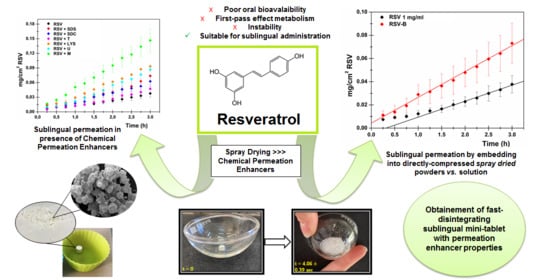
Improvement of Resveratrol Permeation through Sublingual Mucosa: Chemical Permeation Enhancers versus Spray Drying Technique to Obtain Fast-Disintegrating Sublingual Mini-Tablets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. CPEs to Promote RSV Sublingual Permeation
Preliminary Stability Assay of RSV
Ex Vivo Evaluations
- Tissue Preparation
- Ex Vivo Permeation Assay
- Evaluation of RSV Amount Entrapped in the Sublingual Tissue
Determination of the Biopharmaceutical Parameters: Js, Kp, tlag, De and Ac
2.2.2. Preparation and Ex Vivo Evaluation of RSV-Loaded Mini-Tablets
Preparation of RSV-Loaded Pharmaceutical Powders by Spray Drying
Characterization of the Pharmaceutical Powders: Yield Percentage, Drug Loading Percentage (DL%) and Loading Efficacy Percentage (LE%)
Preparation of RSV-Loaded Sublingual Mini-Tablets
Ex Vivo Permeation Studies by Administering RSV-Loaded Sublingual Mini-Tablets
Preparation and Characterization of CPE-Loaded Sublingual Mini-Tablets
2.2.3. Characterization of RSV-B Powder and Sublingual Fast-Disintegrating Mini-Tablets
Scanning Electron Microscopy (SEM) Analysis
Fourier Transform Infrared Spectroscopic in Attenuated Total Reflectance Mode (FTIR-ATR mode) Evaluations
X-ray Diffraction (XRD) Analysis
Disintegration Studies
- In Vitro Disintegration Test
- In Vitro Disintegration Visual Test
- In Vitro Dissolution Test
2.2.4. Data Analysis
3. Results and Discussion
3.1. Evaluation of CPEs to Promote RSV Sublingual Permeation
- Sodium dodecylsulfate (SDS). It is a widely used anionic surfactant consisting of a hydrophobic tail (C12) linked to a hydrophilic sulfate group. It appears as a white crystalline powder, is quite soluble in water and ethanol and is often used in the cosmetic and pharmaceutical fields to promote solubility as well as absorption of actives through epithelial membranes, e.g., skin and gastrointestinal mucosa. The permeation enhancer effects are attributable to the alteration of the ordered state of the extracellular lipids by solubilization [23,24,41];
- Sodium dehydrocolate (SDC). It belongs to the class of biliary salts/acids which are defined as amphipathic ionic biosurfactants with a steroid structure, as they are synthesized in the liver from cholesterol. Thanks to its high biocompatibility, it could be widely used as a permeation enhancer through skin buccal, nasal, lung and intestinal tissues. Moreover, it exerts chemical and enzymatic stabilization of drugs. The absorption enhancement effect is due to the extraction of membrane proteins, interaction with the lipid component of the membranes and the formation of inverse micelles which reversibly increase the fluidity of the apical and basolateral membranes, thus facilitating the passage of drugs [22,42];
- Transcutol® (T). It is also known as diethylene glycol monoethyl ether. It is a clear liquid characterized by low viscosity, a stability between pH 4-9 and a pleasant odor. It acts as a permeation enhancer by improving drug solubility inside the membranes (alteration of the partition coefficient) rather than directly increasing the drug diffusivity [43];
- Lysine hydrochloride (LYS). It is a cationic amino acid that belongs to the twenty essential amino acids and, consequently, it is biocompatible, safe and non-toxic. Its chemical permeation enhancement effect is mainly due to the establishment of ionic interactions with the charged groups of the mucosal membrane, thus increasing the diffusion process. Furthermore, lysine could benefit from the amino acid transporters and consequently direct active substances through the epithelial layers [26,44];
- Urea (U). It is a biocompatible organic compound that appears as a colorless crystalline solid. Numerous studies have depicted its permeation enhancement ability because of its highly moisturizing power (ability to recall and retain water). Moreover, urea is also able to increase the fluidity of the phospholipid bilayer while maintaining the integrity of the membrane protein domains [27];
- Menthol (M). It belongs to the terpenes, which are reported as permeation promoters obtained from natural sources and widely included on the Food and Drug Administration (FDA) list of safe agents. The permeation enhancement power of terpenes is mainly linked to their chemical structure and their physicochemical properties. In particular, menthol is able to increase the interaction with the non-polar membranes and it is also useful as a flavoring agent, thus increasing patients’ compliance [45].
3.2. Preparation and Characterization of RSV-Loaded Mini-Tablets by Spray Drying Technique
3.3. Characterization of RSV-B Powder and Mini-Tablets
- Ability to completely and quickly disintegrate to ensure good patient compliance (DT: 4.06 ± 0.39 s);
- Ability to quickly release the embedded drug thus ensuring the formation of a concentrated in situ solution (RSV released after 15 min: 88.94 ± 8.19%);
- Ability to promote drug solubility (RSV concentration in the donor chamber: 192.44 ± 37.40 μg/mL);
- Ability to ensure a high drug flux through the administration site in order to maximize its entrance into the bloodstream allowing systemic effects (RSV flux after RSV-B administration: 22.26 ± 4.78 μg/cm2∙h−1);
- Ability to promote an immediate absorption, allowing a fast achievement of the desired effects (lag time absence; 8.41-fold increased Kp value with respect to RSV solution 1 mg/mL).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pangeni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. npj Precis. Oncol. 2017, 1, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2017, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Angellotti, G.; Murgia, D.; Presentato, A.; D’Oca, M.C.; Scarpaci, A.G.; Alduina, R.; Raimondi, M.V.; De Caro, V. Antibacterial PEGylated Solid Lipid Microparticles for Cosmeceutical Purpose: Formulation, Characterization, and Efficacy Evaluation. Materials 2020, 13, 2073. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuzhu, G.; Komai-Koma, M.; Leung, B.; Howe, H.S.; McSharry, C.; McInnes, I.; Xu, D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann. Rheum. Dis. 2011, 71, 129–135. [Google Scholar] [CrossRef]
- Nunes, S.; Danesi, F.; Del Rio, D.; Silva, P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr. Res. Rev. 2017, 31, 85–97. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Horne, J.R.; Vohl, M.-C. Biological plausibilitfory for interactions between dietary fat, resveratrol, ACE2, and SARS-CoV illness severity. Am. J. Physiol. Metab. 2020, 318, E830–E833. [Google Scholar] [CrossRef] [Green Version]
- Almeida, L.; Silva, M.V.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.-F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53, S7–S15. [Google Scholar] [CrossRef]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in hu-mans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Robinson, K.; Mock, C.; Liang, D. Pre-formulation studies of resveratrol. Drug Dev. Ind. Pharm. 2014, 41, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther. 2018, 21, 84–90. [Google Scholar] [CrossRef]
- AlAli, A.; Aldawsari, M.; Alalaiwe, A.; Almutairy, B.; Al-Shdefat, R.; Walbi, I.; Fayed, M. Exploitation of Design-of-Experiment Approach for Design and Optimization of Fast-Disintegrating Tablets for Sublingual Delivery of Sildenafil Citrate with Enhanced Bioavailability Using Fluid-Bed Granulation Technique. Pharmaceutics 2021, 13, 870. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.; Dawes, C. The Surface Area of the Adult Human Mouth and Thickness of the Salivary Film Covering the Teeth and Oral Mucosa. J. Dent. Res. 1987, 66, 1300–1302. [Google Scholar] [CrossRef]
- Sallam, N.M.; Sanad, R.A.-B.; Kharshoum, R.M.; Zineldin, M.A. Development of Salbutamol Sulphate fast disintegrating sublingual tablets with enhanced bioavailability and improved clinical efficacy for potential treatment of asthma. J. Drug Deliv. Sci. Technol. 2017, 41, 78–89. [Google Scholar] [CrossRef]
- Aungst, B.J. Absorption Enhancers: Applications and Advances. AAPS J. 2011, 14, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Hassan, N.; Ahad, A.; Ali, M.; Ali, J. Chemical permeation enhancers for transbuccal drug delivery. Expert Opin. Drug Deliv. 2009, 7, 97–112. [Google Scholar] [CrossRef]
- Sohi, H.; Ahuja, A.; Ahmad, F.J.; Khar, R.K. Critical evaluation of permeation enhancers for oral mucosal drug delivery. Drug Dev. Ind. Pharm. 2009, 36, 254–282. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Ali, A.A.; Aboud, H.; Hassan, A.H.; Godah, A.H. Transbuccal delivery of betahistine dihydrochloride from mucoadhesive tablets with a unidirectional drug flow: In vitro, ex vivo and in vivo evaluation. Drug Des. Dev. Ther. 2016, 10, 4031–4045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, S.E.; Marxen, E.; Janfelt, C.; Jacobsen, J. Buccal delivery of small molecules—Impact of levulinic acid, oleic acid, sodium dodecyl sulfate and hypotonicity on ex vivo permeability and spatial distribution in mucosa. Eur. J. Pharm. Biopharm. 2018, 133, 250–257. [Google Scholar] [CrossRef]
- Sandri, G.; Ruggeri, M.; Rossi, S.; Bonferoni, M.C.; Vigani, B.; Ferrari, F. (Trans)buccal drug delivery. In Nanotechnology for Oral Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–250. [Google Scholar]
- Javadzadeh, Y.; Adibkia, K.; Hamishekar, H. Transcutol® (diethylene glycol monoethyl ether): A potential penetration enhancer. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Modification of the Stratum Corneum; Springer: Berlin/Heidelberg, Germany, 2015; pp. 195–205. ISBN 9783662470398. [Google Scholar]
- Morales, J.; Brayden, D. Buccal delivery of small molecules and biologics: Of mucoadhesive polymers, films, and nanoparticles. Curr. Opin. Pharmacol. 2017, 36, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Dodla, S.; Velmurugan, S. Buccal penetration enhancers—An overview. Asian J. Pharm. Clin. Res. 2013, 6, 39–47. [Google Scholar]
- Chen, J.; Jiang, Q.D.; Chai, Y.P.; Zhang, H.; Peng, P.; Yang, X.X. Natural terpenes as penetration enhancers for transdermal drug delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuda, Y.; Irisawa, Y.; Okimoto, K.; Osawa, T.; Yamashita, S. A new formulation for orally disintegrating tablets using a suspension spray-coating method. Int. J. Pharm. 2009, 382, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S.M. Nano spray drying for encapsulation of pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- del Consuelo, I.D.; Pizzolato, G.-P.; Falson, F.; Guy, R.; Jacques, Y. Evaluation of pig esophageal mucosa as a permeability barrier model for buccal tissue. J. Pharm. Sci. 2005, 94, 2777–2788. [Google Scholar] [CrossRef]
- De Caro, V.; Giandalia, G.; Siragusa, M.; Sutera, F.M.; Giannola, L.I. New prospective in treatment of Parkinson’s disease: Studies on permeation of ropinirole through buccal mucosa. Int. J. Pharm. 2012, 429, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Di Prima, G.; Campisi, G.; De Caro, V. Amorphous Ropinirole-Loaded Mucoadhesive Buccal Film: A Potential Patient-Friendly Tool to Improve Drug Pharmacokinetic Profile and Effectiveness. J. Pers. Med. 2020, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Di Prima, G.; Saladino, S.; Bongiovì, F.; Adamo, G.; Ghersi, G.; Pitarresi, G.; Giammona, G. Novel inulin-based mucoadhesive micelles loaded with corticosteroids as potential transcorneal permeation enhancers. Eur. J. Pharm. Biopharm. 2017, 117, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Murgia, D.; Angellotti, G.; Conigliaro, A.; Pavia, F.C.; D’Agostino, F.; Contardi, M.; Mauceri, R.; Alessandro, R.; Campisi, G.; De Caro, V. Development of a Multifunctional Bioerodible Nanocomposite Containing Metronidazole and Curcumin to Apply on L-PRF Clot to Promote Tissue Regeneration in Dentistry. Biomedicines 2020, 8, 425. [Google Scholar] [CrossRef]
- Di Prima, G.; Bongiovì, F.; Palumbo, F.S.; Pitarresi, G.; Licciardi, M.; Giammona, G. Mucoadhesive PEGylated inulin-based self-assembling nanoparticles: In vitro and ex vivo transcorneal permeation enhancement of corticosteroids. J. Drug Deliv. Sci. Technol. 2018, 49, 195–208. [Google Scholar] [CrossRef]
- Di Prima, G.; Conigliaro, A.; De Caro, V. Mucoadhesive Polymeric Films to Enhance Barbaloin Penetration Into Buccal Mucosa: A Novel Approach to Chemoprevention. AAPS PharmSciTech 2019, 20, 1–12. [Google Scholar] [CrossRef]
- Di Prima, G.; Licciardi, M.; Pavia, F.C.; Monte, A.I.L.; Cavallaro, G.; Giammona, G. Microfibrillar polymeric ocular inserts for triamcinolone acetonide delivery. Int. J. Pharm. 2019, 567, 118459. [Google Scholar] [CrossRef]
- Borges, R.; Costa, F.; Pereira, T.; Araújo, R.; Almeida, E.; Da Silva, A. N-Acetyl-cysteine Increases Chemical Stability of Hydroquinone in Pharmaceutical Formulations: A Theoretical and Experimental Approach. J. Braz. Chem. Soc. 2017. [Google Scholar] [CrossRef]
- Dhiman, M.K.; Dhiman, A.; Sawant, K.K. Transbuccal Delivery of 5-Fluorouracil: Permeation Enhancement and Pharmacokinetic Study. AAPS PharmSciTech 2009, 10, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Moghimipour, E.; Ameri, A.; Handali, S. Absorption-Enhancing Effects of Bile Salts. Molecules 2015, 20, 14451–14473. [Google Scholar] [CrossRef] [Green Version]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®—Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef]
- Iyire, A.; Alaayedi, M.; Mohammed, A.R. Pre-formulation and systematic evaluation of amino acid assisted permeability of insulin across in vitro buccal cell layers. Sci. Rep. 2016, 6, 32498. [Google Scholar] [CrossRef]
- Ahad, A.; Aqil, M.; Ali, A. The application of anethole, menthone, and eugenol in transdermal penetration of valsartan: Enhancement and mechanistic investigation. Pharm. Biol. 2015, 54, 1042–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sfondrini, M.F.; Fraticelli, D.; Barbati, M.; Scribante, A.; Gandini, P. Effects of a new pharmaceutical preparation on the soft tissues healing after apthous lesions in pediatric patients. Dent. Cadmos 2012, 80, 334–339. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.S.N.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conven-tional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Pal, K.; Anis, A.; Pramanik, K.; Prabhakar, B. Polymers in Mucoadhesive Drug-Delivery Systems: A Brief Note Review Polymers in Mucoadhesive Drug-Delivery Systems: A Brief Note. Des. Monomers Polym. 2009, 12, 483–495. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Dinge, A.; Nagarsenker, M. Formulation and Evaluation of Fast Dissolving Films for Delivery of Triclosan to the Oral Cavity. AAPS PharmSciTech 2008, 9, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Lê, T. Barel, Paye, Maibach. In Handbook of Cosmetic Science and Technology; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Silva, C.G.; Monteiro, J.; Marques, R.R.; Silva, A.; Martínez, C.; Canle, M.; Faria, J.L. Photochemical and photocatalytic degradation of trans-resveratrol. Photochem. Photobiol. Sci. 2012, 12, 638–644. [Google Scholar] [CrossRef]
- Bayrak, Z.; Tas, C.; Tasdemir, U.; Erol, H.; Ozkan, C.K.; Savaser, A.; Ozkan, Y. Formulation of zolmitriptan sublingual tablets prepared by direct compression with different polymers: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2011, 78, 499–505. [Google Scholar] [CrossRef]
- Vanbillemont, B.; Everaert, H.; De Beer, T. New advances in the characterization of lyophilised orally disintegrating tablets. Int. J. Pharm. 2020, 579, 119153. [Google Scholar] [CrossRef] [PubMed]
- Kalný, M.; Grof, Z.; Štěpánek, F. Microstructure based simulation of the disintegration and dissolution of immediate release pharmaceutical tablets. Powder Technol. 2020, 377, 257–268. [Google Scholar] [CrossRef]











| PVP K90 (%) | PEG200 (%) | Sorbitol (%) | Propylene Glycol (%) | Trans-Resveratrol (%) | |
|---|---|---|---|---|---|
| RSV-A | 59 | 20 | 6 | - | 15 |
| RSV-B | 45 | 20 | 20 | - | 15 |
| RSV-C | 49 | 30 | 6 | - | 15 |
| RSV-D | 35 | 20 | 10 | 10 | 15 |
| PVP K90 (%) | PEG200 (%) | Sorbitol (%) | Trans-Resveratrol (%) | Lysine (%) | Menthol (%) | Urea (%) | |
|---|---|---|---|---|---|---|---|
| RSV-B/LYS-3 | 45 | 20 | 20 | 15 | 3 | - | - |
| RSV-B/M-3 | 45 | 20 | 20 | 15 | - | 3 | - |
| RSV-B/U-3 | 45 | 20 | 20 | 15 | - | - | 3 |
| RSV-B/U-1 | 45 | 20 | 20 | 15 | - | - | 1 |
| RSV-C/LYS-3 | 49 | 30 | 6 | 15 | 3 | - | - |
| Js (mg/cm2∙h−1) = Kp (cm/h) * | tlag (min) | De (mg/cm2) = Ac (cm) * | |
|---|---|---|---|
| RSV | 0.01376 ± 0.00311 | 19 min | 0.02271 ± 0.00554 |
| RSV+T | 0.01829 ± 0.00347 | 29 min | 0.03086 ± 0.00883 |
| RSV+SDC | 0.02139 ± 0.00401 | 11 min | 0.08435 ± 0.00922 |
| RSV+SDS | 0.02718 ± 0.00286 | 26 min | 0.04108 ± 0.00510 |
| RSV+U | 0.03226 ± 0.00220 | 22 min | 0.03203 ± 0.00622 |
| RSV+LYS | 0.03247 ± 0.00067 | 5 min | 0.03384 ± 0.01013 |
| RSV+M | 0.04780 ± 0.00896 | – | 0.03472 ± 0.00553 |
| Composition | Yield % | DL% | LE% |
|---|---|---|---|
| RSV-A | 76.0 | 16.54 ± 1.06 | 110.29 ± 7.09 |
| RSV-B | 82.0 | 11.06 ± 0.67 | 73.71 ± 4.50 |
| RSV-C | 64.4 | 15.41 ± 0.44 | 102.73 ± 2.95 |
| RSV-D | 67.6 | 15.67 ± 0.33 | 102.44 ± 2.22 |
| Formulation | Weight (mg) | RSV per Mini-Tablet (mg) |
|---|---|---|
| RSV-A mini-tablet | 29.96 ± 0.38 | 4.96 ± 0.06 |
| RSV-B mini-tablet | 30.60 ± 0.47 | 3.28 ± 0.05 |
| RSV-C mini-tablet | 30.59 ± 0.12 | 4.71 ± 0.02 |
| RSV-D mini-tablet | 30.82 ± 0.40 | 4.74 ± 0.06 |
| Sample | Js (μg/cm2∙h−1) | Kp (cm/h) | tlag (min) | De (μg/cm2) | Ac (cm) | (RSV)DONOR (μg/mL) |
|---|---|---|---|---|---|---|
| RSV | 13.76 ± 3.11 | 0.01376 ± 0.00311 | 19 | 22.71 ± 5.54 | 0.02271 ± 0.00554 | 1000 (STD) |
| RSV-A | 1.95 ± 0.64 | 0.05944 ± 0.01981 | NO | 7.12 ± 1.58 | 0.21702 ± 0.07829 | 32.74 ± 4.60 |
| RSV-B | 22.26 ± 4.78 | 0.11566 ± 0.01422 | NO | 25.19 ± 9.36 | 0.13087 ± 0.08584 | 192.44 ± 37.40 |
| RSV-C | 3.70 ± 0.35 | 0.22115 ± 0.02894 | NO | 7.13 ± 1.07 | 0.42688 ± 0.09983 | 16.71 ± 2.17 |
| RSV-D | 3.95 ± 0.55 | 0.03502 ± 0.00932 | NO | 81.18 ± 5.22 | 0.71939 ± 0.29361 | 112.85 ± 16.76 |
| Sample | Spray-Dried Powders | Mini-Tablets | |||
|---|---|---|---|---|---|
| Yield % | DL% | LE% | Weight (mg) | RSV (mg) | |
| RSV-B/M-3 | 73.2 | 13.40 ± 0.34 | 89.33 ± 2.27 | 30.43 ± 0.60 | 4.08 ± 0.08 |
| RSV-B/LYS-3 | 74.8 | 15.15 ± 1.41 | 101.02 ± 9.39 | 31.18 ± 1.07 | 4.72 ± 0.16 |
| RSV-B/U-3 | 75.4 | 15.03 ± 0.76 | 100.22 ± 5.07 | 30.20 ± 0.38 | 4.58 ± 0.06 |
| RSV-B/U-1 | 83.2 | 11.02 ± 1.33 | 73.47 ± 8.87 | 29.11 ± 0.31 | 4.41 ± 0.05 |
| RSV-C/LYS-3 | 73.2 | 15.75 ± 1.70 | 105.00 ± 11.33 | 31.38 ± 0.60 | 4.75 ± 0.09 |
| Sample | Js (μg/cm2∙h-1) | Kp (cm/h) | tlag (min) | De (μg/cm2) | Ac (cm) | (RSV)DONOR (μg/mL) |
|---|---|---|---|---|---|---|
| RSV-B/M-3 | 3.84 ± 1.61 | 0.08023 ± 0.05225 | NO | 14.28 ± 3.21 | 0.29870 ± 0.08721 | 47.82 ± 6.56 |
| RSV-B/LYS-3 | 7.96 ± 1.00 | 0.16635 ± 0.03022 | NO | 2.84 ± 1.00 | 0.05941 ± 0.02586 | 47.89 ± 8.25 |
| RSV-B/U-3 | 7.17 ± 1.77 | 0.05240 ± 0.01536 | NO | 23.94 ± 6.78 | 0.17504 ± 0.10061 | 136.76 ± 41.63 |
| RSV-B/U-1 | 3.82 ± 0.18 | 0.05127 ± 0.00547 | NO | 14.36 ± 4.91 | 0.19267 ± 0.07104 | 74.55 ± 8.35 |
| RSV-C/LYS-3 | 7.40 ± 0.96 | 0.22531 ± 0.02123 | NO | 5.90 ± 2.16 | 0.17979 ± 0.06396 | 32.83 ± 4.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Prima, G.; Angellotti, G.; Scarpaci, A.G.; Murgia, D.; D’agostino, F.; Campisi, G.; De Caro, V. Improvement of Resveratrol Permeation through Sublingual Mucosa: Chemical Permeation Enhancers versus Spray Drying Technique to Obtain Fast-Disintegrating Sublingual Mini-Tablets. Pharmaceutics 2021, 13, 1370. https://doi.org/10.3390/pharmaceutics13091370
Di Prima G, Angellotti G, Scarpaci AG, Murgia D, D’agostino F, Campisi G, De Caro V. Improvement of Resveratrol Permeation through Sublingual Mucosa: Chemical Permeation Enhancers versus Spray Drying Technique to Obtain Fast-Disintegrating Sublingual Mini-Tablets. Pharmaceutics. 2021; 13(9):1370. https://doi.org/10.3390/pharmaceutics13091370
Chicago/Turabian StyleDi Prima, Giulia, Giuseppe Angellotti, Amalia Giulia Scarpaci, Denise Murgia, Fabio D’agostino, Giuseppina Campisi, and Viviana De Caro. 2021. "Improvement of Resveratrol Permeation through Sublingual Mucosa: Chemical Permeation Enhancers versus Spray Drying Technique to Obtain Fast-Disintegrating Sublingual Mini-Tablets" Pharmaceutics 13, no. 9: 1370. https://doi.org/10.3390/pharmaceutics13091370