäter, A.; Hitos, K.; Jaff, M.R.; Nordanstig, J.; et al. A structured review of antithrombotic therapy in peripheral artery disease with a focus on revascularization: A TASC (intersociety consensus for the management of peripheral artery disease) Initiative. Circulation 2017, 135, 2534–2555. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causesof-death (accessed on 1 September 2021).
- Barquera, S.; Pedroza-Tobías, A.; Medina, C.; Hernández-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef]
- De Wijs-Meijler, D.P.; Duncker, D.J.; Tibboel, D.; Schermuly, R.T.; Weissmann, N.; Merkus, D.; Reiss, I.K.M. Oxidative Injury of the pulmonary circulation in the perinatal period: Short- and long-term consequences for the human cardiopulmonary system. Pulm. Circ. 2017, 7, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; Clair, D.K.S. Regulation of Superoxide Dismutase Genes: Implications in Disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef] [Green Version]
- Tapeinos, C.; Pandit, A. Physical, chemical, and biological structures based on ros-sensitive moieties that are able to respond to oxidative microenvironments. Adv. Mater. 2016, 28, 5553–5585. [Google Scholar] [CrossRef]
- Wu, T.; Chen, X.; Wang, Y.; Xiao, H.; Peng, Y.; Lin, L.; Xia, W.; Long, M.; Tao, J.; Shuai, X. Aortic Plaque-targeted andrographolide delivery with oxidation-sensitive micelle effectively treats atherosclerosis via simultaneous ros capture and anti-inflammation. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2215–2226. [Google Scholar] [CrossRef]
- Takahashi, S. Triglyceride Rich Lipoprotein -LPL-VLDL Receptor and Lp(a)-VLDL Receptor pathways for macrophage foam cell formation. J. Atheroscler. Thromb. 2017, 24, 552–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-L.; Sun, G.-Y.; Zhang, Y.; He, J.-J.; Zheng, S.; Lin, J.-N. Tormentic acid inhibits H2O2-Induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NF-ΚB signaling pathway. Mol. Med. Rep. 2016, 14, 3559–3564. [Google Scholar] [CrossRef] [Green Version]
- Velasquez, J.C.; Weiss, D.; Joseph, G.; Landazuri, N.; Taylor, W.R. Abstract 3961: Macrophage Catalase Overexpression Inhibits Atherosclerosis and Vascular Inflammation. Circulation 2008, 118, S510. [Google Scholar]
- Fruehauf, J.P.; Meyskens, F.L. Reactive Oxygen species: A breath of life or death? Clin. Cancer Res. 2007, 13, 789–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive Oxygen species: Key regulators in vascular health and diseases: Ros in vascular diseases. Br. J. Pharm. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Lippert, A.R.; Van de Bittner, G.C.; Chang, C.J. Boronate Oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc. Chem. Res. 2011, 44, 793–804. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The role of hydrogen peroxide in redox-dependent signaling: Homeostatic and pathological responses in mammalian cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S.; Song, C.G.; Kang, P.M. Targeting oxidative stress using nanoparticles as a theranostic strategy for cardiovascular diseases. Antioxid. Redox. Sign. 2019, 30, 733–746. [Google Scholar] [CrossRef]
- Nishida, K.; Otsu, K. Inflammation and metabolic cardiomyopathy. Cardiovasc. Res. 2017, 113, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnár, Z. Neonatal hypoxia ischaemia: Mechanisms, Models, and therapeutic challenges. Front. Cell. Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Tomaniak, M.; Katagiri, Y.; Modolo, R.; de Silva, R.; Khamis, R.Y.; Bourantas, C.V.; Torii, R.; Wentzel, J.J.; Gijsen, F.J.H.; van Soest, G.; et al. Vulnerable plaques and patients: State-of-the-Art. Eur. Heart J. 2020, 41, 2997–3004. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Raimondo, D.; Pecoraro, R.; Arnao, V.; Pinto, A.; Licata, G. Atherosclerosis as an inflammatory disease. Curr. Pharm. Des. 2012, 18, 4266–4288. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.; Wang, X.; Hu, J.; Liu, S. Enzyme-responsive polymeric vesicles for bacterial-strain-selective delivery of antimicrobial agents. Angew. Chem. Int. Ed. 2016, 55, 1760–1764. [Google Scholar] [CrossRef]
- Ge, Z.; Liu, S. Functional Block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chem. Soc. Rev. 2013, 42, 7289. [Google Scholar] [CrossRef]
- Luo, L.; Wu, W.; Sun, D.; Dai, H.-B.; Wang, Y.; Zhong, Y.; Wang, J.-X.; Maruf, A.; Nurhidayah, D.; Zhang, X.-J.; et al. Acid-Activated Melittin for targeted and safe antitumor therapy. Bioconjug. Chem. 2018, 29, 2936–2944. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Tong, R.; Tang, L.; Ma, L.; Tu, C.; Baumgartner, R.; Cheng, J. Smart Chemistry in polymeric nanomedicine. Chem. Soc. Rev. 2014, 43, 6982–7012. [Google Scholar] [CrossRef]
- Lee, S.H.; Gupta, M.K.; Bang, J.B.; Bae, H.; Sung, H.-J. Current progress in reactive oxygen species (ROS)-responsive materials for biomedical applications. Adv. Healthc. Mater. 2013, 2, 908–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, H.-L.; Chiang, W.-L.; Maiti, B.; Liao, Z.-X.; Ho, Y.-C.; Shim, M.S.; Chuang, E.-Y.; Xia, Y.; Sung, H.-W. Nanoparticles with Dual responses to oxidative stress and reduced PH for Drug release and anti-inflammatory applications. ACS Nano 2014, 8, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, J.; Liu, B. Conjugated-Polyelectrolyte-Based polyprodrug: Targeted and image-guided photodynamic and chemotherapy with on-demand drug release upon irradiation with a single light source. Angew. Chem. Int. Ed. 2014, 126, 7291–7296. [Google Scholar] [CrossRef]
- Wang, Y.; Shim, M.S.; Levinson, N.S.; Sung, H.-W.; Xia, Y. Stimuli-Responsive Materials for controlled release of theranostic agents. Adv. Funct. Mater. 2014, 24, 4206–4220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.; Wang, Y.; Wijaya, A.; Liu, B.; Maruf, A.; Wang, J.; Xu, J.; Liao, X.; Wu, W.; Wang, G. ROS-Responsive biomimetic nanoparticles for potential application in targeted anti-atherosclerosis. Regen. Biomater. 2021, 8, rbab033. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An Emerging treatment modality for cancer. Nat. Rev. Drug. Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Song, C.-C.; Du, F.-S.; Li, Z.-C. Oxidation-Responsive Polymers for biomedical applications. J. Mater. Chem. B 2014, 2, 3413–3426. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Y.; Chen, K.; Zhang, X.; Xu, X.; Shi, Q.; Han, S.; Chen, X.; Gong, H.; Li, X.; et al. Biocompatible Reactive oxygen species (ros)-responsive nanoparticles as superior drug delivery vehicles. Adv. Healthc. Mater. 2015, 4, 69–76. [Google Scholar] [CrossRef]
- Deng, H.; Zhao, X.; Liu, J.; Deng, L.; Zhang, J.; Liu, J.; Dong, A. Reactive oxygen species (ROS) responsive PEG-PCL nanoparticles with pH-controlled negative-to-positive charge reversal for intracellular delivery of doxorubicin. J. Mater. Chem. B 2015, 3, 9397–9408. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Su, G.-M.; Chen, C.-K.; Chiang, Y.-T.; Lo, C.-L. Specific Cancer Cytosolic Drug Delivery Triggered by Reactive Oxygen Species-Responsive Micelles. Biomacromolecules 2016, 17, 3040–3047. [Google Scholar] [CrossRef]
- Jayrajsinh, S. Montmorillonite Nanoclay as a Multifaceted Drug-Delivery Carrier: A Review. J. Drug Deliv. Sci. Technol. 2017, 39, 200–209. [Google Scholar] [CrossRef]
- Aamer, K. Rheological Studies of PLLA-PEO-PLLA triblock copolymer hydrogels. Biomaterials 2004, 25, 1087–1093. [Google Scholar] [CrossRef]
- Reddy, S.T.; Rehor, A.; Schmoekel, H.G.; Hubbell, J.A.; Swartz, M.A. In Vivo Targeting of Dendritic Cells in Lymph Nodes with Poly(Propylene Sulfide) Nanoparticles. J. Control. Release 2006, 112, 26–34. [Google Scholar] [CrossRef]
- Han, P.; Ma, N.; Ren, H.; Xu, H.; Li, Z.; Wang, Z.; Zhang, X. Oxidation-Responsive Micelles based on a selenium-containing polymeric superamphiphile. Langmuir 2010, 26, 14414–14418. [Google Scholar] [CrossRef]
- Ma, N.; Li, Y.; Xu, H.; Wang, Z.; Zhang, X. Dual Redox Responsive Assemblies Formed from Diselenide Block Copolymers. J. Am. Chem. Soc. 2010, 132, 442–443. [Google Scholar] [CrossRef]
- Tan, X.; Yu, Y.; Liu, K.; Xu, H.; Liu, D.; Wang, Z.; Zhang, X. Single-Molecule Force spectroscopy of selenium-containing amphiphilic block copolymer: Toward disassembling the polymer micelles. Langmuir 2012, 28, 9601–9605. [Google Scholar] [CrossRef]
- Ren, H.; Wu, Y.; Ma, N.; Xu, H.; Zhang, X. Side-Chain selenium-containing amphiphilic block copolymers: Redox-controlled self-assembly and disassembly. Soft Matter 2012, 8, 1460–1466. [Google Scholar] [CrossRef]
- Ma, N.; Li, Y.; Ren, H.; Xu, H.; Li, Z.; Zhang, X. Selenium-Containing Block copolymers and their oxidation-responsive aggregates. Polym. Chem. 2010, 1, 1609. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, J.; Park, S.; Khang, G.; Kang, P.M.; Lee, D. Inflammation-Responsive antioxidant nanoparticles based on a polymeric prodrug of vanillin. Biomacromolecules 2013, 14, 1618–1626. [Google Scholar] [CrossRef]
- Yang, S.C.; Bhide, M.; Crispe, I.N.; Pierce, R.H.; Murthy, N. Polyketal copolymers: A new acid-sensitive delivery vehicle for treating acute inflammatory diseases. Bioconjug. Chem. 2008, 19, 1164–1169. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Jo, S.D.; Seah, G.L.; Kim, I.; Nam, Y.S. ROS-Induced Biodegradable polythioketal nanoparticles for intracellular delivery of anti-cancer therapeutics. J. Ind. Eng. Chem. 2015, 21, 1137–1142. [Google Scholar] [CrossRef]
- Su, Z.; Chen, M.; Xiao, Y.; Sun, M.; Zong, L.; Asghar, S.; Dong, M.; Li, H.; Ping, Q.; Zhang, C. ROS-Triggered and regenerating anticancer nanosystem: An Effective strategy to subdue tumor’s multidrug resistance. J. Control. Release 2014, 196, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-C.; Ji, R.; Du, F.-S.; Liang, D.-H.; Li, Z.-C. Oxidation-Accelerated Hydrolysis of the ortho ester-containing acid-labile polymers. ACS Macro Lett. 2013, 2, 273–277. [Google Scholar] [CrossRef]
- Jourden, J.L.M.; Daniel, K.B.; Cohen, S.M. Investigation of self-immolative linkers in the design of hydrogen peroxide activated metalloprotein inhibitors. Chem. Commun. 2011, 47, 7968. [Google Scholar] [CrossRef] [Green Version]
- Vaccari, L.; Canton, D.; Zaffaroni, N.; Villa, R.; Tormen, M.; di Fabrizio, E. Porous silicon as drug carrier for controlled delivery of doxorubicin anticancer agent. Microelectron. Eng. 2006, 83, 1598–1601. [Google Scholar] [CrossRef]
- Salonen, J.; Laitinen, L.; Kaukonen, A.M.; Tuura, J.; Björkqvist, M.; Heikkilä, T.; Vähä-Heikkilä, K.; Hirvonen, J.; Lehto, V.-P. Mesoporous Silicon microparticles for oral drug delivery: Loading and release of five model drugs. J. Control. Release 2005, 108, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.C.; Park, J.-H.; Park, J.; Segal, E.; Cunin, F.; Sailor, M.J. Oxidation-Triggered release of fluorescent molecules or drugs from mesoporous Si microparticles. ACS Nano 2008, 2, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Gupta, M.K.; Lee, S.H.; Crowder, S.W.; Wang, X.; Hofmeister, L.H.; Nelson, C.E.; Bellan, L.M.; Duvall, C.L.; Sung, H.-J. Oligoproline-Derived Nanocarrier for Dual Stimuli-Responsive Gene Delivery. J. Mater. Chem. B 2015, 3, 7271–7280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.S.; Koblin, R.L.; Zachman, A.L.; Perrien, D.S.; Hofmeister, L.H.; Giorgio, T.D.; Sung, H.-J. Physiologically Relevant oxidative degradation of oligo(proline) cross-linked polymeric scaffolds. Biomacromolecules 2011, 12, 4357–4366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, C.; Corbett, G.; Moss, A.C. Systematic review and meta-analysis: Serum Infliximab levels during maintenance therapy and outcomes in inflammatory bowel disease. J. Crohns Colitis 2016, 10, 619–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.; Sanoj Rejinold, N.; Lee, D.; Jon, S.; Kim, Y.-C. Protease-Activatable Cell-penetrating peptide possessing ROS-Triggered phase transition for enhanced cancer therapy. J. Control. Release 2017, 264, 89–101. [Google Scholar] [CrossRef]
- Lee, D.; Bae, S.; Ke, Q.; Lee, J.; Song, B.; Karumanchi, S.A.; Khang, G.; Choi, H.S.; Kang, P.M. Hydrogen peroxide-responsive copolyoxalate nanoparticles for detection and therapy of ischemia–reperfusion injury. J. Control. Release 2013, 172, 1102–1110. [Google Scholar] [CrossRef] [Green Version]
- Saravanakumar, G.; Lee, J.; Kim, J.; Kim, W.J. Visible Light-Induced Singlet oxygen-mediated intracellular disassembly of polymeric micelles co-loaded with a photosensitizer and an anticancer drug for enhanced photodynamic therapy. Chem. Commun. 2015, 51, 9995–9998. [Google Scholar] [CrossRef] [Green Version]
- Baugh, S.D.P.; Yang, Z.; Leung, D.K.; Wilson, D.M.; Breslow, R. Cyclodextrin dimers as cleavable carriers of photodynamic sensitizers. J. Am. Chem. Soc. 2001, 123, 12488–12494. [Google Scholar] [CrossRef]
- Li, Q.; Chen, X.; Yue, X.; Huang, D.; Wang, X. Construction and Transformation of stimuli-responsive vesicles from the ferrocene derivative supramolecular amphiphiles. Colloids Surf. A Physicochem. Eng. Asp. 2012, 409, 98–104. [Google Scholar] [CrossRef]
- Sun, D.; Chen, J.; Wang, Y.; Ji, H.; Peng, R.; Jin, L.; Wu, W. Advances in refunctionalization of erythrocyte-based nanomedicine for enhancing cancer-targeted drug delivery. Theranostics 2019, 9, 6885–6900. [Google Scholar] [CrossRef]
- Ami, A.; Markovi, Z.; Markovi, J.M.D.; Milenkovi, D.; Stepani, V. Antioxidative potential of ferulic acid phenoxyl radical. Phytochemistry 2019, 170, 112218. [Google Scholar] [CrossRef]
- Chmielowski, R.A.; Abdelhamid, D.S.; Faig, J.J.; Petersen, L.K.; Gardner, C.R.; Uhrich, K.E.; Joseph, L.B.; Moghe, P.V. Athero-Inflammatory nanotherapeutics: Ferulic acid-based poly(anhydride-ester) nanoparticles attenuate foam cell formation by regulating macrophage lipogenesis and reactive oxygen species generation. Acta Biomater. 2017, 57, 85–94. [Google Scholar] [CrossRef]
- Ziegler, M.; Wallert, M.; Lorkowski, S.; Peter, K. Cardiovascular and Metabolic protection by vitamin E: A matter of treatment strategy? Antioxidants 2020, 9, 935. [Google Scholar] [CrossRef]
- Wallert, M.; Ziegler, M.; Wang, X.; Maluenda, A.; Peter, K. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biol. 2019, 26, 101292. [Google Scholar] [CrossRef]
- Bizeau, J.; Tapeinos, C.; Marella, C.; Larranaga, A.; Pandit, A. Synthesis and characterization of hyaluronic acid coated manganese dioxide microparticles that act as ROS scavengers. Colloids Surf. B Biointerfaces 2017, 159, 30–38. [Google Scholar] [CrossRef]
- Luo, X.L.; Xu, J.J.; Wei, Z.; Chen, H.Y. A novel glucose ENFET Based on the special reactivity of MnO2 nanoparticles. Biosens. Bioelectron. 2004, 19, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Adjei, I.M.; Brown, S.B.; Liseth, O.; Sharma, B. Manganese Dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials 2019, 224, 119467. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Naik, P. Synthesis and biomedical applications of cerium oxide nanoparticle—A review. Biotechnol. Rep. 2018, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Y.; Zhao, W.; Xu, Z.; Little, P.J.; Whittaker, A.K.; Zhang, R.; Ta, H.T. Novel iron oxide-cerium oxide core-shell nanoparticles as a potential theranostic material for ROS related inflammatory diseases. J. Mater. Chem. B 2018, 6, 4937–4951. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Behnam, B.; Banach, M.; Sahebkar, A. The yin and yang of carbon nanomaterials in atherosclerosis. Biotechnol. Adv. 2018, 36, 2232–2247. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef] [Green Version]
- Katsuki, S.; Matoba, T.; Nakashiro, S.; Sato, K.; Koga, J.-I.; Nakano, K.; Nakano, Y.; Egusa, S.; Sunagawa, K.; Egashira, K. Nanoparticle-mediated delivery of pitavastatin inhibits atherosclerotic plaque destabilization/rupture in mice by regulating the recruitment of inflammatory monocytes. Circulation 2014, 129, 896–906. [Google Scholar] [CrossRef]
- Tang, J.; Lobatto, M.E.; Hassing, L.; van deer Staay, S.; van Rijs, S.M.; Calcagno, C.; Braza, M.S.; Baxter, S.; Fay, F.; Sanchez-Gaytan, B.L.; et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 2015, 1, e1400223. [Google Scholar] [CrossRef] [Green Version]
- Mu, D.; Li, J.; Qi, Y.; Sun, X.; Liu, Y.; Shen, S.; Li, Y.; Xu, B.; Zhang, B. Hyaluronic acid-coated polymeric micelles with hydrogen peroxide scavenging to encapsulate statins for alleviating atherosclerosis. J. Nanobiotechnol. 2020, 18, 179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, F.; Chen, Y.; Dou, Y.; Tao, H.; Zhang, D.; Wang, R.; Li, X.; Zhang, J. Structure-Property correlations of reactive oxygen species-responsive and hydrogen peroxide-eliminating materials with anti-oxidant and anti-inflammatory activities. Chem. Mater. 2017, 29, 8221–8238. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zhao, W.; Dou, Y.; An, H.; Tao, H.; Xu, X.; Jia, Y.; Lu, S.; Zhang, J.; et al. Targeted therapy of atherosclerosis by a broad-spectrum reactive oxygen species scavenging nanoparticle with intrinsic anti-inflammatory activity. ACS Nano 2018, 12, 8943–8960. [Google Scholar] [CrossRef]
- Li, L.; Guo, J.; Wang, Y.; Xiong, X.; Zhang, J. A Broad-Spectrum ROS-eliminating material for prevention of inflammation and drug-induced organ toxicity. Adv. Sci. 2018, 5, 1800781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Li, D.; Tao, H.; Li, G.; Liu, R.; Dou, Y.; Jin, T.; Li, L.; Huang, J.; Hu, H.; et al. Cyclodextrin-Derived intrinsically bioactive nanoparticles for treatment of acute and chronic inflammatory diseases. Adv. Mater. 2019, 31, 1904607. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Guan, S.; Chang, C.; Wu, S.; Wong, W. A novel antiinflammatory Role for andrographolide in asthma via inhibition of the nuclear factor-kB pathway. Am. J. Respir. Crit. Care Med. 2012, 179, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Burgos, R.A.; Alarcon, N.P.; Quiroga, J.; Manosalva, C.; Hancke, J. Andrographolide, an anti-inflammatory multitarget drug: All roads lead to cellular metabolism. Molecules 2020, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Napoli, A.; Valentini, M.; Tirelli, N.; Müller, M.; Hubbell, J.A. Oxidation-Responsive polymeric vesicles. Nat. Mater. 2004, 3, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.D.; Liu, Y.-G.; Kim, T.; Bobbala, S.; Yi, S.; Zhang, X.; Choi, J.; Scott, E.A. Celastrol-Loaded PEG-b-PPS Nanocarriers as an anti-inflammatory treatment for atherosclerosis. Biomater. Sci. 2019, 7, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Chen, Y.; Zhang, X.; Xu, X.; Chen, Y.; Guo, J.; Zhang, D.; Wang, R.; Li, X.; Zhang, J. Non-Proinflammatory and responsive nanoplatforms for targeted treatment of atherosclerosis. Biomaterials 2017, 143, 93. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, Y.; Zhao, M.; Gao, W.; Zhang, H.; Li, S.; He, B.; Pu, Y. A reactive oxygen species–responsive prodrug micelle with efficient cellular uptake and excellent bioavailability. J. Mater. Chem. B 2018, 6, 1076–1084. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Xia, Y.; Liu, S.; Lu, Y. Preparation of ROS-Responsive core crosslinked polycarbonate micelles with thioketal linkage. Colloid Surf. B 2020, 195, 111276. [Google Scholar] [CrossRef]
- Hou, X.; Lin, H.; Zhou, X.; Cheng, Z.; Li, Y.; Liu, X.; Zhao, F.; Zhu, Y.; Zhang, P.; Chen, D. Novel dual ROS-Sensitive and CD44 Receptor targeting nanomicelles based on oligomeric hyaluronic acid for the efficient therapy of atherosclerosis. Carbohyd. Polym. 2020, 232, 115787. [Google Scholar] [CrossRef]
- Hong, L.; Sureda, A.; Devkota, H.P.; Pittalà, V.; Nabavi, S.M. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 2019, 38, 107343. [Google Scholar] [CrossRef]
- Yang, L.; Zang, G.; Li, J.; Li, X.; Li, Y.; Zhao, Y. Cell-Derived Biomimetic nanoparticles as a novel drug delivery system for atherosclerosis: Predecessors and perspectives. Regen. Biomater. 2020, 7, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.-B.; Lee, S.M.Y.; Wang, R. Treatment of Atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef]
- Peng, R.; Ji, H.; Jin, L.; Lin, S.; Huang, Y.; Xu, K.; Yang, Q.; Sun, D.; Wu, W. Macrophage-Based therapies for atherosclerosis management. J. Immunol. Res. 2020, 2020, 8131754. [Google Scholar] [CrossRef] [Green Version]
- Maruf, A.; Wang, Y.; Luo, L.; Zhong, Y.; Nurhidayah, D.; Liu, B.; Tang, D.; Rouf, M.A.; Zhang, H.; Yin, Y.; et al. Nanoerythrocyte membrane-enveloped ROS-responsive 5-aminolevulinic Acid prodrug nanostructures with robust atheroprotection. Part. Part. Syst. Charact. 2020, 37, 2000021. [Google Scholar] [CrossRef]
- Shen, M.; Li, H.; Yao, S.; Wu, X.; Liu, S.; Yang, Q.; Zhang, Y.; Du, J.; Qi, S.; Li, Y. Shear stress and ROS-Responsive biomimetic micelles for atherosclerosis via ROS consumption. Mater. Sci. Eng. C 2021, 126, 112164. [Google Scholar] [CrossRef]
- Mkinen, P.; Ruotsalainen, A.K.; Yl-Herttuala, S. Nucleic acid-based therapies for atherosclerosis. Curr. Atheroscler. Rep. 2020, 22, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, L.; Niu, X.; Dang, X.; Pan, L. mTOR enhances foam cell formation by suppressing the autophagy pathway. DNA Cell Biol. 2014, 33, 198–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Zhao, Y.; Li, X.; Sun, Y.; Cai, M.; Cao, W.; Liu, Z.; Tong, L.; Cui, G.; Tang, B. H2O2-responsive and plaque-penetrating nanoplatform for mTOR Gene silencing with robust anti-atherosclerosis efficacy. Chem. Sci. 2018, 9, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.; Bai, H.; Wang, Z.; Qiu, Y.; Kong, L.-L.; Sun, F.; Wang, D.; Yin, H.; Zhang, X.; Mu, H.; et al. A Hyaluronan-Based Nanosystem enables combined anti-inflammation of mTOR gene silencing and pharmacotherapy. Carbohyd. Polym. 2018, 195, 339–348. [Google Scholar] [CrossRef]
- Wang, Q.; Lou, R.; Yin, Q.; Yang, R.; Li, S.; Zhou, J. A nano-detection system based on a chemical probe for early diagnosis of atherosclerosis in situ. Analyst 2021, 146, 4674–4682. [Google Scholar] [CrossRef]
- Manea, S.-A.; Vlad, M.-L.; Rebleanu, D.; Lazar, A.-G.; Fenyo, I.M.; Calin, M.; Simionescu, M.; Manea, A. Detection of vascular reactive oxygen species in experimental atherosclerosis by high-resolution near-infrared fluorescence imaging using VCAM-1-targeted liposomes entrapping a fluorogenic redox-sensitive probe. Oxid. Med. Cell. Longev. 2021, 2021, 6685612. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Song, J.W.; Kim, H.J.; Kim, C.-S.; Song, Y.J.; Yang, D.H.; Yoo, H.; Kim, J.W.; Park, K. In vivo Imaging of reactive oxygen species (ROS)-Producing pro-inflammatory macrophages in murine carotid atheromas using a CD44-targetable and ROS-responsive nanosensor. J. Ind. Eng. Chem. 2020, 92, 158–166. [Google Scholar] [CrossRef]
- Rao, N.V.; Rho, J.G.; Um, W.; Pramod Kumar, E.K.; Nguyen, V.Q.; Oh, B.H.; Kim, W.; Park, J.H. Hyaluronic acid nanoparticles as nanomedicine for treatment of inflammatory diseases. Pharmaceutics 2020, 12, 931. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Kim, I.; Park, T.G. Fluorescent gold nanoprobe sensitive to intracellular reactive oxygen species. Adv. Funct. Mater. 2009, 19, 1884–1890. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Kim, I.-H.; Kim, K.; Choi, Y. ROS-Responsive activatable photosensitizing agent for imaging and photodynamic therapy of activated macrophages. Theranostics 2014, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Jeong, W.Y.; Kang, M.S.; Lee, H.; Lee, J.H.; Kim, J.; Han, D.W.; Kim, K.S. Recent Trends in photoacoustic imaging techniques for 2D nanomaterial-based phototherapy. Biomedicines 2021, 9, 80. [Google Scholar] [CrossRef]
- Gao, W.; Li, X.; Liu, Z.; Fu, W.; Sun, Y.; Cao, W.; Tong, L.; Tang, B. A redox-responsive self-assembled nanoprobe for photoacoustic inflammation imaging to assess atherosclerotic plaque vulnerability. Anal. Chem. 2019, 91, 1150–1156. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, B.; Zhuang, W.; He, H.; Su, X.; Wang, Y. Two-Photon AIE probe conjugated theranostic nanoparticles for tumor bioimaging and PH-sensitive drug delivery. Nano Res. 2019, 12, 1703–1712. [Google Scholar] [CrossRef]
- Situ, B.; Gao, M.; He, X.; Li, S.; He, B.; Guo, F.; Kang, C.; Liu, S.; Yang, L.; Jiang, M. A Two-Photon AIEgen for Simultaneous Dual-Color Imaging of Atherosclerotic Plaques. Mater. Horiz. 2019, 6, 546–553. [Google Scholar] [CrossRef]
- Ma, B.; Xu, H.; Zhuang, W.; Wang, Y.; Li, G.; Wang, Y. Reactive oxygen species responsive theranostic nanoplatform for two-photon aggregation-induced emission imaging and therapy of acute and chronic inflammation. ACS Nano 2020, 14, 5862–5873. [Google Scholar] [CrossRef]
- Tenório-Neto, E.T.; Lima, D.; Guilherme, M.R.; Lima-Tenório, M.K.; Scariot, D.B.; Nakamura, C.V.; Kunita, M.H.; Rubira, A.F. Synthesis and drug release profile of a dual-responsive poly(ethylene glycol) hydrogel nanocomposite. RSC Adv. 2017, 7, 27637–27644. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Xu, H.; Zhuang, W.; Wang, Y.; Li, G.; Wang, Y. ROS responsive nanoplatform with two-photon AIE Imaging for atherosclerosis diagnosis and “two-pronged” therapy. Nano Micro Small 2020, 16, 2003253. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Peng, R.; Jin, L.; Ma, J.; Yang, Q.; Sun, D.; Wu, W. Recent Advances in ROS-Sensitive Nano-Formulations for Atherosclerosis Applications. Pharmaceutics 2021, 13, 1452. https://doi.org/10.3390/pharmaceutics13091452
Ji H, Peng R, Jin L, Ma J, Yang Q, Sun D, Wu W. Recent Advances in ROS-Sensitive Nano-Formulations for Atherosclerosis Applications. Pharmaceutics. 2021; 13(9):1452. https://doi.org/10.3390/pharmaceutics13091452
Chicago/Turabian StyleJi, Hao, Renyi Peng, Libo Jin, Jiahui Ma, Qinsi Yang, Da Sun, and Wei Wu. 2021. "Recent Advances in ROS-Sensitive Nano-Formulations for Atherosclerosis Applications" Pharmaceutics 13, no. 9: 1452. https://doi.org/10.3390/pharmaceutics13091452