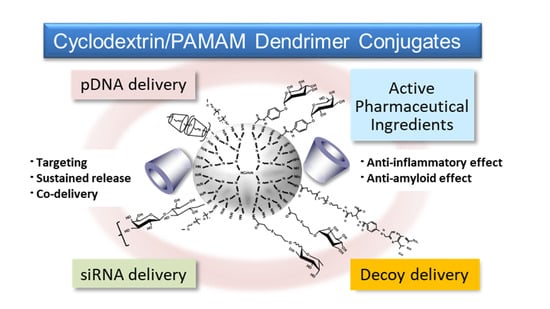
Twenty Years of Research on Cyclodextrin Conjugates with PAMAM Dendrimers
Abstract
:1. Introduction
2. Cyclodextrins Conjugates with PAMAM Dendrimers for Gene and Oligonucleotide Drug Delivery
3. Parent CD/PAMAM Dendrimer Conjugates (CDE) for Delivery Gene and Oligonucleotide Drugs
3.1. CDE for pDNA Delivery
3.2. CDE for Short Hairpin RNA Expressing pDNA (shpDNA) Delivery
3.3. CDE for siRNA Delivery
3.4. α-CDE (G3, DSC2) for the Other Negatively Charged Drug Delivery
4. GUG-β-CD/Dendrimer Conjugates (GUG-β-CDE) as pDNA and siRNA Delivery
4.1. GUG-β-CDE for pDNA Delivery
4.2. GUG-β-CDE for siRNA Delivery
5. Glactose- and Lactose-Appended α-CDE for pDNA and siRNA Delivery
5.1. Glactose- and Lactose-Appended α-CDE for Gene Delivery
5.2. Galactose- and Lactose-Appended α-CDE for siRNA Delivery
5.3. PEG-Lac-α-CDE (G3) for pDNA and siRNA Delivery
6. Mannose-Appended α-CDE for pDNA and siRNA Carrier
6.1. Mannose-Appended α-CDE for pDNA Carrier
6.2. Mannose-Appended α-CDE for siRNA Delivery
7. Fucose-Appended α-CDE for Decoy DNA Delivery
8. Folate-Appended α-CDE
8.1. Folate-Appended α-CDE for pDNA Delivery
8.2. Fol-PEG-α-CDE for siRNA Delivery
8.3. Fol-PEG-GUG-β-CDE for siRNA Delivery
9. Ternary Complex of CDE with Low-Molecular-Weight Sacran
9.1. Lac-α-CDE with Low-Molecular-Weight Sacran for siRNA Delivery
9.2. Fol-PEG-α-CDE with Low-Molecular-Weight Sacran for siRNA Delivery
10. Co-Delivery of siRNA and Low-Molecular-Weight Antitumor Drug
11. Supramolecular with PEG-Appended CDE as Sustained Release System of pDNA
12. GUG-β-CDE for Genome Editing
13. CDE as Active Pharmaceutical Ingredients (API)
13.1. CDE as an Anti-Inflammatory Agent
13.2. CDE for Treatment of Amyloidosis
14. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Valeur, E.; Guéret, S.M.; Adihou, H.; Gopalakrishnan, R.; Lemurell, M.; Waldmann, H.; Grossmann, T.N.; Plowright, A.T. New Modalities for Challenging Targets in Drug Discovery. Angew. Chem. Int. Ed. 2017, 56, 10294–10323. [Google Scholar] [CrossRef]
- Brown, D.G.; Wobst, H.J. A Decade of FDA-Approved Drugs (2010–2019): Trends and Future Directions. J. Med. Chem. 2021, 64, 2312–2338. [Google Scholar] [CrossRef]
- Chung, Y.H.; Beiss, V.; Fiering, S.N.; Steinmetz, N.F. COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS Nano 2020, 14, 12522–12537. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Siegwart, D.J.; Anderson, D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019, 144, 133–147. [Google Scholar] [CrossRef]
- Ickenstein, L.M.; Garidel, P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin. Drug Deliv. 2019, 16, 1205–1226. [Google Scholar] [CrossRef]
- Debacker, A.J.; Voutila, J.; Catley, M.; Blakey, D.; Habib, N. Delivery of Oligonucleotides to the Liver with GalNAc: From Research to Registered Therapeutic Drug. Mol. Ther. 2020, 28, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bono, N.; Ponti, F.; Mantovani, D.; Candiani, G. Non-Viral in Vitro Gene Delivery: It is Now Time to Set the Bar! Pharmaceutics 2020, 12, 183. [Google Scholar] [CrossRef] [Green Version]
- Szejtli, J. Medicinal applications of cyclodextrins. Med. Res. Rev. 1994, 14, 353–386. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, E.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 years of cyclodextrin discovery for health, food, agriculture, and the industry: A review. Environ. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Ikuta, D.; Hirata, Y.; Wakamori, S.; Shimada, H.; Tomabechi, Y.; Kawasaki, Y.; Ikeuchi, K.; Hagimori, T.; Matsumoto, S.; Yamada, H. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science 2019, 364, 674–677. [Google Scholar] [CrossRef]
- Watanabe, H.; Nishimoto, T.; Chaen, H.; Fukuda, S. A novel glucanotransferase that produces a cyclomaltopentaose cyclized by an α-1,6 linkage. J. Appl. Glycosci. 2007, 54, 109–118. [Google Scholar] [CrossRef]
- Saenger, W.; Jacob, J.; Gessler, K.; Steiner, T.; Hoffmann, D.; Sanbe, H.; Koizumi, K.; Smith, S.M.; Takaha, T. Structures of the Common Cyclodextrins and Their Larger AnaloguesBeyond the Doughnut. Chem. Rev. 1998, 98, 1787–1802. [Google Scholar] [CrossRef]
- Dhiman, P.; Bhatia, M. Pharmaceutical applications of cyclodextrins and their derivatives. J. Incl. Phenom. Macro. Chem. 2020, 98, 171–186. [Google Scholar] [CrossRef]
- Xu, C.; Yu, B.; Qi, Y.; Zhao, N.; Xu, F.-J. Versatile Types of Cyclodextrin-Based Nucleic Acid Delivery Systems. Adv. Healthc. Mater. 2021, 10, e2001183.1. [Google Scholar] [CrossRef]
- Ajisaka, N.; Hara, K.; Mikuni, K.; Hara, K.; Hashimoto, H. Effects of Branched Cyclodextrins on the Solubility and Stability of Terpenes. Biosci. Biotechnol. Biochem. 2000, 64, 731–734. [Google Scholar] [CrossRef]
- Tavornvipas, S.; Hirayama, F.; Arima, H.; Uekama, K.; Ishiguro, T.; Oka, M.; Hamayasu, K.; Hashimoto, H. 6-O-α-(4-O-α-D-glucuronyl)-D-glucosyl-β-cyclodextrin: Solubilizing ability and some cellular effects. Int. J. Pharm. 2002, 249, 199–209. [Google Scholar] [CrossRef]
- Jono, H.; Anno, T.; Motoyama, K.; Misumi, Y.; Tasaki, M.; Oshima, T.; Mori, Y.; Mizuguchi, M.; Ueda, M.; Shono, M.; et al. Cyclodextrin, a novel therapeutic tool for suppressing amyloidogenic transthyretin misfolding in transthyretin-related amyloidosis. Biochem. J. 2011, 437, 35–42. [Google Scholar] [CrossRef]
- Mousazadeh, H.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Zarghami, N. Cyclodextrin based natural nanostructured carbohydrate polymers as effective non-viral siRNA delivery systems for cancer gene therapy. J. Control. Release 2020, 330, 1046–1070. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ding, X.; Fu, Y.; Xiang, C.; Yuan, Y.; Zhang, Y.; Yu, P. Cyclodextrins based delivery systems for macro biomolecules. Eur. J. Med. Chem. 2020, 212, 113105. [Google Scholar] [CrossRef] [PubMed]
- Ghodke, S.; Mahajan, P.; Gupta, K.; Avadhani, C.V.; Dandekar, P.; Jain, R. Biodegradable Polyester of Poly (Ethylene glycol)-sebacic Acid as a Backbone for β-Cyclodextrin-polyrotaxane: A Promising Gene Silencing Vector. Curr. Gene Ther. 2019, 19, 274–287. [Google Scholar] [CrossRef]
- Arisaka, Y.; Yui, N. Molecular mobility of polyrotaxane-based biointerfaces alters inflammatory responses and polarization in Kupffer cell lines. Biomater. Sci. 2021, 9, 2271–2278. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Liu, Y.H.; Liu, Y. Cyclodextrin-Based Multistimuli-Responsive Supramolecular Assemblies and Their Biological Functions. Adv. Mater. 2020, 32, e1806158. [Google Scholar] [CrossRef] [PubMed]
- Pun, S.H.; Davis, M.E. Development of a nonviral gene delivery vehicle for systemic application. Bioconjugate Chem. 2002, 13, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.J.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Tai, Z.; Ma, J.; Ding, J.; Pan, H.; Chai, R.; Zhu, C.; Cui, Z.; Chen, Z.; Zhu, Q. Aptamer-Functionalized Dendrimer Delivery of Plasmid-Encoding lncRNA MEG3 Enhances Gene Therapy in Castration-Resistant Prostate Cancer. Int. J. Nanomed. 2020, 15, 10305–10320. [Google Scholar] [CrossRef]
- Kheiriabad, S.; Ghaffari, M.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM Dendrimers as a Delivery System for Small Interfering RNA. Methods Mol. Biol. 2020, 2115, 91–106. [Google Scholar]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM dendrimer-cell membrane interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Petersen, J.F.; Ficker, M.; Janaszewska, A.; Christensen, J.B.; Klajnert, B.; Bryszewska, M. Surface modification of PAMAM dendrimer improves its biocompatibility. Nanomedicine 2012, 8, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Nam, K.; Park, J.Y.; Kim, J.B.; Lee, J.K.; Park, J.S. Enhanced transfection efficiency of PAMAM dendrimer by surface modification with L-arginine. J. Control. Release 2004, 99, 445–456. [Google Scholar] [CrossRef]
- Kolhatkar, R.B.; Kitchens, K.M.; Swaan, P.W.; Ghandehari, H. Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjugate Chem. 2007, 18, 2054–2060. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.C.I.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Hirayama, F.; Uekama, K. Cyclodextrin-based controlled drug release system. Adv. Drug Deliv. Rev. 1999, 36, 125–141. [Google Scholar] [CrossRef]
- Arima, H.; Kihara, F.; Hirayama, F.; Uekama, K. Enhancement of gene expression by polyamidoamine dendrimer conjugates with α-, β-, and γ-cyclodextrins. Bioconjugate Chem. 2001, 12, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Kihara, F.; Arima, H.; Tsutsumi, T.; Hirayama, F.; Uekama, K. Effects of structure of polyamidoamine dendrimer on gene transfer efficiency of the dendrimer conjugate with α-cyclodextrin. Bioconjugate Chem. 2002, 13, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Kihara, F.; Arima, H.; Tsutsumi, T.; Hirayama, F.; Uekama, K. In vitro and in vivo gene transfer by an optimized α-cyclodextrin conjugate with polyamidoamine dendrimer. Bioconjugate Chem. 2003, 14, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.B.; Guthrie, E.H.; Huang, M.H.G.; Taxman, D.J. Short hairpin RNA (shRNA): Design, delivery, and assessment of gene knockdown. Methods Mol. Biol. 2010, 629, 141–158. [Google Scholar]
- Tsutsumi, T.; Hirayama, F.; Uekama, K.; Arima, H. Potential use of polyamidoamine dendrimer/α-cyclodextrin conjugate (generation 3, G3) as a novel carrier for short hairpin RNA-expressing plasmid DNA. J. Pharm. Sci. 2008, 97, 3022–3034. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Hirayama, F.; Uekama, K.; Arima, H. Evaluation of polyamidoamine dendrimer/α-cyclodextrin conjugate (generation 3, G3) as a novel carrier for small interfering RNA (siRNA). J. Control. Release 2007, 119, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Arima, H.; Tsutsumi, T.; Yoshimatsu, A.; Ikeda, H.; Motoyama, K.; Higashi, T.; Hirayama, F.; Uekama, K. Inhibitory effect of siRNA complexes with polyamidoamine dendrimer/α-cyclodextrin conjugate (generation 3, G3) on endogenous gene expression. Eur. J. Pharm. Sci. 2011, 44, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, H.; Monde, K.; Anraku, K.; Koga, R.; Hayashi, Y.; Ciftci, H.I.; DeMirci, H.; Higashi, T.; Motoyama, K.; Arima, H.; et al. A clue to unprecedented strategy to HIV eradication: “Lock-in and apoptosis”. Sci. Rep. 2017, 7, 8957. [Google Scholar] [CrossRef]
- Anno, T.; Motoyama, K.; Higashi, T.; Hirayama, F.; Uekama, K.; Arima, H. Preparation and evaluation of polyamidoamine dendrimer (G2)/branched-β-cyclodextrin conjugate as a novel gene transfer carrier. J. Incl. Phenom. Macro. Chem. 2010, 70, 339–344. [Google Scholar] [CrossRef]
- Anno, T.; Higashi, T.; Motoyama, K.; Hirayama, F.; Uekama, K.; Arima, H. Potential use of glucuronylglucosyl-β-cyclodextrin/dendrimer conjugate (G2) as a DNA carrier in vitro and in vivo. J. Drug Target. 2012, 20, 272–280. [Google Scholar] [CrossRef]
- Anno, T.; Higashi, T.; Motoyama, K.; Hirayama, F.; Uekama, K.; Arima, H. Possible enhancing mechanisms for gene transfer activity of glucuronylglucosyl-β-cyclodextrin/dendrimer conjugate. Int. J. Pharm. 2012, 426, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Anno, T.; Higashi, T.; Hayashi, Y.; Motoyama, K.; Jono, H.; Ando, Y.; Arima, H. Potential use of glucuronylglucosyl-β-cyclodextrin/dendrimer conjugate (G2) as a siRNA carrier for the treatment of familial amyloidotic polyneuropathy. J. Drug Target. 2014, 22, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, A.F.; Ohyama, A.; Higashi, T.; Motoyama, K.; Khaled, K.A.; Sarhan, H.A.; Hussein, A.K.; Arima, H. Preparation and evaluation of polyamidoamine dendrimer conjugate with glucuronylglucosyl-β-cyclodextrin (G3) as a novel carrier for siRNA. J. Drug Target. 2014, 22, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Zatloukal, K.; Cotton, M.; Mechtler, K.; Wagner, E. Gene transfer into hepatocytes using asialoglycoprotein receptor mediated endocytosis of DNA complexed with an artificial tetra-antennary galactose ligand. Bioconjugate Chem. 1992, 3, 533–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, K.; Arima, H.; Tsutsumi, T.; Hirayama, F.; Uekama, K. Enhancing effects of galactosylated dendrimer/alpha-cyclodextrin conjugates on gene transfer efficiency. Biol. Pharm. Bull. 2005, 28, 500–505. [Google Scholar] [CrossRef] [Green Version]
- Arima, H.; Yamashita, S.; Mori, Y.; Hayashi, Y.; Motoyama, K.; Hattori, K.; Takeuchi, T.; Jono, H.; Ando, Y.; Hirayama, F.; et al. In vitro and in vivo gene delivery mediated by Lactosylated dendrimer/α-cyclodextrin conjugates (G2) into hepatocytes. J. Control. Release 2010, 146, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Mori, Y.; Yamashita, S.; Hayashi, Y.; Jono, H.; Ando, Y.; Hirayama, F.; Uekama, K.; Arima, H. In vitro gene delivery mediated by lactosylated dendrimer (generation 3, G3)/α-cyclodextrin conjugates into hepatocytes. J. Incl. Phenom. Macro. Chem. 2011, 70, 333–338. [Google Scholar] [CrossRef]
- Hayashi, Y.; Mori, Y.; Higashi, T.; Motoyama, K.; Jono, H.; Sah, D.W.; Ando, Y.; Arima, H. Systemic delivery of transthyretin siRNA mediated by lactosylated dendrimer/α-cyclodextrin conjugates into hepatocyte for familial amyloidotic polyneuropathy therapy. Amyloid 2012, 19 (Suppl. S1), 47–49. [Google Scholar] [CrossRef]
- Hayashi, Y.; Mori, Y.; Yamashita, S.; Motoyama, K.; Higashi, T.; Jono, H.; Ando, Y.; Arima, H. Potential use of lactosylated dendrimer (G3)/α-cyclodextrin conjugates as hepatocyte-specific siRNA carriers for the treatment of familial amyloidotic polyneuropathy. Mol. Pharm. 2012, 9, 1645–1653. [Google Scholar] [CrossRef]
- Hayashi, Y.; Higashi, T.; Motoyama, K.; Mori, Y.; Jono, H.; Ando, Y.; Arima, H. Design and evaluation of polyamidoamine dendrimer conjugate with PEG, α-cyclodextrin and lactose as a novel hepatocyte-selective gene carrier in vitro and in vivo. J. Drug Target. 2013, 21, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Higashi, T.; Motoyama, K.; Jono, H.; Ando, Y.; Onodera, R.; Arima, H. Hepatocyte-targeted delivery of siRNA polyplex with PEG-modified lactosylated dendrimer/cyclodextrin conjugates for transthyretin-related amyloidosis therapy. Biol. Pharm. Bull. 2019, 42, 1679–1688. [Google Scholar] [CrossRef]
- Martinez-Pomares, L. The mannose receptor. J. Leukoc. Biol. 2012, 92, 1177–1186. [Google Scholar] [CrossRef]
- Feinberg, H.; Jegouzo, S.A.; Lasanajak, Y.; Smith, D.F.; Drickamer, K.; Weis, W.I.; Taylor, M.E. Structural analysis of carbohydrate binding by the macrophage mannose receptor CD206. J. Biol. Chem. 2021, 296, 100368. [Google Scholar] [CrossRef]
- Lee, R.T.; Hsu, T.-L.; Huang, S.K.; Hsieh, S.L.; Wong, C.-H.; Lee, Y.C. Survey of immune-related, mannose/fucose-binding C-type lectin receptors reveals widely divergent sugar-binding specificities. Glycobiology 2011, 21, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Fiani, M.L.; Barreca, V.; Sargiacomo, M.; Ferrantelli, F.; Manfredi, F.; Federico, M. Exploiting Manipulated Small Extracellular Vesicles to Subvert Immunosuppression at the Tumor Microenvironment through Mannose Receptor/CD206 Targeting. Int. J. Mol. Sci. 2020, 21, 6318. [Google Scholar] [CrossRef]
- Arima, H.; Wada, K.; Kihara, F.; Tsutsumi, T.; Hirayama, F.; Uekama, K. Cell-Specific gene transfer by α-cyclodextrin conjugates with mannosylated polyamidoamine dendrimers. J. Incl. Phenom. Macro. Chem. 2002, 44, 361–364. [Google Scholar] [CrossRef]
- Wada, K.; Arima, H.; Tsutsumi, T.; Chihara, Y.; Hattori, K.; Hirayama, F.; Uekama, K. Improvement of gene delivery mediated by mannosylated dendrimer/α-cyclodextrin conjugates. J. Control. Release 2005, 104, 397–413. [Google Scholar] [CrossRef]
- Arima, H.; Chihara, Y.; Arizono, M.; Yamashita, S.; Wada, K.; Hirayama, F.; Uekama, K. Enhancement of gene transfer activity mediated by mannosylated dendrimer/alpha-cyclodextrin conjugate (generation 3, G3). J. Control. Release 2006, 116, 64–74. [Google Scholar] [CrossRef]
- Chihara, Y.; Arima, H.; Arizono, M.; Waka, K.; Hirayama, F.; Uekama, K. Serum-resistant Gene Transfer Activity of Mannosylated Dendrimer/α-Cyclodextrin Conjugate (G3). J. Incl. Phenom. Macro. Chem. 2006, 56, 89–93. [Google Scholar] [CrossRef]
- Kovacs, L.; Cabral, P.; Chammas, R. Mannose receptor 1 expression does not determine the uptake of high-density mannose dendrimers by activated macrophages populations. PLoS ONE 2020, 15, e0240455. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Mitsuyasu, R.; Akao, C.; Tanaka, T.; Ohyama, A.; Sato, N.; Higashi, T.; Arima, H. Design and Evaluation of Thioalkylated Mannose-Modified Dendrimer (G3)/α-Cyclodextrin Conjugates as Antigen-Presenting Cell-Selective siRNA Carriers. AAPS J. 2014, 16, 1298–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolios, G.; Valatas, V.; Kouroumalis, E. Role of Kupffer cells in the pathogenesis of liver disease. World J. Gastroenterol. 2006, 12, 7413–7420. [Google Scholar] [CrossRef]
- Sun, B.; Karin, M. NF-κB signaling, liver disease and hepatoprotective agents. Oncogene 2008, 27, 6228–6244. [Google Scholar] [CrossRef] [Green Version]
- Motoyama, K.; Mitsuyasu, R.; Akao, C.; Abu Hashim, I.I.; Sato, N.; Tanaka, T.; Higashi, T.; Arima, H. Potential Use of Thioalkylated Mannose-Modified Dendrimer (G3)/α-Cyclodextrin Conjugate as an NF-κB siRNA Carrier for the Treatment of Fulminant Hepatitis. Mol. Pharm. 2015, 12, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Opanasopit, P.; Nishikawa, M.; Yamashita, F.; Takakura, Y.; Hashida, M. Pharmacokinetic analysis of lectin-dependent biodistribution of fucosylated bovine serum albumin: A possible carrier for Kupffer cells. J. Drug Target. 2001, 9, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Akao, C.; Tanaka, T.; Onodera, R.; Ohyama, A.; Sato, N.; Motoyama, K.; Higashi, T.; Arima, H. Potential use of fucose-appended dendrimer/α-cyclodextrin conjugates as NF-κB decoy carriers for the treatment of lipopolysaccharide-induced fulminant hepatitis in mice. J. Control. Release 2014, 193, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Akao, C.; Mitsuyasu, R.; Higashi, T.; Arima, H. Fucose-appended dendrimer/α-cyclodextrin conjugate as a novel NF-κB decoy carrier to Kupffer cells infulminant hepatitis mice induced by lipopolysaccharide. Asian J. Pharm. Sci. 2016, 11, 152–153. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2017, 9, 790–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.B.; Ganapathy, P.S.; Bozard, R.B.; Ganapathy, V. Chapter 35-Folate Transport in Retina and Consequences on Retinal Structure and Function of Hyperhomocysteinemia. In Handbook of Nutrition, Diet and the Eye; Academic Press: Cambridge, MA, USA, 2014; pp. 349–359. [Google Scholar]
- Arima, H.; Arizono, M.; Higashi, T.; Yoshimatsu, A.; Ikeda, H.; Motoyama, K.; Hattori, K.; Takeuchi, T.; Hirayama, F.; Uekama, K. Potential use of folate-polyethylene glycol (PEG)-appended dendrimer (G3) conjugate with α-cyclodextrin as DNA carriers to tumor cells. Cancer Gene Ther. 2012, 19, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Trivedi, P.; Jain, N.K. Advances in siRNA delivery in cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 274–283. [Google Scholar] [CrossRef]
- Arima, H.; Yoshimatsu, A.; Ikeda, H.; Ohyama, A.; Motoyama, K.; Higashi, T.; Tsuchiya, A.; Niidome, T.; Katayama, Y.; Hattori, K.; et al. Folate-PEG-appended dendrimer conjugate with α-cyclodextrin as a novel cancer cell-selective siRNA delivery carrier. Mol. Pharm. 2012, 9, 2591–2604. [Google Scholar] [CrossRef]
- Ohyama, A.; Higashi, T.; Motoyama, K.; Arima, H. In Vitro and In Vivo Tumor-Targeting siRNA Delivery Using Folate-PEG-appended Dendrimer (G4)/α-Cyclodextrin Conjugates. Bioconjugate Chem. 2016, 27, 521–532. [Google Scholar] [CrossRef]
- Arima, H.; Motoyama, K.; Higashi, T. Potential therapeutic application of dendrimer/cyclodextrin conjugates with targeting ligands as advanced carriers for gene and oligonucleotide drugs. Ther. Deliv. 2017, 8, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.F.A.; Higashi, T.; Motoyama, K.; Ohyama, A.; Onodera, R.; Khaled, K.A.; Sarhan, H.A.; Hussein, A.K.; Arima, H. Targeted siRNA delivery to tumor cells by folate-PEG-appended dendrimer/glucuronylglucosyl-β-cyclodextrin conjugate. J. Incl. Phenom. Macro. Chem. 2018, 93, 41–52. [Google Scholar] [CrossRef]
- Hattori, Y.; Nakamura, A.; Arai, S.; Nishigaki, M.; Ohkura, H.; Kawano, K.; Maitani, Y.; Yonemochi, E. In vivo siRNA delivery system for targeting to the liver by poly-l-glutamic acid-coated lipoplex. Results Pharma Sci. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okajima, M.K.; Miyazato, S.; Kaneko, T. Cyanobacterial megamolecule sacran efficiently forms LC Gels with very heavy metal ions. Langmuir 2009, 25, 8526–8531. [Google Scholar] [CrossRef]
- Okeyoshi, K.; Okajima, M.K.; Kaneko, T. The cyanobacterial polysaccharide sacran: Characteristics, structures, and preparation of LC gels. Polym. J. 2021, 53, 81–91. [Google Scholar] [CrossRef]
- Hayashi, Y.; Higashi, T.; Motoyama, K.; Jono, H.; Ando, Y.; Arima, H. In vitro and in vivo siRNA delivery to hepatocyte utilizing ternary complexation of lactosylated dendrimer/cyclodextrin conjugates, siRNA and low-molecular-weight sacran. Int. J. Biol. Macromol. 2018, 107, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, A.; Higashi, T.; Motoyama, K.; Arima, H. Ternary complexes of folate-PEG-appended dendrimer (G4)/α-cyclodextrin conjugate, siRNA and low-molecular-weight polysaccharide sacran as a novel tumor-selective siRNA delivery system. Int. J. Biol. Macromol. 2017, 99, 21–28. [Google Scholar] [CrossRef]
- Khelghati, N.; Soleimanpour Mokhtarvand, J.; Mir, M.; Alemi, F.; Asemi, Z.; Sadeghpour, A.; Maleki, M.; Samadi Kafil, H.; Jadidi-Niaragh, F.; Majidinia, M.; et al. The importance of co-delivery of nanoparticle-siRNA and anticancer agents in cancer therapy. Chem. Biol. Drug Des. 2021, 97, 997–1015. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.F.A.; Higashi, T.; Motoyama, K.; Ohyama, A.; Onodera, R.; Khaled, K.A.; Sarhan, H.A.; Hussein, A.K.; Arima, H. In vitro and in vivo co-delivery of siRNA and doxorubicin by folate-PEG-appended dendrimer/glucuronylglucosyl-β-cyclodextrin conjugate. AAPS J. 2019, 21, 54. [Google Scholar] [CrossRef]
- Higashi, T.; Iohara, D.; Motoyama, K.; Arima, H. Supramolecular Pharmaceutical Sciences: A Novel Concept Combining Pharmaceutical Sciences and Supramolecular Chemistry with a Focus on Cyclodextrin-Based Supermolecules. Chem. Pharm. Bull. 2018, 66, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Motoyama, K.; Hayashida, K.; Higashi, T.; Arima, H. Polypseudorotaxanes of pegylated alpha-cyclodextrin/polyamidoamine dendrimer conjugate with cyclodextrins as a sustained release system for DNA. Bioorganic Med. Chem. 2012, 20, 1425–1433. [Google Scholar] [CrossRef]
- Morita, K.; Motoyama, K.; Higashi, T.; Hayashida, K.; Abu Hashim Mohamed, I.I.; Arima, H. Sustained release system of siRNA complex with polyethyleneglycol-appended β-cyclodextrin/dendrimer conjugate from cyclodextrin polypseudorotaxane. J. Drug Des. Res. 2017, 4, 1056. [Google Scholar]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, D.B.T.; Platt, R.J.; Zhang, F. Therapeutic genome editing: Prospects and challenges. Nat. Med. 2015, 21, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Uchida, E. Regulations and safety assessment of genome editing technologies for human gene therapy. Transl. Regul. Sci. 2020, 2, 107–114. [Google Scholar] [CrossRef]
- Xu, X.; Wan, T.; Xin, H.; Li, D.; Pan, H.; Wu, J.; Ping, Y. Delivery of CRISPR/Cas9 for therapeutic genome editing. J. Gene Med. 2019, 21, e3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, T.; Cheng, Q.; Min, Y.-L.; Olson, E.N.; Siegwart, D.J. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat. Commun. 2020, 11, 3232. [Google Scholar] [CrossRef] [PubMed]
- Taharabaru, T.; Yokoyama, R.; Higashi, T.; Mohammed, A.F.A.; Inoue, M.; Maeda, Y.; Niidome, T.; Onodera, R.; Motoyama, K. Genome Editing in a Wide Area of the Brain Using Dendrimer-Based Ternary Polyplexes of Cas9 Ribonucleoprotein. ACS Appl. Mater. Interfaces 2020, 12, 21386–21397. [Google Scholar] [CrossRef]
- Bom, A.; Bradley, M.; Cameron, K.; Clark, J.K.; Van Egmond, J.; Feilden, H.; MacLean, E.J.; Muir, A.W.; Palin, R.; Rees, D.C.; et al. A novel concept of reversing neuromuscular block: Chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew. Chem. Int. Ed. 2002, 41, 266–270. [Google Scholar] [CrossRef]
- Safety and Efficacy of Intravenous Trappsol Cyclo (HPBCD) in Niemann-Pick Type C Patients. Available online: https://ichgcp.net/clinical-trials-registry/NCT02912793 (accessed on 28 February 2021).
- Kameyama, K.; Motoyama, K.; Tanaka, N.; Yamashita, Y.; Higashi, T.; Arima, H. Induction of mitophagy-mediated antitumor activity with folate-appended methyl-β-cyclodextrin. Int. J. Nanomed. 2017, 12, 3433–3446. [Google Scholar] [CrossRef] [Green Version]
- Motoyama, K.; Sako, A.; Abu Hashim, I.I.; Higashi, T.; Arima, H. Effects of dendrimer/cyclodextrin conjugates as gene transfer carriers on nitric oxide production from macrophages. J. Pharm. Pharmacol. 2016, 68, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Ueda, M.; Higashi, T.; Anno, T.; Fujisawa, K.; Motoyama, K.; Mizuguchi, M.; Ando, Y.; Jono, H.; Arima, H. Therapeutic Potential of Polyamidoamine Dendrimer for Amyloidogenic Transthyretin Amyloidosis. ACS Chem. Neurosci. 2019, 10, 2584–2590. [Google Scholar] [CrossRef] [PubMed]












| CD | Glucose Unit | Molecular Weight | Cavity Size (Å) | Cavity Volume (Å 2) | Solubility (g/100 mL) 1 | Surface Tension (mN/m) |
|---|---|---|---|---|---|---|
| α-CD | 6 | 973 | 4.7–5.3 | 174 | 14.5 | 73 |
| β-CD | 7 | 1135 | 6.0–6.5 | 262 | 1.85 | 73 |
| γ-CD | 8 | 1297 | 7.5–8.3 | 427 | 23.2 | 73 |
| GUG-β-CD | 9 | 1473 | 6.0–6.5 | 262 | >200 | 73 |
| Pamameter | Comparison |
|---|---|
| Physicochemical properties | |
| Complexation ability | α-CDE > GUG-β-CDE |
| Particle sizes | α-CDE ≈ GUG-β-CDE |
| ζ-Potential | α-CDE ≈ GUG-β-CDE |
| Stability against nuclease | α-CDE ≈ GUG-β-CDE |
| Cellular association | α-CDE ≈ GUG-β-CDE |
| Endocytosis pathway | α-CDE ≈ GUG-β-CDE |
| Endolysosomal escaping ability | α-CDE < GUG-β-CDE |
| Nuclear localization ability | α-CDE < GUG-β-CDE |
| Decompaction ability | α-CDE < GUG-β-CDE |
| Properties | Fol-PEG-α-CDE (G3, DSC2.4, DSF4, DSP4) | Fol-PEG-α-CDE (G4, DSC2.9, DSF2, DSP2) |
|---|---|---|
| Optimal charge (N/P) ratio | 20 | 10 |
| RNAi effect in the presence of serum | up to 10% FBS | up to 50% FBS |
| Complexation ability with siRNA | + | ++ |
| In vitro RNAi effect | 50–60% | 50–70% |
| Cellular uptake | + | ++ |
| FR-α selectivity | +++ | +++ |
| Interferon response | − | − |
| CDE | G 1 | DSC 2 | DSL 3 | DSP 4 | Payload | System | Reference |
|---|---|---|---|---|---|---|---|
| α-CDE | 2 | 1, 1.2 | - | - | pDNA | Complex | [37,38,45,46,47,52,62,63,64,65] |
| α-CDE | 3 | 1, 1.1, 5.4 | - | - | pDNA | Complex | [38,39] |
| α-CDE | 3 | 2.4 | - | - | pDNA | Complex | [38,39,42,44,53,78] |
| α-CDE | 3 | 2.4 | - | - | shRNA | Complex | [42] |
| α-CDE | 3 | 2.4 | - | - | siRNA | Complex | [42,43,78] |
| α-CDE | 3 | 2.4 | - | - | L-HIPRO | Complex | [44] |
| α-CDE | 4 | 1 | - | - | pDNA | Complex | [38] |
| β-CDE | 2 | 1, 1.3 | - | - | pDNA | Complex | [37,45,46,47] |
| γ-CDE | 4 | 1 | - | - | pDNA | Complex | [37] |
| PEG 5-α-CDE | 2 | 1.5 | - | 4 | pDNA | PPRX 5 | [92] |
| PEG-α-CDE | 2 | 1 | - | 3 | siRNA | PPRX 5 | [93] |
| Gal 6-α-CDE | 2 | 1 | 4 | - | pDNA | PPRX 5 | [51] |
| Lac 7-α-CDE | 2 | 1 | 1.2, 2.6, 4.6, 6.2, 10.2 | - | pDNA | Complex | [52] |
| Lac-α-CDE | 2 | 2 | 1 | - | pDNA | ternary complex with sacran | [87] |
| Lac-α-CDE | 3 | 2.4 | 1.2, 2.6, 4.1, 6.1 | - | pDNA | Complex | [53] |
| Lac-α-CDE | 3 | 2.4 | 1.2 | - | siRNA | Complex | [54,55] |
| PEG-Lac-α-CDE | 3 | 2.0 | 1.2 | 2.1, 4.0, 6.2 | pDNA | Complex | [56] |
| PEG-Lac-α-CDE | 3 | 2.0 | 1 | 2 | pDNA | Complex | [57] |
| Man 8-α-CDE | 2 | 1 | 1, 3, 5 | pDNA | Complex | [62] | |
| Man-α-CDE | 2 | 1.1 | 1.1, 3.3, 4.9, 8.3 | pDNA | Complex | [63] | |
| Man-α-CDE | 3 | 2.2 | 5, 10, 13, 20 | pDNA | Complex | [64,65] | |
| Man-S-α-CDE | 3 | 2 | 4 | siRNA | Complex | [67,69] | |
| Fuc 9-S-α-CDE | 2 | 1 | 2 | decoy DNA | Complex | [72,73] | |
| Fol 10-α-CDE | 3 | 2.4 | 2, 5, 7 | pDNA | Complex | [78] | |
| Fol-PEG-α-CDE | 3 | 2.4 | 2, 5, 7 | 2,5,7 | pDNA | Complex | [78] |
| Fol-PEG-α-CDE | 3 | 2 | 2, 4, 7 | 2, 4, 7 | siRNA | Complex | [80] |
| Fol-PEG-α-CDE | 4 | 2.9 | 2 | 2 | siRNA | Complex | [81] |
| Fol-PEG-α-CDE | 4 | 3 | 2 | 2 | siRNA | ternary complex with sacran | [88] |
| GUG 11-β-CDE | 2 | 1.8 | pDNA | Complex | [45,46,47,48] | ||
| GUG-β-CDE | 2 | 1.8, 2.5, 3.0, 5.0 | siRNA | Complex | [48] | ||
| GUG-β-CDE | 3 | 1.6, 3.0, 3.7, 5.0, 8.6 | pDNA | Complex | [49] | ||
| GUG-β-CDE | 3 | 1.5, 3, 6.5 | Cas9 RNP | Complex | [99] | ||
| Fol-PEG-GUG-β-CDE | 3 | 3.7 | 3.9, 6.7, 7.3 | 3.9, 6.7, 7.3 | siRNA | Complex | [83] |
| Fol-PEG-GUG-β-CDE | 3 | 3.7 | 6.7 | 6.7 | siRNA, doxorubicin | ternary complex | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arima, H. Twenty Years of Research on Cyclodextrin Conjugates with PAMAM Dendrimers. Pharmaceutics 2021, 13, 697. https://doi.org/10.3390/pharmaceutics13050697
Arima H. Twenty Years of Research on Cyclodextrin Conjugates with PAMAM Dendrimers. Pharmaceutics. 2021; 13(5):697. https://doi.org/10.3390/pharmaceutics13050697
Chicago/Turabian StyleArima, Hidetoshi. 2021. "Twenty Years of Research on Cyclodextrin Conjugates with PAMAM Dendrimers" Pharmaceutics 13, no. 5: 697. https://doi.org/10.3390/pharmaceutics13050697