Tablet Disintegration and Dispersion under In Vivo-like Hydrodynamic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Powder Manufacturing
2.3. Particle Size Analysis
2.4. Tablet Manufacturing
2.5. Tablet Water Uptake and Swelling Analysis
2.6. Standard Tablet Disintegration Analysis
2.7. Novel Tablet Disintegration and Dispersion Analysis
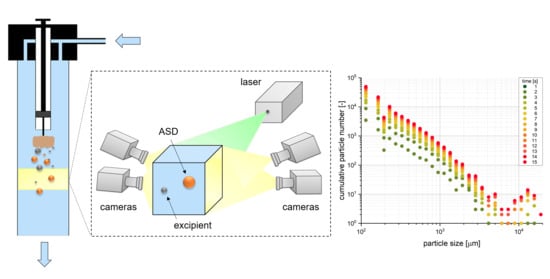
2.7.1. Experimental Set-Up
2.7.2. Experimental Procedure
2.7.3. Disintegration Time Analysis
2.7.4. Dispersion Analysis
3. Results and Discussion
3.1. Tablet Water Uptake and Swelling
3.2. Standard Tablet Disintegration Analysis
3.3. Verification of Novel Method for Tablet Disintegration and Dispersion Analysis
3.4. Application of Novel Method for Tablet Disintegration and Dispersion Analysis
3.4.1. Investigation of Fluid Velocity
3.4.2. Investigation of Tablet Formulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almoazen, H. Felton L. Remington: Essentials of Pharmaceutics. Am. J. Pharm. Educ. 2013, 77, 233. [Google Scholar] [CrossRef] [Green Version]
- Markl, D.; Zeitler, J.A. A review of disintegration mechanisms and measurement techniques. Pharm. Res. 2017, 34, 890–917. [Google Scholar] [CrossRef] [Green Version]
- Quodbach, J.; Kleinebudde, P. A critical review on tablet disintegration. Pharm. Dev. Technol. 2016, 21, 763–774. [Google Scholar] [CrossRef]
- Desai, P.M.; Liew, C.V.; Heng, P.W.S. Review of disintegrants and the disintegration phenomena. J. Pharm. Sci. 2016, 105, 2545–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litster, J. Dispersion, disintegration, and dissolution. In Design and Processing of Particulate Products; Cambridge University Press: Cambridge, UK, 2016; pp. 294–322. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention. <701> Disintegration. In United States Pharmacopeia and National Formulary (USP 43-NF 38); United States Pharmacopeial Convention: Rockville, MD, USA, 2019. [Google Scholar]
- Council of Europe, 2.9.1. Disintegration of tablets and capsules. In European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2021. [Google Scholar]
- Society of Japanese Pharmacopoeia, 6.09. Disintegration test. In The Japanese Pharmacopeia, 17th ed.; Society of Japanese Pharmacopoeia: Tokyo, Japan, 2021. [Google Scholar]
- Radwan, A.; Wagner, M.; Amidon, G.L.; Langguth, P. Bio-predictive tablet disintegration: Effect of water diffusivity, fluid flow, food composition and test conditions. Eur. J. Pharm. Sci. 2014, 57, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kindgen, S.; Wachtel, H.; Abrahamsson, B.; Langguth, P. Computational fluid dynamics simulation of hydrodynamics and stresses in the PhEur/USP disintegration tester under fed and fasted fluid characteristics. J. Pharm. Sci. 2015, 104, 2956–2968. [Google Scholar] [CrossRef] [Green Version]
- Butler, J.; Hens, B.; Vertzoni, M.; Brouwers, J.; Berben, P.; Dressman, J.; Andreas, C.J.; Schaefer, K.J.; Mann, J.; McAllister, M.; et al. In vitro models for the prediction of in vivo performance of oral dosage forms: Recent progress from partnership through the IMI OrBiTo collaboration. Eur. J. Pharm. Biopharm. 2019, 136, 70–83. [Google Scholar] [CrossRef]
- Mudie, D.M.; Amidon, G.L.; Amidon, G.E. Physiological parameters for oral delivery and in vitro testing. Mol. Pharm. 2010, 7, 1388–1405. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.M.; Mah, K.K.; Seluakumaran, K. Gastric motility: (Migrating motor complex, receptive relaxation, accommodation, retropulsion). In Defining Physiology: Principles, Themes, Concepts. Volume 2: Neurophysiology and Gastrointestinal Systems; Springer International Publishing: Cham, Switzerland, 2020; pp. 21–22. [Google Scholar] [CrossRef]
- Imai, Y.; Kobayashi, I.; Ishida, S.; Ishikawa, T.; Buist, M.; Yamaguchi, T. Antral recirculation in the stomach during gastric mixing. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G536–G542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausken, T.; Ødegaard, S.; Matre, K.; Berstad, A. Antroduodenal motility and movements of luminal contents studied by duplex sonography. Gastroenterology 1992, 102, 1583–1590. [Google Scholar] [CrossRef]
- Katori, N.; Aoyagi, N.; Terao, T. Estimation of agitation intensity in the GI tract in humans and dogs based on in vitro/in vivo correlation. Pharm. Res. 1995, 12, 237–243. [Google Scholar] [CrossRef]
- O’Grady, G.; Du, P.; Cheng, L.K.; Egbuji, J.U.; Lammers, W.J.E.P.; Windsor, J.A.; Pullan, A.J. Origin and propagation of human gastric slow-wave activity defined by high-resolution mapping. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G585–G592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulby, P.; Moore, R.; Gowland, P.; Spiller, R.C. Fat delays emptying but increases forward and backward antral flow as assessed by flow-sensitive magnetic resonance imaging. Neurogastroenterol. Motil. 1999, 11, 27–36. [Google Scholar] [CrossRef]
- Sarna, S.K.; Soergel, K.H.; Koch, T.R.; Stone, J.E.; Wood, C.M.; Ryan, R.P.; Arndorfer, R.C.; Cavanaugh, J.H.; Nellans, H.N.; Lee, M.B. Gastrointestinal motor effects of erythromycin in humans. Gastroenterology 1991, 101, 1488–1496. [Google Scholar] [CrossRef]
- Malagelada, J.R. Where do we stand on gastric motility? Scand. J. Gastroenterol. 1990, 25, 42–51. [Google Scholar] [CrossRef]
- Pal, A.; Indireshkumar, K.; Schwizer, W.; Abrahamsson, B.; Fried, M.; Brasseur, J.G. Gastric flow and mixing studied using computer simulation. Proc. Biol. Sci. 2004, 271, 2587–2594. [Google Scholar] [CrossRef] [Green Version]
- Ferrua, M.J.; Singh, R.P. Modeling the fluid dynamics in a human stomach to gain insight of food digestion. J. Food Sci. 2010, 75, R151–R162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrua, M.J.; Singh, R.P. Understanding the fluid dynamics of gastric digestion using computational modeling. Procedia Food Sci. 2011, 1, 1465–1472. [Google Scholar] [CrossRef] [Green Version]
- Kindgen, S.; Rach, R.; Nawroth, T.; Abrahamsson, B.; Langguth, P. A novel disintegration tester for solid dosage forms enabling adjustable hydrodynamics. J. Pharm. Sci. 2016, 105, 2402–2409. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, J.; Tomas, J.; Lizoňová, D.; Schöngut, M.; Dammer, O.; Pekárek, T.; Beránek, J.; Štěpánek, F. Investigation of tablet disintegration pathways by the combined use of magnetic resonance imaging, texture analysis and static light scattering. Int. J. Pharm. 2020, 587, 119719. [Google Scholar] [CrossRef]
- Coutant, C.A.; Skibic, M.J.; Doddridge, G.D.; Kemp, C.A.; Sperry, D.C. In vitro monitoring of dissolution of an immediate release tablet by focused beam reflectance measurement. Mol. Pharm. 2010, 7, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gupta, A.; Sayeed, V.A.; Khan, M.A. Process analytical technology to understand the disintegration behavior of alendronate sodium tablets. J. Pharm. Sci. 2013, 102, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Wren, S.; Reynolds, G. Linking dissolution to disintegration in immediate release tablets using image analysis and a population balance modelling approach. Pharm. Res. 2012, 29, 198–208. [Google Scholar] [CrossRef]
- Worlitschek, J.; Hocker, T.; Mazzotti, M. Restoration of PSD from chord length distribution data using the method of projections onto convex sets. Part. Part. Syst. Charact. 2005, 22, 81–98. [Google Scholar] [CrossRef]
- Bai, G.; Armenante, P.M.; Plank, R.V.; Gentzler, M.; Ford, K.; Harmon, P. Hydrodynamic investigation of USP dissolution test apparatus II. J. Pharm. Sci. 2007, 96, 2327–2349. [Google Scholar] [CrossRef] [PubMed]
- Quodbach, J.; Kleinebudde, P. A new apparatus for real-time assessment of the particle size distribution of disintegrating tablets. J. Pharm. Sci. 2014, 103, 3657–3665. [Google Scholar] [CrossRef]
- Mesnier, X.; Althaus, T.O.; Forny, L.; Niederreiter, G.; Palzer, S.; Hounslow, M.J.; Salman, A.D. A novel method to quantify tablet disintegration. Powder Technol. 2013, 238, 27–34. [Google Scholar] [CrossRef]
- Rajkumar, A.D.; Reynolds, G.K.; Wilson, D.; Wren, S.; Hounslow, M.J.; Salman, A.D. Investigating the effect of processing parameters on pharmaceutical tablet disintegration using a real-time particle imaging approach. Eur. J. Pharm. Biopharm. 2016, 106, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of increased bioavailability through amorphous solid dispersions: A review. Drug Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef]
- Demuth, B.; Nagy, Z.K.; Balogh, A.; Vigh, T.; Marosi, G.; Verreck, G.; Van Assche, I.; Brewster, M.E. Downstream processing of polymer-based amorphous solid dispersions to generate tablet formulations. Int. J. Pharm. 2015, 486, 268–286. [Google Scholar] [CrossRef]
- Goddeeris, C.; Willems, T.; Van den Mooter, G. Formulation of fast disintegrating tablets of ternary solid dispersions consisting of TPGS 1000 and HPMC 2910 or PVPVA 64 to improve the dissolution of the anti-HIV drug UC 781. Eur. J. Pharm. Sci. 2008, 34, 293–302. [Google Scholar] [CrossRef]
- Zhang, W.; Noland, R.; Chin, S.; Petkovic, M.; Zuniga, R.; Santarra, B.; Conklin, B.; Hou, H.H.; Nagapudi, K.; Gruenhagen, J.A.; et al. Impact of polymer type, ASD loading and polymer-drug ratio on ASD tablet disintegration and drug release. Int. J. Pharm. 2021, 592, 120087. [Google Scholar] [CrossRef]
- DiNunzio, J.C.; Schilling, S.U.; Coney, A.W.; Hughey, J.R.; Kaneko, N.; McGinity, J.W. Use of highly compressible Ceolus™ microcrystalline cellulose for improved dosage form properties containing a hydrophilic solid dispersion. Drug Dev. Ind. Pharm. 2012, 38, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenz, J.; Bunjes, H.; Kwade, A.; Juhnke, M. An improved method for the simultaneous determination of water uptake and swelling of tablets. Int. J. Pharm. 2021, 595, 120229. [Google Scholar] [CrossRef]
- Kristoffersen, A.S.; Erga, S.R.; Hamre, B.; Frette, O. Testing fluorescence lifetime standards using two-photon excitation and time-domain instrumentation: Rhodamine B, Coumarin 6 and Lucifer Yellow. J. Fluoresc. 2014, 24, 1015–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieneke, B. Volume self-calibration for 3D particle image velocimetry. Exp. Fluids 2008, 45, 549–556. [Google Scholar] [CrossRef]
- Wieneke, B. Improvements for volume self-calibration. Meas. Sci. Technol. 2018, 29, 084002. [Google Scholar] [CrossRef]
- Lenz, J.; Finke, J.H.; Bunjes, H.; Kwade, A.; Juhnke, M. Tablet formulation development focusing on the functional behaviour of water uptake and swelling. Int. J. Pharm. X 2021, 3, 100103. [Google Scholar] [CrossRef]
- Szafraniec-Szczęsny, J.; Antosik-Rogóż, A.; Kurek, M.; Gawlak, K.; Górska, A.; Peralta, S.; Knapik-Kowalczuk, J.; Kramarczyk, D.; Paluch, M.; Jachowicz, R. How does the addition of Kollidon(®)VA64 inhibit the recrystallization and improve Ezetimibe dissolution from amorphous solid dispersions? Pharmaceutics 2021, 13, 147. [Google Scholar] [CrossRef]
- Galeano, E.; Linares, V.; Yarce, C.; Echeverri, J.D.; Salamanca, C. Relationship between degree of polymeric ionisation and hydrolytic degradation of Eudragit® E polymers under extreme acid conditions. Polymers 2019, 11, 1010. [Google Scholar] [CrossRef] [Green Version]
- Quodbach, J.; Kleinebudde, P. Systematic classification of tablet disintegrants by water uptake and force development kinetics. J. Pharm. Pharmacol. 2014, 66, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Quodbach, J.; Moussavi, A.; Tammer, R.; Frahm, J.; Kleinebudde, P. Tablet disintegration studied by high-resolution real-time magnetic resonance imaging. J. Pharm. Sci. 2014, 103, 249–255. [Google Scholar] [CrossRef]
- Desai, P.M.; Liew, C.V.; Heng, P.W.S. Understanding disintegrant action by visualization. J. Pharm. Sci. 2012, 101, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Bisharat, L.; Blaibleh, A.; Pavoni, L.; Cespi, M. A simple and inexpensive image analysis technique to study the effect of disintegrants concentration and diluents type on disintegration. J. Pharm. Sci. 2018, 107, 2643–2652. [Google Scholar] [CrossRef]
- Berardi, A.; Bisharat, L.; Quodbach, J.; Abdel Rahim, S.; Perinelli, D.R.; Cespi, M. Advancing the understanding of the tablet disintegration phenomenon—An update on recent studies. Int. J. Pharm. 2021, 602, 120390. [Google Scholar] [CrossRef] [PubMed]












| Formulation | MCC/Eudragit EPO | MCC/Kollidon VA64 | ||||
|---|---|---|---|---|---|---|
| average fluid velocity | disintegrant type | disintegrant content | tablet porosity | disintegrant type | disintegrant content | tablet porosity |
| standard 55 mm/s | +++ | + | + | + | + | ++ |
| modified 9 mm/s | ++ | +++ | ++ | +++ | ++ | ++ |
| PE Particles Nominal Size Range (µm) | x10,3 (µm) | x50,3 (µm) | x90,3 (µm) | |||
|---|---|---|---|---|---|---|
| LD | 3D Tomo-LIF | LD | 3D Tomo-LIF | LD | 3D Tomo-LIF | |
| 45–53 | 40 | 65 | 52 | 94 | 69 | 136 |
| 125–150 | 106 | 168 | 145 | 207 | 198 | 463 |
| 355–425 | 283 | 359 | 380 | 446 | 500 | 490 |
| 710–850 | 579 | 871 | 791 | 957 | 1048 | 1313 |
| Formulation | Composition (% w/w) | Porosity (-) | DT (s) | |
|---|---|---|---|---|
| SDT | 3D Tomo-LIF | |||
| MCC/Kollidon VA64 | 80/20 | 0.3 | >600 | >600 |
| MCC/Kollidon VA64 | 80/20 | 0.2 | >600 | >600 |
| MCC/Kollidon VA64/NaCMCXL | 78/20/2 | 0.3 | 187 | 38 |
| MCC/Kollidon VA64/PVPP | 78/20/2 | 0.3 | >600 | >600 |
| MCC/Kollidon VA64/NaCMCXL | 78/20/2 | 0.2 | >600 | >600 |
| MCC/Kollidon VA64/PVPP | 78/20/2 | 0.2 | >600 | >600 |
| MCC/Kollidon VA64/NaCMCXL | 75/20/5 | 0.3 | 68 | 27 |
| MCC/Kollidon VA64/PVPP | 75/20/5 | 0.3 | >600 | 42 |
| MCC/Kollidon VA64/NaCMCXL | 75/20/5 | 0.2 | 389 | 314 |
| MCC/Kollidon VA64/PVPP | 75/20/5 | 0.2 | >600 | >600 |
| MCC/Eudragit EPO | 80/20 | 0.3 | >600 | >600 |
| MCC/Eudragit EPO | 80/20 | 0.2 | >600 | >600 |
| MCC/Eudragit EPO/NaCMCXL | 78/20/2 | 0.3 | >600 | 15 |
| MCC/Eudragit EPO/PVPP | 78/20/2 | 0.3 | >600 | >600 |
| MCC/Eudragit EPO/NaCMCXL | 78/20/2 | 0.2 | >600 | 76 |
| MCC/Eudragit EPO/PVPP | 78/20/2 | 0.2 | >600 | >600 |
| MCC/Eudragit EPO/NaCMCXL | 75/20/5 | 0.3 | 56 | 16 |
| MCC/Eudragit EPO/PVPP | 75/20/5 | 0.3 | >600 | 16 |
| MCC/Eudragit EPO/NaCMCXL | 75/20/5 | 0.2 | 85 | 26 |
| MCC/Eudragit EPO/PVPP | 75/20/5 | 0.2 | 418 | >600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenz, J.; Fuest, F.; Finke, J.H.; Bunjes, H.; Kwade, A.; Juhnke, M. Tablet Disintegration and Dispersion under In Vivo-like Hydrodynamic Conditions. Pharmaceutics 2022, 14, 208. https://doi.org/10.3390/pharmaceutics14010208
Lenz J, Fuest F, Finke JH, Bunjes H, Kwade A, Juhnke M. Tablet Disintegration and Dispersion under In Vivo-like Hydrodynamic Conditions. Pharmaceutics. 2022; 14(1):208. https://doi.org/10.3390/pharmaceutics14010208
Chicago/Turabian StyleLenz, Jan, Frederik Fuest, Jan Henrik Finke, Heike Bunjes, Arno Kwade, and Michael Juhnke. 2022. "Tablet Disintegration and Dispersion under In Vivo-like Hydrodynamic Conditions" Pharmaceutics 14, no. 1: 208. https://doi.org/10.3390/pharmaceutics14010208