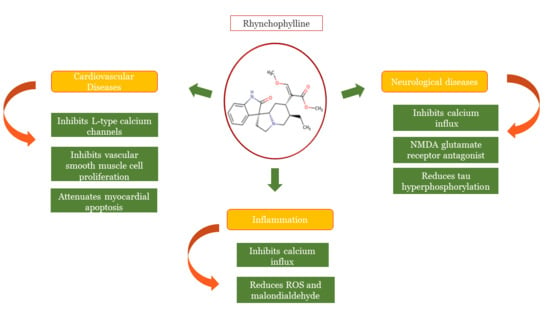
Recent Advances in the Anti-Inflammatory Activity of Plant-Derived Alkaloid Rhynchophylline in Neurological and Cardiovascular Diseases
Abstract
:1. Introduction
2. Rhynchophylline and Nervous Disorders
3. Rhynchophylline in Atherosclerosis and Other Cardiovascular Diseases
4. Pharmacological Effects of Rhynchophylline in Other Diseases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Rhy | Rhynchophylline |
| IsoRhy | Isorhynchophylline |
| oxLDL | Oxidized low-density lipoprotein |
| EPC | Endothelial progenitor cell |
| NSAID | Non-steroidal anti-inflammatory drugs |
| COX | Cyclooxygenase |
| IL-1 | Interleukin-1 |
| IL-8 | Interleukin-8 |
| TNF-α | Tumor necrosis factor α |
| IFN-γ | Interferon γ |
| hsCRP | High-Sensitivity C-reactive protein |
| ROS | Reactive oxygen species |
| JNKp | c-Jun amino terminal kinase phosphorylation |
| MAPK | Mitogen activated protein kinase |
| Cyp A | Cyclophilin A |
| MMP | Mitochondrial membrane potential |
| MMP | Matrix metalloproteinases |
| Nurr1 | Nuclear receptor-related-1 |
| p-CREB | cAMP response element-binding protein |
| MCP-1 | Monocyte chemoattractant protein-1 |
| ICAM-1 | Intracellular adhesion molecule-1 |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| NF-kB | Nuclear factor kappa-B |
| LOX-1 | Lectin like ox-LDL receptor-1 |
| SR-A1 | Scavenger receptor-1 |
| CD36 | Cluster of differentiation 36 |
| LPS | Lipopolysaccharide |
| CNS | Central nervous system |
| EphA4 | Ephrin type A-receptor 4-precursor |
| NMDA | N-methyl-D-aspartate |
| BDNF | Brain-derived nucleophilic factor |
| JNKp | c-Jun amino terminal kinase phosphorylation |
| CPP | Conditioned Place Preference |
| EBI | Early Brain Injury |
| TXA2 | Thromboxane A2 |
| TXB2 | Thromboxane B2 |
| AMPK | AMP-activated protein kinase |
| SHR | Spontaneous hypertensive rats |
| GLP-1 RAs | Glucagon-like peptide-1 receptor agonists |
| SGLT-2 | sodium-glucose cotransporter-2 |
| AGE | Advanced glycation end products |
| PCKSK9 | Proprotein convertase subtilisin-kexin type 9 |
| RAGE | Receptor for advanced glycation end-products |
| DM | Diabetes mellitus |
| ASCVD | atherosclerotic cardiovascular diseases |
| Ang | Angiotensin |
| MA | Methamphetamine |
| KA | Kainic acid |
| EPC | Endothelial progenitor cell |
| EGFR | Endothelial growth factor receptor |
References
- Ng, Y.P.; Or, T.C.; Ip, N.Y. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem. Int. 2015, 89, 260–270. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.-X.; Chen, X.-P.; Xu, R.-X. Pharmacological actions of Uncaria alkaloids, rhynchophylline and isorhynchophylline. Acta Pharmacol. Sin. 2003, 24, 97–101. [Google Scholar]
- Abdel-Fattah, M.A.; Matsumoto, K.; Tabata, K.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H. Effects of Uncaria tomentosa Total Alkaloid and its Components on Experimental Amnesia in Mice: Elucidation Using the Passive Avoidance Test. J. Pharm. Pharmacol. 2010, 52, 1553–1561. [Google Scholar] [CrossRef]
- Suhaimi, F.W.; Yusoff, N.H.; Hassan, R.; Mansor, S.M.; Navaratnam, V.; Müller, C.; Hassan, Z. Neurobiology of Kratom and its main alkaloid mitragynine. Brain Res. Bull. 2016, 126, 29–40. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Jivan, J.K.; Andurkar, S.V. Pharmacology of kratom: An emerging botanical agent with stimulant, analgesic and opioid-like effects. J. Am. Osteopat. Assoc. 2012, 112, 792–799. [Google Scholar]
- Qu, J.; Gong, T.; Ma, B.; Zhang, L.; Kano, Y.; Yuan, D. Comparative Study of Fourteen Alkaloids from Uncaria rhynchophylla Hooks and Leaves Using HPLC-Diode Array Detection-Atmospheric Pressure Chemical Ionization/MS Method. Chem. Pharm. Bull. 2012, 60, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Lin, C.; Ma, J.; Hu, L.; Lin, G.; Wang, X. Determination of Rhynchophylline in Rat Plasma by Liquid Chromatography Mass Spectrometry and Its Application. J. Chromatogr. Sci. 2013, 52, 661–665. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Ip, S.-P.; Liu, L.; Xian, Y.-F.; Lin, Z.-X. Uncaria rhynchophylla and its Major Constituents on Central Nervous System: A Review on Their Pharmacological Actions. Curr. Vasc. Pharmacol. 2020, 18, 346–357. [Google Scholar] [CrossRef]
- Liu, H.-M.; Feng, X.-Z. Oxindole alkaloids from Uncaria sinensis. Phytochemistry 1993, 33, 707–710. [Google Scholar] [CrossRef]
- Ma, B.; Wu, C.-F.; Yang, J.-Y.; Wang, R.; Kano, Y.; Yuan, D. Three New Alkaloids from the Leaves ofUncaria rhynchophylla. Helvetica Chim. Acta 2009, 92, 1575–1585. [Google Scholar] [CrossRef]
- Montoro, P.; Carbone, V.; de Dioz, Z.Q.J.; De Simone, F.; Pizza, C. Identification and quantification of components in extracts of Uncaria tomentosa by HPLC-ES/MS. Phytochem. Anal. Int. J. Plant. Chem. Biochem. Tech. 2004, 15, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, J.D.; Ridsdale, C.E. Alkaloids of Uncaria. V. their occurrence and chemotaxonomy. LLOYDIA 1978, 41, 503–570. [Google Scholar]
- Yamanaka, E.; Kimizuka, Y.; Aimi, N.; Sakai, S.; Haginiwa, J. Studies of Plants Containing Indole Alkaloids. IX. Quantitative Analysis on the Tertiary Alkaloids in Various Parts of Uncaria rhynchophylla MIQ. Yakugaku Zasshi 1983, 103, 1028–1033. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yang, C.; Wu, D. Studies on the Chemical Constituents of Sharpleaf Gambirplant (Uncaria rhynchophylla)(II). Chin. Tradit. Herb. Drugs 1998, 29, 649–651. [Google Scholar]
- Yung, K.K.L.; Mo, Z.; Guo, Y.; Luo, C.; Tu, G.; Li, C.; Liu, Y.; Liu, W. Rhynchophylline downregulates phosphorylated camp response element binding protein, nuclear receptor-related-1, and brain-derived neurotrophic factor expression in the hippocampus of ketamine-induced conditioned place preference rats. Pharmacogn. Mag. 2018, 14, 81–86. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Y.; Lv, X.; Yu, X.; Li, X.; Li, H.; Wang, Y.; Lu, D.; Qi, R.; Wang, H. Rhynchophylline prevents cardiac dysfunction and improves survival in lipopolysaccharide-challenged mice via suppressing macrophage I-κBα phosphorylation. Int. Immunopharmacol. 2012, 14, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-Y.; Chen, J.; Zhou, S.-W.; Mo, Z.-X. Individual and combined effects of rhynchophylline and ketamine on proliferation, NMDAR1 and GluA2/3 protein expression in PC12 cells. Fitoterapia 2013, 85, 125–129. [Google Scholar] [CrossRef]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide attenuates inflammatory response in membranous glomerulo-nephritis rat via downregulation of NF-κB signaling pathway. Kidney Blood Press. Res. 2016, 41, 901–910. [Google Scholar] [CrossRef]
- Chertov, O.; Yang, D.; Howard, O.M.Z.; Oppenheim, J.J. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol. Rev. 2000, 177, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Placha, D.; Jampilek, J. Chronic Inflammatory Diseases, Anti-Inflammatory Agents and Their Delivery Nanosystems. Pharmaceutics 2021, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.D.R.; Arantes, M.B.; de Faria Pereira, S.M.; Da Cruz, L.L.; de Souza Passos, M.; De Moraes, L.P.; Vieira, I.J.C.; de Oliveira, D.B. Plants as Sources of Anti-Inflammatory Agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Leite, C.; Nunes, C.; Jamal, S.K.; Cuccovia, I.M.; Reis, S. Nonsteroidal Anti-Inflammatory Therapy: A Journey Toward Safety. Med. Res. Rev. 2017, 37, 802–859. [Google Scholar] [CrossRef]
- Sandoval, A.C.; Fernandes, D.R.; Silva, E.A.; Júnior, A.T. O uso indiscriminado dos Anti-Inflamatórios Não Esteroidais (AINES). Saúde Ciência Ação 2017, 3, 48–69. [Google Scholar] [CrossRef] [Green Version]
- Sostres, C.; Lanas, Á. Appropriate prescription, adherence and safety of non-steroidal anti-inflammatory drugs. Med. Clínica 2016, 146, 267–272. [Google Scholar] [CrossRef]
- Patel, D.P.; Schenk, J.M.; Darke, A.; Myers, J.B.; Brant, W.O.; Hotaling, J.M. Non-steroidal anti-inflammatory drug (NSAID) use is not associated with erectile dysfunction risk: Results from the Prostate Cancer Prevention Trial. BJU Int. 2016, 117, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Chakraborti, A.K.; Garg, S.K.; Kumar, R.; Motiwala, H.F.; Jadhavar, P.S. Progress in COX-2 inhibitors: A journey so far. Curr. Med. Chem. 2010, 17, 1563–1593. [Google Scholar] [CrossRef]
- Silverstein, F.E.; Faich, G.; Goldstein, J.L.; Simon, L.S.; Pincus, T.; Whelton, A.; Makuch, R.; Eisen, G.; Agrawal, N.M.; Stenson, W.F.; et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS study: A randomized controlled trial. JAMA 2000, 284, 1247–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar]
- Cushnie, T.T.; Cushnie, B.; Lamb, A. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zheng, T.-T.; Li, X.; Liang, Y.; Wang, L.-J.; Huang, Y.-C.; Xiao, H.-T. Plant-Derived Alkaloids: The Promising Disease-Modifying Agents for Inflammatory Bowel Disease. Front. Pharmacol. 2019, 10, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ti, H.; Zhuang, Z.; Yu, Q.; Wang, S. Progress of Plant Medicine Derived Extracts and Alkaloids on Modulating Viral Infections and Inflammation. Drug Des. Dev. Ther. 2021, ume 15, 1385–1408. [Google Scholar] [CrossRef]
- Küpeli, E.; Koşar, M.; Yeşilada, E.; Baser, K.H.C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002, 72, 645–657. [Google Scholar] [CrossRef]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.T.; Šmejkal, K.; Malaník, M.; et al. Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics. Theranostics 2019, 9, 1923–1951. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Baggioni, A. Berberine and Its Role in Chronic Disease. Adv. Exp. Med. Biol. 2016, 928, 27–45. [Google Scholar] [CrossRef]
- Yenisetti, S.C. Beneficial role of coffee and caffeine in neurodegenerative diseases: A minireview. AIMS Public Health 2016, 3, 407. [Google Scholar]
- Xu, W.; Liu, J.; Ma, D.; Yuan, G.; Lu, Y.; Yang, Y. Capsaicin reduces Alzheimer-associated tau changes in the hippocampus of type 2 diabetes rats. PLoS ONE 2017, 12, e0172477. [Google Scholar] [CrossRef]
- Magdy, S.; Gamal, M.; Samir, N.F.; Rashed, L.; Aboulhoda, B.E.; Mohammed, H.S.; Sharawy, N. IκB kinase inhibition remodeled connexins, pannexin-1, and excitatory amino-acid transporters expressions to promote neuroprotection of galantamine and morphine. J. Cell. Physiol. 2021, 14. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Zhou, S.-W. Isorhynchophylline: A plant alkaloid with therapeutic potential for cardiovascular and central nervous system diseases. Fitoterapia 2012, 83, 617–626. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, S. Antihypertensive and neuroprotective activities of rhynchophylline: The role of rhynchophylline in neurotransmission and ion channel activity. J. Ethnopharmacol. 2010, 132, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E. The Epidemiology of Epilepsy. Neuroepidemiology 2019, 54, 185–191. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2010, 7, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P.; Camandola, S. NF-κB in neuronal plasticity and neurodegenerative disorders. J. Clin. Investig. 2001, 107, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Novelli, A.; Tasker, R.A.R. Excitatory amino acids in epilepsy: From the clinics to the laboratory. Amino Acids 2007, 32, 295–297. [Google Scholar] [CrossRef]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.L.; Ho, T.Y.; Su, S.Y.; Lo, W.Y.; Liu, C.H.; Tang, N.Y. Uncaria rhynchophylla and rhynchophylline inhibit c-Jun N-terminal kinase phosphorylation and nuclear factor-κB activity in kainic acid-treated rats. Am. J. Chin. Med. 2009, 37, 351–360. [Google Scholar] [CrossRef]
- Kang, T.-H.; Murakami, Y.; Matsumoto, K.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H. Rhynchophylline and isorhynchophylline inhibit NMDA receptors expressed in Xenopus oocytes. Eur. J. Pharmacol. 2002, 455, 27–34. [Google Scholar] [CrossRef]
- Shimada, Y.; Goto, H.; Itoh, T.; Sakakibara, I.; Kubo, M.; Sasaki, H.; Terasawa, K. Evaluation of the Protective Effects of Alkaloids Isolated from the Hooks and Stems of Uncaria sinensis on Glutamate-induced Neuronal Death in Cultured Cerebellar Granule Cells from Rats. J. Pharm. Pharmacol. 2010, 51, 715–722. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Chen, M.-F.; Li, T.-C.; Li, S.-C.; Tang, N.-Y.; Pon, C.-Z.; Lin, J.-G. Anticonvulsant Effect of Uncaria rhynchophylla (Miq) Jack. Am. J. Chin. Med. 1999, 27, 257–264. [Google Scholar] [CrossRef]
- Geng, H.; Chen, X.; Wang, C. Systematic elucidation of the pharmacological mechanisms of Rhynchophylline for treating epilepsy via network pharmacology. BMC Complement. Med. Ther. 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Krasnova, I.N.; Cadet, J.L. Methamphetamine toxicity and messengers of death. Brain Res. Rev. 2009, 60, 379–407. [Google Scholar] [CrossRef] [Green Version]
- Riddle, E.L.; Fleckenstein, A.E.; Hanson, G.R. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. AAPS J. 2006, 8, E413–E418. [Google Scholar] [CrossRef]
- Xu, D.D.; Hoeven, R.; Rong, R.; Cho, W.C.-S. Rhynchophylline Protects Cultured Rat Neurons against Methamphetamine Cytotoxicity. Evid. Based Complement. Altern. Med. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Peng, Q.-X.; Lin, X.-L.; Luo, C.-H.; Jiang, M.-J.; Mo, Z.-X.; Yung, K.K.-L. Effect of rhynchophylline on the expression of p-CREB and sc-Fos in triatum and hippocampal CA1 area of methamphetamine-induced conditioned place preference rats. Fitoterapia 2014, 92, 16–22. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Mo, Z.-X.; Zhou, S.-W. Effect of rhynchophylline on central neurotransmitter levels in amphetamine-induced conditioned place preference rat brain. Fitoterapia 2010, 81, 844–848. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Mo, Z.-X.; Zhou, S.-W. Rhynchophylline down-regulates NR2B expression in cortex and hippocampal CA1 area of amphetamine-induced conditioned place preference rat. Arch. Pharmacal Res. 2010, 33, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R. (Ed.) Protein Aggregation and Fibrillogenesis in Cerebral and Systemic Amyloid Disease; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Heneka, M.T.; Carson, M.J.; Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Sperling, R.; Mormino, E.; Johnson, K. The Evolution of Preclinical Alzheimer’s Disease: Implications for Prevention Trials. Neuron 2014, 84, 608–622. [Google Scholar] [CrossRef] [Green Version]
- Millington, C.; Sonego, S.; Karunaweera, N.; Rangel, A.; Aldrich-Wright, J.R.; Campbell, I.L.; Gyengesi, E.; Münch, G. Chronic neuroinflammation in Alzheimer’s disease: New perspectives on animal models and promising candidate drugs. BioMed Res. Int. 2014, 2014, 309129. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ma, X.; Wei, S.; Qiu, D.; Wilson, I.W.; Wu, P.; Tang, Q.; Liu, L.; Dong, S.; Zu, W. De novo transcriptome sequencing and digital gene expression analysis predict biosynthetic pathway of rhynchophylline and isorhynchophylline from Uncaria rhynchophylla, a non-model plant with potent anti-alzheimer’s properties. BMC Genom. 2014, 15, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xian, Y.-F.; Lin, Z.-X.; Mao, Q.-Q.; Chen, J.-N.; Su, Z.-R.; Lai, X.-P.; Ip, P.S.-P. Isorhynchophylline Protects PC12 Cells Against Beta-Amyloid-Induced Apoptosis via PI3K/Akt Signaling Pathway. Evid. Based Complement. Altern. Med. 2013, 2013, 163057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xian, Y.-F.; Mao, Q.-Q.; Wu, J.C.Y.; Su, Z.-R.; Chen, J.-N.; Lai, X.-P.; Ip, S.-P.; Lin, Z.-X. Isorhynchophylline Treatment Improves the Amyloid-β-Induced Cognitive Impairment in Rats via Inhibition of Neuronal Apoptosis and Tau Protein Hyperphosphorylation. J. Alzheimer’s Dis. 2014, 39, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.F.; Lin, Z.X.; Mao, Q.Q.; Hu, Z.; Zhao, M.; Che, C.T.; Ip, S.P. Bioassay-guided isolation of neuroprotective compounds from Uncaria rhynchophylla against beta-amyloid-induced neurotoxicity. Evid. Based Complement. Altern. Med. 2012, 2012, 802625. [Google Scholar] [CrossRef]
- Fu, A.K.Y.; Hung, K.-W.; Huang, H.; Gu, S.; Shen, Y.; Cheng, E.Y.L.; Ip, F.C.F.; Huang, X.; Fu, W.-Y.; Ip, N.Y. Blockade of EphA4 signaling ameliorates hippocampal synaptic dysfunctions in mouse models of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2014, 111, 9959–9964. [Google Scholar] [CrossRef] [Green Version]
- Zeng, P.; Wang, X.-M.; Ye, C.-Y.; Su, H.-F.; Tian, Q. The Main Alkaloids in Uncaria rhynchophylla and Their Anti-Alzheimer’s Disease Mechanism Determined by a Network Pharmacology Approach. Int. J. Mol. Sci. 2021, 22, 3612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, X.; Xian, Y.; Zhu, L.; Lin, G.; Lin, Z.-X. Evidence on Integrating Pharmacokinetics to Find Truly Therapeutic Agent for Alzheimer’s Disease: Comparative Pharmacokinetics and Disposition Kinetics Profiles of Stereoisomers Isorhynchophylline and Rhynchophylline in Rats. Evid. Based Complement. Altern. Med. 2019, 2019, 4016323. [Google Scholar] [CrossRef] [Green Version]
- Imamura, S.; Tabuchi, M.; Kushida, H.; Nishi, A.; Kanno, H.; Yamaguchi, T.; Sekiguchi, K.; Ikarashi, Y.; Kase, Y. The Blood–Brain Barrier Permeability of Geissoschizine Methyl Ether in Uncaria Hook, a Galenical Constituent of the Traditional Japanese Medicine Yokukansan. Cell. Mol. Neurobiol. 2011, 31, 787–793. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Xu, J.; Song, X.; Huang, H.; Feng, Y.; Fu, C. Rhynchophylline Loaded-mPEG-PLGA nanoparticles coated with tween-80 for preliminary study in Alzheimer’s Disease. Int. J. Nanomed. 2020, 15, 1149. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gao, J.; Peng, M.; Meng, H.; Ma, H.; Cai, P.; Xu, Y.; Zhao, Q.; Si, G. A Review on Central Nervous System Effects of Gastrodin. Front. Pharmacol. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-S.; Liu, M.-F.; Ji, X.-Y.; Jiang, C.-R.; Li, Z.-L.; OuYang, B. Gastrodin combined with rhynchophylline inhibits cerebral ischaemia-induced inflammasome activation via upregulating miR-21–5p and miR-331–5p. Life Sci. 2019, 239, 116935. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Chen, L.; Chen, X.; He, J.; Lv, P.; Ge, H. Rhynchophylline attenuates migraine in trigeminal nucleus caudalis in nitroglycerin-induced rat model by inhibiting MAPK/NF-κB signaling. Mol. Cell. Biochem. 2019, 461, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol. Ther. 2008, 120, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, R.; Zhu, S.; Zhang, R.; Ma, S. Rhynchophylline Attenuates LPS-induced Pro-inflammatory Responses through Down-regulation of MAPK/NF-κB Signaling Pathways in Primary Microglia. Phytother. Res. 2012, 26, 1528–1533. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Zhu, S.; Xu, T.; Lu, J.; Han, H.; Zhou, C.; Yan, J. The role of rhynchophylline in alleviating early brain injury following subarachnoid hemorrhage in rats. Brain Res. 2016, 1631, 92–100. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Sedighi, M.; Bahmani, M.; Asgary, S.; Beyranvand, F. A review of plant-based compounds and medicinal plants effective on atherosclerosis. J. Res. Med. Sci. 2017, 22, 30. [Google Scholar] [CrossRef]
- Ross, R.; Mack, T.; Paganini-Hill, A.; Arthur, M.; Henderson, B. Menopausal Oestrogen Therapy and Protection from Death from Ischaemic Heart Disease. Lancet 1981, 317, 858–860. [Google Scholar] [CrossRef]
- Ridker, P.M. C-Reactive Protein and the Prediction of Cardiovascular Events Among Those at Intermediate Risk: Moving an Inflammatory Hypothesis Toward Consensus. J. Am. Coll. Cardiol. 2007, 49, 2129–2138. [Google Scholar] [CrossRef] [Green Version]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Goyal, T.; Mehta, J.L. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovasc. Drugs Ther. 2011, 25, 419. [Google Scholar] [CrossRef] [PubMed]
- Weij, J.; Toet, K.; Zadelaar, S.; Wielinga, P.Y.; Kleemann, R.; Rensen, P.C.; Kooistra, T. Anti-inflammatory salicylate beneficially modulates pre-existing atherosclerosis through quenching of NF-κB activity and lowering of cholesterol. Atherosclerosis 2010, 213, 241–246. [Google Scholar] [CrossRef]
- Goldfine, A.B.; Shoelson, S.E. Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. J. Clin. Investig. 2017, 127, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, P.; Qiu, L.; Liu, Y.; Liu, X.-L.; Zheng, J.-L.; Xue, H.-Y.; Liu, W.-H.; Liu, D.; Li, J. Metformin Treatment Was Associated with Decreased Mortality in COVID-19 Patients with Diabetes in a Retrospective Analysis. Am. J. Trop. Med. Hyg. 2020, 103, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M. Diabetes mellitus and cardiovascular disease: Emerging therapeutic approaches. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 558–568. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Zhu, H.; Wang, Z.; Chen, J.-A.; Wu, J.; Zhu, Y.; Gu, X. Novel rhynchophylline analogues as microvascular relaxation agents for the treatment of microvascular dysfunction caused by diabetes. Eur. J. Med. Chem. 2017, 139, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-J.; Chang, L.-L.; Wu, J.; Pan, H.-M.; Zhang, Q.-Y.; Wang, M.-J.; Xin, X.-M.; Luo, S.-S.; Chen, J.-A.; Gu, X.-F.; et al. A Novel Rhynchophylline Analog, Y396, Inhibits Endothelial Dysfunction Induced by Oxidative Stress in Diabetes Through Epidermal Growth Factor Receptor. Antioxid. Redox Signal. 2020, 32, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Lu, F.; Lin, B.; Zhou, Y.; Liu, T.; Sun, Y. Stability of Alkaloids during Drying Process and Their Effect on Anticoagulating Activity of Uncariae Ramulus Cum Uncis. J. Anal. Methods Chem. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.X.; Jin, R.M.; Wang, Q.; Zhang, H.G. Effects of Rhynchophylline on Guinea Pig Atria. J. Chin. Pharm. Sci. 1995, 4, 144. [Google Scholar]
- Jin, R.M.; Chen, C.X.; Li, Y.K.; Xu, P.K. Effect of rhyncophylline on platelet aggregation and experimental thrombosis. Yao Xue Xue Bao Acta Pharm. Sin. 1991, 26, 246–249. [Google Scholar]
- Trinder, M.; Francis, G.A.; Brunham, L.R. Association of Monogenic vs Polygenic Hypercholesterolemia with Risk of Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2020, 5, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Xu, K.; Che, D.; Huang, Z.; Jahan, N.; Wang, S. Endothelium-independent vasodilator effect of isocorynoxeine in vitro isolated from the hook of Uncaria rhynchophylla (Miquel). Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-Y.; Zeng, X.-R.; Cheng, J.; Wen, J.; Inoue, I.; Yang, Y. Rhynchophylline-induced vasodilation in human mesenteric artery is mainly due to blockage of L-type calcium channels in vascular smooth muscle cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.-F.; Liu, L.-M.; Liu, Y.-Y.; Liu, J.; Yan, L.; Pan, C.-S.; Wang, M.-X.; Wang, C.-S.; Fan, J.-Y.; Gao, Y.-S.; et al. Inhibitory effect of rhynchophylline on contraction of cerebral arterioles to endothelin 1: Role of rho kinase. J. Ethnopharmacol. 2014, 155, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, C.; Hamilton, P.K.; Pumb, R.; Harbinson, M.; McVeigh, G.E. Endothelial Function in Hypertension: Victim or Culprit? J. Clin. Hypertens. 2015, 17, 651–654. [Google Scholar] [CrossRef]
- Goligorsky, M.S. Endothelial Progenitor Cells: From Senescence to Rejuvenation. Semin. Nephrol. 2014, 34, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Mudyanadzo, T. Endothelial Progenitor Cells and Cardiovascular Correlates. Cureus 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Sun, A.S.; Yu, L.M.; Wu, Q.; Gong, Q.H. Effects of isorhynchophylline on angiotensin II-induced proliferation in rat vascular smooth muscle cells. J. Pharm. Pharmacol. 2008, 60, 1673–1678. [Google Scholar] [CrossRef]
- He, N.; Sun, A.; Wu, Q.; Huang, X.; Shi, J. Inhibitory effect of rhynchophylline on cardiomyocyte hypertrophy induced by angiotensin II. Chin. J. Pharmacol. Toxicol. 2010, 24, 255–260. [Google Scholar]
- Lin, L.; Zhang, L.; Li, X.-T.; Ji, J.-K.; Chen, X.-Q.; Li, Y.-L.; Li, C. Rhynchophylline Attenuates Senescence of Endothelial Progenitor Cells by Enhancing Autophagy. Front. Pharmacol. 2020, 10. [Google Scholar] [CrossRef]
- Hao, H.F.; Liu, L.M.; Pan, C.S.; Wang, C.S.; Gao, Y.S.; Fan, J.Y.; Han, J.Y. Rhynchophylline ameliorates endothelial dysfunction via Src-PI3K/Akt-eNOS cascade in the cultured intrarenal arteries of spontaneous hypertensive rats. Front. Physiol. 2017, 15, 928. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Jiang, F.; Li, Y.-L.; Jiang, Y.-H.; Yang, W.-Q.; Sheng, J.; Xu, W.-J.; Zhu, Q.-J. Rhynchophylla total alkaloid rescues autophagy, decreases oxidative stress and improves endothelial vasodilation in spontaneous hypertensive rats. Acta Pharmacol. Sin. 2018, 39, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Dai, G.-H.; Sun, J.-C.; Qi, D.-M. Effects of rhynchophylla alkaloids on vascular adventitial fibroblast apoptosis and proliferation in the thoracic aorta of spontaneously hypertensive rats. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi jiehe zazhi Chin. J. Integr. Tradit. West. Med. 2012, 32, 1233–1237. [Google Scholar]
- Yuan, D.; Ma, B.; Yang, J.-Y.; Xie, Y.-Y.; Wang, L.; Zhang, L.-J.; Kano, Y.; Wu, C.-F. Anti-inflammatory effects of rhynchophylline and isorhynchophylline in mouse N9 microglial cells and the molecular mechanism. Int. Immunopharmacol. 2009, 9, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, C.-M.; Hattori, M. Metabolism and Pharmacokinetics of Rhynchophylline in Rats. Biol. Pharm. Bull. 2010, 33, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Y.; Li, H.M.; Wang, H.D.; Peng, X.M.; Wang, Y.P.; Lu, D.X.; Qi, R.B.; Hu, C.F.; Jiang, J.W. Pretreatment with berberine and yohimbine protects against LPS-induced myocardial dysfunction via inhibition of cardiac I-κBα phosphorylation and apoptosis in mice. Shock 2011, 35, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Cui, L.; Li, P.; Wang, Y.; Zhang, X.; Guo, M. Rhynchophylline ameliorates myocardial ischemia/reperfusion injury through the modulation of mitochondrial mechanisms to mediate myocardial apoptosis. Mol. Med. Rep. 2019, 19, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Tang, N.Y.; Liu, C.H.; Hsieh, C.L. Antiepileptic effect of Uncaria rhynchophylla and Rhynchophylline involved in the initiation of c-jun N-terminal kinase phosphorylation of MAPK signal pathways in acute seizures of kainic acid-treated rats. Evid. Based Complementary Altern. Med. 2013, 2013, 961289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saglani, S.; Lloyd, C. Novel concepts in airway inflammation and remodelling in asthma. Eur. Respir. J. 2015, 46, 1796–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, W.; Zhao, L.; Lei, J.; Xie, J.; Xiao, Y.; Zhang, Z. Effect of the BMPR-II-SMAD3/MRTF complex on proliferation and migration of ASMCs and the mechanism in asthma. bioRxiv 2020, 1–34. [Google Scholar] [CrossRef]
- Sun, A.S.; Huang, X.Z.; Liu, W.G.; Zhang, X.D.; Ke, M.M. The anti-asthma effect of rhynchophylla total alkaloids (translated). Gui Zhou Yi Yao 1983, 2. (In Chinese) [Google Scholar]
- Li, H.; Bi, Q.; Cui, H.; Lv, C.; Wang, M. Suppression of autophagy through JAK2/STAT3 contributes to the therapeutic action of rhynchophylline on asthma. BMC Complement. Med. Ther. 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, H.; Zhao, Y.; Lv, C.; Zhou, G. Rhynchophylline attenuates allergic bronchial asthma by inhibiting transforming growth factor β1 mediated Smad and mitogen activated protein kinase signaling transductions in vivo and in vitro. Exp. Ther. Med. 2019, 17, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xu, J.; Mu, X.; Hu, Y.; Hu, G.; Duan, H.; Zhang, T.; Lin, H.; Zhang, W. Effects of rhynchophylline and isorhynchophylline on nitric oxide and endothelin-1 secretion from RIMECs induced by Listeriolysin O in vitro. Vet. Microbiol. 2010, 143, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Coats, A.B.; Tuazon, J.P.; Jo, M.; Borlongan, C.V. Rhynchophylline promotes stem cell autonomous metabolic homeostasis. Cytotherapy 2020, 22, 106–113. [Google Scholar] [CrossRef] [PubMed]



| Markers Expressed by Activated Macrophages/Endothelial Cells in Early Stage of Plaque Formation | Markers Expressed by Activated Macrophages/Endothelial Cells in Later Stage of Plaque Formation |
|---|---|
| E-selectin | VCAM-1 |
| E-Cadherin | Fibrinogen |
| P-selectin | MCP-1 |
| MCP-1 | MPO |
| MMP | CD14 |
| VCAM-1 | CD16 |
| LOX | CCR2 |
| CD36 | |
| Inflammatory markers | Inflammatory markers |
| Cyp A | CRP |
| hs-CRP | TNF-α |
| TNF-α | IL-18 |
| IL-1 | |
| IL-6 |
| Diseases | Study Highlights of Rhy | References |
|---|---|---|
| Epilepsy | Noncompetitive NMDA glutamate receptor antagonist | Hsieh et al., 2009 [49] |
| Inhibited calcium influx and prevented glutamate-induced neuronal death in vitro | Kang et al., 2002 [50] | |
| Attenuated JNKp expression and NFkB activation | Shimada et al., 1999 [51] | |
| Addiction | Blocked calcium channel through inhibition of ionotropic glutamate receptor | Xu et al., 2012 [56] |
| Decreased the levels of p Fos, Nurr, p-CREB, and BDNF in the hippocampus | Liu et al., 2014 [57] Yung et al., 2018 [15] | |
| Alzheimer’s | Reduced Ca2+ overload and tau protein hyperphosphorylation | Xian et al., 2012 [67] |
| Inhibitor of ephrin type A-receptor 4-precursor (EphA4) tyrosine kinase | Fu et al., 2014 [68] | |
| Nanoparticle T80-NPS-RIN improved bioavailability and bioaccumulation | Xu et al., 2020 [72] | |
| Migraine | Inhibited MAPK/NF-kB pathway against oxidative stress | Lai et al., 2019 [75] |
| Reduced the concentrations of ROS and malondialdehyde (MDA) in the hippocampus | Song et al., 2012 [77] | |
| Diabetes | Rhy analog G2 ameliorated diabetes-induced endothelial dysfunction in mesenteric arteries and upregulated eNOS expression | Guo et al., 2017 [88] |
| Novel Rhy analog, Y396 inhibited the tyrosine kinase activity of EGFR and down regulated Nox2 and Nox4 | Wang et al., 2020 [89] | |
| Hypertension | Vasodilatory effect by decreasing calcium sensitivity in smooth muscles or through inhibition of L-type calcium channels | Li et al., 2013 [96] Hao et al., 2014 [97] |
| Inhibited vascular smooth muscle cell proliferation and reduced Ang II-induced cardiomyocyte hypertrophy | Zhang et al., 2008 [101] He et al., 2010 [102] | |
| Improved endothelial-dependent relaxation in renal arteries from spontaneous hypertensive rats via Src-PI3K/Akt-eNOS signaling cascade | Hao et al., 2017 [104] | |
| Septic shock | Suppressed IκBα phosphorylation, inhibited myocardial TNF-α and IL-1β in unfiltered macrophages during endotoxemia | Yung et al., 2018 [15] |
| Increased cell viability and attenuated apoptosis in myocardial ischemia-reperfusion (MI/R)-induced cardiomyocytes | Qin et al., 2019 [110] | |
| Asthma | Reduced asthmatic inflammation by suppressing JAK2/STAT3 signaling pathway thereby reducing oxidativestress and suppressing autophagy-related proteins | Li et al., 2021 [115] |
| Blocked Smad and MAPK signal transduction pathways | Wang et al., 2019 [116] | |
| Listeriosis | Increased the cell viability of intestinal microvascular endothelial cells and upregulated NO levels but inhibited endothelin-1(ET-1) release | Chen et al., 2010 [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geetha, R.G.; Ramachandran, S. Recent Advances in the Anti-Inflammatory Activity of Plant-Derived Alkaloid Rhynchophylline in Neurological and Cardiovascular Diseases. Pharmaceutics 2021, 13, 1170. https://doi.org/10.3390/pharmaceutics13081170
Geetha RG, Ramachandran S. Recent Advances in the Anti-Inflammatory Activity of Plant-Derived Alkaloid Rhynchophylline in Neurological and Cardiovascular Diseases. Pharmaceutics. 2021; 13(8):1170. https://doi.org/10.3390/pharmaceutics13081170
Chicago/Turabian StyleGeetha, Rajeswari Gopal, and Surya Ramachandran. 2021. "Recent Advances in the Anti-Inflammatory Activity of Plant-Derived Alkaloid Rhynchophylline in Neurological and Cardiovascular Diseases" Pharmaceutics 13, no. 8: 1170. https://doi.org/10.3390/pharmaceutics13081170