The Influence of Omeprazole on the Dissolution Processes of pH-Dependent Magnetic Tablets Assessed by Pharmacomagnetography
Abstract
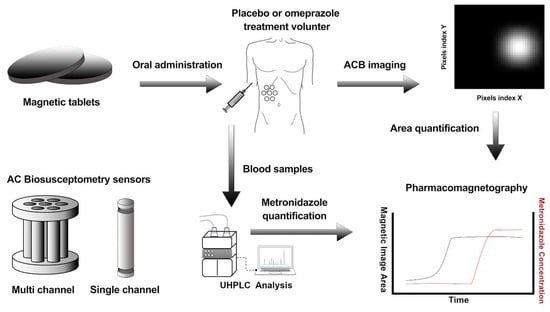
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. pH-Dependent Magnetic Coated Tablets
2.3. Alternate Current Biosusceptometry
2.4. In Vitro Studies
2.4.1. USP 2 Dissolution Procedure
2.4.2. Multichannel ACB System Measurements (MC-ACB)
2.5. In Vivo Study Protocol
2.6. Pharmacokinetic Analysis
2.7. Magnetic Data Analysis
2.8. Statistical Analysis
3. Results
3.1. Tablet Quality Control Assays
3.2. In Vitro Studies
3.3. In Vivo study Protocols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huanbutta, K.; Yunsir, A.; Sriamornsak, P.; Sangnim, T. Development and in vitro/in vivo evaluation of tamarind seed gum-based oral disintegrating tablets after fabrication by freeze drying. J. Drug Deliv. Sci. Technol. 2019, 54, 101298. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sinica. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Debotton, N.; Dahan, A. Applications of Polymers as Pharmaceutical Excipients in Solid Oral Dosage Forms. Med. Res. Rev. 2017, 37, 52–97. [Google Scholar] [CrossRef]
- Silchenko, S.; Nessah, N.; Li, J.; Li, L.B.; Huang, Y.; Owen, A.J.; Hidalgo, I.J. In vitro dissolution absorption system (IDAS2): Use for the prediction of food viscosity effects on drug dissolution and absorption from oral solid dosage forms. Eur. J. Pharm. Sci. 2020, 143, 105164. [Google Scholar] [CrossRef]
- Koziolek, M.; Gorke, K.; Neumann, M.; Garbacz, G.; Weitschies, W. Development of a bio-relevant dissolution test device simulating mechanical aspects present in the fed stomach. Eur. J. Pharm. Sci. 2014, 57, 250–256. [Google Scholar] [CrossRef]
- Koziolek, M.; Schneider, F.; Grimm, M.; Modebeta, C.; Seekamp, A.; Roustom, T.; Siegmund, W.; Weitschies, W. Intragastric pH and pressure profiles after intake of the high-caloric, high-fat meal as used for food effect studies. J. Control. Release 2015, 220, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Abuhelwa, A.Y.; Williams, D.B.; Upton, R.N.; Foster, D.J. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm. 2017, 112, 234–248. [Google Scholar] [CrossRef] [PubMed]
- van Seyen, M.; Colbers, A.; Abbink, E.J.; Drenth, J.P.H.; Burger, D.M. Concomitant Intake of Coca-Cola to Manage the Drug-Drug Interaction Between Velpatasvir and Omeprazole Studied in Healthy Volunteers. Clin. Pharmacol. Ther. 2019, 106, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Bellia, G.F.; Gil-Ramirez, G.; Baron, M.G.; Croxton, R.; Gonzalez-Rodriguez, J. Analysis of omeprazole and esomeprazole obtained from traditional pharmacies and unlicensed internet websites using Raman spectroscopy, 1H-NMR and chemometric analysis. Vib. Spectrosc. 2020, 106, 102996. [Google Scholar] [CrossRef]
- Abuhelwa, A.Y.; Mudge, S.; Upton, R.N.; Foster, D.J.R. Mechanistic Assessment of the Effect of Omeprazole on the In Vivo Pharmacokinetics of Itraconazole in Healthy Volunteers. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 201–215. [Google Scholar] [CrossRef]
- Ronchi, F.; Sereno, A.; Paide, M.; Sacre, P.; Guillaume, G.; Stephenne, V.; Goole, J.; Amighi, K. Development and evaluation of an omeprazole-based delayed-release liquid oral dosage form. Int. J. Pharm. 2019, 567, 118416. [Google Scholar] [CrossRef]
- van der Bol, J.M.; Loos, W.J.; de Jong, F.A.; van Meerten, E.; Konings, I.R.; Lam, M.H.; de Bruijn, P.; Wiemer, E.A.; Verweij, J.; Mathijssen, R.H. Effect of omeprazole on the pharmacokinetics and toxicities of irinotecan in cancer patients: A prospective cross-over drug-drug interaction study. Eur. J. Cancer 2011, 47, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zeng, S.; Yu, L.S.; Zhou, Q. Pharmacokinetic drug interaction profile of omeprazole with adverse consequences and clinical risk management. Ther. Clin. Risk Manag. 2013, 9, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Attia, T.Z.; Yamashita, T.; Tsujino, H.; Derayea, S.M.; Tsutsumi, Y.; Uno, T. Effect of Drug Combination on Omeprazole Metabolism by Cytochrome P450 2C19 in Helicobacter pylori Eradication Therapy. Chem. Pharm. Bull. 2019, 67, 810–815. [Google Scholar] [CrossRef] [Green Version]
- Jaruratanasirikul, S.; Sriwiriyajan, S. Effect of omeprazole on the pharmacokinetics of itraconazole. Eur. J. Clin. Pharmacol. 1998, 54, 159–161. [Google Scholar] [CrossRef]
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric coating of oral solid dosage forms as a tool to improve drug bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Khan, S.; Nawaz, S.; Ranjha, N.M.; Haider, M.S.; Khan, M.M.; Dar, E.; Nawaz, A. Enhanced gastric retention and drug release via development of novel floating microspheres based on Eudragit E100 and polycaprolactone: Synthesis and in vitro evaluation. Des. Monomers Polym. 2017, 20, 419–433. [Google Scholar] [CrossRef] [Green Version]
- Cora, L.A.; Romeiro, F.G.; Paixao, F.C.; Americo, M.F.; Oliveira, R.B.; Baffa, O.; Miranda, J.R. Enteric coated magnetic HPMC capsules evaluated in human gastrointestinal tract by AC biosusceptometry. Pharm Res. 2006, 23, 1809–1816. [Google Scholar] [CrossRef]
- Weitschies, W.; Blume, H.; Monnikes, H. Magnetic marker monitoring: High resolution real-time tracking of oral solid dosage forms in the gastrointestinal tract. Eur. J. Pharm. Biopharm. 2010, 74, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Cora, L.A.; Americo, M.F.; Oliveira, R.B.; Serra, C.H.; Baffa, O.; Evangelista, R.C.; Oliveira, G.F.; Miranda, J.R. Biomagnetic methods: Technologies applied to pharmaceutical research. Pharm. Res. 2011, 28, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Cora, L.A.; Romeiro, F.G.; Stelzer, M.; Americo, M.F.; Oliveira, R.B.; Baffa, O.; Miranda, J.R. AC biosusceptometry in the study of drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1223–1241. [Google Scholar] [CrossRef]
- Pinto, L.A.; Corá, L.A.; Rodrigues, G.S.; Prospero, A.G.; Soares, G.A.; de Andreis, U.; de Arruda Miranda, J.R. Pharmacomagnetography to evaluate the performance of magnetic enteric-coated tablets in the human gastrointestinal tract. Eur. J. Pharm. Biopharm. 2021, 161, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Soares, G.A.; Prospero, A.G.; Calabresi, M.F.; Rodrigues, D.S.; Simoes, L.G.; Quini, C.C.; Matos, R.R.; Pinto, L.A.; Sousa-Junior, A.A.; Bakuzis, A.F.; et al. Multichannel AC Biosusceptometry System to Map Biodistribution and Assess the Pharmacokinetic Profile of Magnetic Nanoparticles by Imaging. IEEE Trans. Nanobiosci. 2019, 18, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A. The concept of dissolution efficiency. J. Pharm. Pharmacol. 1975, 27, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Cora, L.A.; Andreis, U.; Romeiro, F.G.; Americo, M.F.; Oliveira, R.B.; Baffa, O.; Miranda, J.R. Magnetic images of the disintegration process of tablets in the human stomach by ac biosusceptometry. Phys. Med. Biol. 2005, 50, 5523–5534. [Google Scholar] [CrossRef]
- Campos, M.L.; de Moura Alonso, J.; Dos Santos Martins, E.; Oliveira, J.A.; Hussni, C.A.; Peccinini, R.G. Ceftriaxone pharmacokinetics by new simple and sensitive ultra-high-performance liquid chromatography method. Diagn. Microbiol. Infect. Dis. 2017, 88, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Perkins, A.C.; Wilson, C.G.; Frier, M.; Blackshaw, P.E.; Juan, D.; Dansereau, R.J.; Hathaway, S.; Li, Z.; Long, P.; Spiller, R.C. Oesophageal transit, disintegration and gastric emptying of a film-coated risedronate placebo tablet in gastro-oesophageal reflux disease and normal control subjects. Aliment. Pharmacol. Ther. 2001, 15, 115–121. [Google Scholar] [CrossRef]
- Corá, L.A.; Américo, M.F.; Romeiro, F.G.; Oliveira, R.B.; Miranda, J.R.A. Pharmaceutical applications of AC Biosusceptometry. Eur. J. Pharm. Biopharm. 2010, 74, 67–77. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Davit, B.M.; Kanfer, I.; Tsang, Y.C.; Cardot, J.-M. BCS Biowaivers: Similarities and Differences Among EMA, FDA, and WHO Requirements. AAPS J. 2016, 18, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.A.; Davit, B.M.; Polli, J.E. Impact of Biopharmaceutics Classification System-based biowaivers. Mol. Pharm 2010, 7, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Dressman, J.B.; Berardi, R.R.; Dermentzoglou, L.C.; Russell, T.L.; Schmaltz, S.P.; Barnett, J.L.; Jarvenpaa, K.M. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm. Res. 1990, 7, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Jessa, M.J.; Goddard, A.F.; Barrett, D.A.; Shaw, P.N.; Spiller, R.C. The effect of omeprazole on the pharmacokinetics of metronidazole and hydroxymetronidazole in human plasma, saliva and gastric juice. Br. J. Clin. Pharm. 1997, 44, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Chun, J.; Park, J.Y.; Hong, S.W.; Lee, J.Y.; Kang, J.W.; Hwang, S.; Ko, S.-B.; Im, J.P.; Kim, J.S. Metronidazole-induced encephalopathy in a patient with Crohn’s disease. Intest. Res. 2017, 15, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Katz, P.O.; Xue, S.; Castell, D.O. Control of intragastric pH with omeprazole 20 mg, omeprazole 40 mg and lansoprazole 30 mg. Aliment. Pharmacol. Ther. 2001, 15, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Löbenberg, R.; Chacra, N.B.; Stippler, E.S.; Shah, V.P.; DeStefano, A.J.; Hauck, W.W.; Williams, R.L. Toward global standards for comparator pharmaceutical products: Case studies of amoxicillin, metronidazole, and zidovudine in the Americas. AAPS J. 2012, 14, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Yin, O.Q.P.; Giles, F.J.; Baccarani, M.; le Coutre, P.; Chiparus, O.; Gallagher, N.; Saglio, G.; Hughes, T.P.; Hochhaus, A.; Kantarjian, H.M.; et al. Concurrent use of proton pump inhibitors or H2 blockers did not adversely affect nilotinib efficacy in patients with chronic myeloid leukemia. Cancer Chemother. Pharm. 2012, 70, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Rubbens, J.; Brouwers, J.; Tack, J.; Augustijns, P. Gastrointestinal dissolution, supersaturation and precipitation of the weak base indinavir in healthy volunteers. Eur. J. Pharm. Biopharm. 2016, 109, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, E.; Lask, G.; Martin, S. Sonic hedgehog pathway inhibition in the treatment of advanced basal cell carcinoma. Curr. Treat. Options Oncol. 2019, 20, 1–12. [Google Scholar] [CrossRef]
- Carpenter, R.L.; Ray, H. Efficacy and safety of sonic hedgehog pathway inhibitors in cancer. Drug Saf. 2019, 42, 263. [Google Scholar] [CrossRef] [PubMed]
- Sandhiya, S.; Melvin, G.; Kumar, S.S.; Dkhar, S.A. The dawn of hedgehog inhibitors: Vismodegib. J. Pharmcol. Pharmcother. 2013, 4, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Quinlan, M.; Glenn, K.; Boss, H.; Picard, F.; Castro, H.; Sellami, D. Effect of esomeprazole, a proton pump inhibitor on the pharmacokinetics of sonidegib in healthy volunteers. Br. J. Clin. Pharm. 2016, 82, 1022–1029. [Google Scholar] [CrossRef] [Green Version]
- Smelick, G.S.; Heffron, T.P.; Chu, L.; Dean, B.; West, D.A.; DuVall, S.L.; Lum, B.L.; Budha, N.; Holden, S.N.; Benet, L.Z. Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug–drug interaction potential for molecular targeted agents in clinical development. Mol. Pharm. 2013, 10, 4055–4062. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.H.; Ou, X.L.; Cao, D.Z.; Yu, Q.; Yu, T.; Hu, J.M.; Zhu, F.; Sun, Y.L.; Fu, X.L.; Su, H. Efficacy of omeprazole and amoxicillin with either clarithromycin or metronidazole on eradication of Helicobacter pylori in Chinese peptic ulcer patients. World J. Gastroenterol. 2005, 11, 2477–2481. [Google Scholar] [CrossRef] [PubMed]
- Gee, S.C.; Hagemann, T.M. Palatability of liquid anti-infectives: Clinician and student perceptions and practice outcomes. J. Pediatric Pharmacol. Ther. 2007, 12, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Ruff, M.E.; Schotik, D.A.; Bass, J.W.; Vincent, J.M. Antimicrobial drug suspensions: A blind comparison of taste of fourteen common pediatric drugs. Pediatric Infect. Dis. J. 1991, 10, 30–33. [Google Scholar] [CrossRef]
- Uchida, T.; Tanigake, A.; Miyanaga, Y.; Matsuyama, K.; Kunitomo, M.; Kobayashi, Y.; Ikezaki, H.; Taniguchi, A. Evaluation of the bitterness of antibiotics using a taste sensor. J. Pharm. Pharmacol. 2003, 55, 1479–1485. [Google Scholar] [CrossRef]
- Keeley, A.; Teo, M.; Ali, Z.; Frost, J.; Ghimire, M.; Rajabi-Siahboomi, A.; Orlu, M.; Tuleu, C. In Vitro Dissolution Model Can Predict the in Vivo Taste Masking Performance of Coated Multiparticulates. Mol Pharm 2019, 16, 2095–2105. [Google Scholar] [CrossRef]
- Al-kasmi, B.; Alsirawan, M.H.D.B.; Paradkar, A.; Nattouf, A.-H.; El-Zein, H. Aqueous and pH dependent coacervation method for taste masking of paracetamol via amorphous solid dispersion formation. Sci. Rep. 2021, 11, 8907. [Google Scholar] [CrossRef]
- Weitschies, W.; Friedrich, C.; Wedemeyer, R.S.; Schmidtmann, M.; Kosch, O.; Kinzig, M.; Trahms, L.; Sorgel, F.; Siegmund, W.; Horkovics-Kovats, S.; et al. Bioavailability of amoxicillin and clavulanic acid from extended release tablets depends on intragastric tablet deposition and gastric emptying. Eur. J. Pharm. Biopharm. 2008, 70, 641–648. [Google Scholar] [CrossRef]





| pH 1.2 | pH 2.0 | pH 3.0 | pH 4.5 | |
|---|---|---|---|---|
| (min) | 0.58 ± 0.20 | 1.16 ± 0.40 | 1.33 ± 0.52 a | 3.16 ± 0.40 a,b,c |
| DE (%) | 90.93 ± 8.11 | 94.21 ± 4.55 | 92.21 ± 2.51 | 92.50 ± 5.08 |
| pH 1.2 | pH 2.0 | pH 3.0 | pH 4.5 | |
|---|---|---|---|---|
| (min) | 2.19 ± 0.10 | 3.03 ± 0.18 a | 4.01 ± 0.15 a,b | 9.54 ± 1.42 a,b,c |
| Control | Omeprazole | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Tlag (min) | Tmax (min) | Cmax (µg/m) | AUC0–60 (µg min/mL) | AUC0–300 (µg min/mL) | T50 (min) | GRT (min) | Tlag (min) | Tmax (min) | Cmax (µg/mL) | AUC0–60 (µg min/mL) | AUC0–300 (µg min/mL) | T50 (min) | GRT (min) |
| 1 | 18.00 | 90.00 | 1.99 | 38.76 | 500.91 | 7.00 | 35.00 | 12.00 | 120.00 | 7.64 | 32.99 | 527.69 | 10.00 | 45.00 |
| 2 | 15.00 | 150.00 | 1.69 | 37.28 | 420.08 | 10.00 | 40.00 | NA | 0 | 0 | 0 | 0 | NA | 35.00 |
| 3 | 18.00 | 120.00 | 2.49 | 59.759 | 556.34 | 7.00 | 45.00 | NA | 0 | 0 | 0 | 0 | NA | 40.00 |
| 4 | 18.00 | 150.00 | 2.42 | 18.90 | 534.65 | 8.00 | 40.00 | 45.00 | 180.00 | 1.83 | 18.86 | 512.21 | NA | 35.00 |
| 5 | 30.00 | 90.00 | 2.57 | 55.35 | 531.90 | 23.00 | 45.00 | NA | 0 | 0 | 0 | 0 | NA | 75.00 |
| 6 | 15.00 | 90.00 | 2.80 | 83.36 | 506.36 | 12.00 | 105.00 | 150.00 | 240.00 | 2.68 | 5.31 | 305.61 | NA | 120.00 |
| 7 | 15.00 | 120.00 | 3.10 | 62.49 | 718.44 | 10.00 | 120.00 | 21.00 | 120.00 | 5.42 | 54.39 | 773.49 | 10.00 | 120.00 |
| 8 | 24.00 | 120.00 | 1.55 | 27.72 | 345.15 | 18.00 | 60.00 | NA | 0 | 0 | 0 | 0 | NA | 40.00 |
| 9 | 24.00 | 90.00 | 1.91 | 26.52 | 545.47 | 15.00 | 40.00 | 45.00 | 150.00 | 3.09 | 21.09 | 507.09 | NA | 35.00 |
| 10 | 18.00 | 120.00 | 2.20 | 40.62 | 484.92 | 12.00 | 45.00 | 45.00 | 90.00 | 2.46 | 14.09 | 452.09 | NA | 35.00 |
| 11 | 18.00 | 120.00 | 1.80 | 27.86 | 353.96 | 8.00 | 35.00 | NA | 0 | 0 | 0 | 0 | NA | 50.00 |
| 12 | 18.00 | 120.00 | 1.52 | 36.68 | 363.83 | 10.00 | 45.00 | 15.00 | 90.00 | 9.55 | 35.58 | 441.03 | 10.00 | 35.00 |
| Mean | 19.25 | 115.00 | 2.17 | 42.76 | 488.50 | 11.67 | 54.58 | 27.75 | 82.5 | 1.333 | 15.19 | 293.3 | - | 55.42 |
| SD | 4.51 | 21.53 | 0.51 | 18.55 | 106.20 | 4.85 | 28.00 | 42.79 | 83.03 | 1.25 | 18.02 | 279 | - | 32.22 |
| Median | 18.00 | 120.00 | 2.09 | 38.02 | 503.60 | 10.00 | 45.00 | 13.5 | 90.00 | 1.86 | 9.7 | 373.3 | - | 40.00 |
| IQ | 15.75-22.5 | 90-120 | 1.718-2.55 | 27.76–57.03 | 377.9–543 | 40–56.25 | 0–45 | 0–142.5 | 0–2.173 | 0–30.02 ** | 0–510.9 * | 35–98.75 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, G.A.; Pires, D.W.; Pinto, L.A.; Rodrigues, G.S.; Prospero, A.G.; Biasotti, G.G.A.; Bittencourt, G.N.; Stoppa, E.G.; Corá, L.A.; Oliveira, R.B.; et al. The Influence of Omeprazole on the Dissolution Processes of pH-Dependent Magnetic Tablets Assessed by Pharmacomagnetography. Pharmaceutics 2021, 13, 1274. https://doi.org/10.3390/pharmaceutics13081274
Soares GA, Pires DW, Pinto LA, Rodrigues GS, Prospero AG, Biasotti GGA, Bittencourt GN, Stoppa EG, Corá LA, Oliveira RB, et al. The Influence of Omeprazole on the Dissolution Processes of pH-Dependent Magnetic Tablets Assessed by Pharmacomagnetography. Pharmaceutics. 2021; 13(8):1274. https://doi.org/10.3390/pharmaceutics13081274
Chicago/Turabian StyleSoares, Guilherme A., Deivid W. Pires, Leonardo A. Pinto, Gustavo S. Rodrigues, André G. Prospero, Gabriel G. A. Biasotti, Gabriela N. Bittencourt, Erick G. Stoppa, Luciana A. Corá, Ricardo B. Oliveira, and et al. 2021. "The Influence of Omeprazole on the Dissolution Processes of pH-Dependent Magnetic Tablets Assessed by Pharmacomagnetography" Pharmaceutics 13, no. 8: 1274. https://doi.org/10.3390/pharmaceutics13081274