Continuous Twin Screw Granulation: A Review of Recent Progress and Opportunities in Formulation and Equipment Design
Abstract
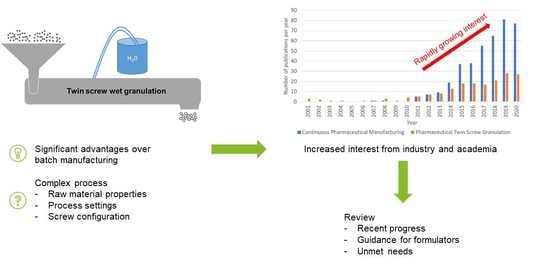
:1. Introduction
2. Influence of Raw Material Properties
2.1. Fillers
2.1.1. Commonly Used Fillers and Filler Combinations
2.1.2. Microcrystalline Cellulose
2.1.3. Lactose
2.1.4. Mannitol
2.2. Binders
2.2.1. Immediate Release
2.2.2. Sustained Release
2.3. Surfactants
2.4. APIs
3. Influence of Process Settings
3.1. L/S Ratio
3.2. Screw Speed
3.3. Throughput
3.4. Barrel Temperature
3.5. Screw Design
4. Recommendations and Research Opportunities
4.1. Formulation
4.2. Equipment Design and Process Control
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- U.S. Food and Drug Administration. Quality Considerations for Continuous Manufacturing—Guidance for Industry—Draft Guidance; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- Portier, C.; Pandelaere, K.; Delaet, U.; Vigh, T.; Kumar, A.; Di Pretoro, G.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Continuous twin screw granulation: Influence of process and formulation variables on granule quality attributes of model formulations. Int. J. Pharm. 2020, 576, 118981. [Google Scholar] [CrossRef]
- Teżyk, M.; Milanowski, B.; Ernst, A.; Lulek, J. Recent progress in continuous and semi-continuous processing of solid oral dosage forms: A review. Drug Dev. Ind. Pharm. 2016, 42, 1195–1214. [Google Scholar] [CrossRef]
- Lee, S.L.; O’Connor, T.F.; Yang, X.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Moore, C.M.V.; Yu, L.X.; Woodcock, J. Modernizing Pharmaceutical Manufacturing: From Batch to Continuous Production. J. Pharm. Innov. 2015, 10, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Karttunen, A.P.; Hörmann, T.R.; De Leersnyder, F.; Ketolainen, J.; De Beer, T.; Hsiao, W.K.; Korhonen, O. Measurement of residence time distributions and material tracking on three continuous manufacturing lines. Int. J. Pharm. 2019, 563, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, J.; Peeters, E.; Fonteyne, M.; Cappuyns, P.; Delaet, U.; Van Assche, I.; De Beer, T.; Remon, J.P.; Vervaet, C. Use of a continuous twin screw granulation and drying system during formulation development and process optimization. Eur. J. Pharm. Biopharm. 2015, 89, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Madarász, L.; Nagy, Z.K.; Hoffer, I.; Szabó, B.; Csontos, I.; Pataki, H.; Démuth, B.; Szabó, B.; Csorba, K.; Marosi, G. Real-time feedback control of twin-screw wet granulation based on image analysis. Int. J. Pharm. 2018, 547, 360–367. [Google Scholar] [CrossRef] [PubMed]
- De Leersnyder, F.; Vanhoorne, V.; Kumar, A.; Vervaet, C.; De Beer, T. Evaluation of an in-line NIR spectroscopic method for the determination of the residence time in a tablet press. Int. J. Pharm. 2019, 565, 358–366. [Google Scholar] [CrossRef]
- Pauli, V.; Roggo, Y.; Kleinebudde, P.; Krumme, M. Real-time monitoring of particle size distribution in a continuous granulation and drying process by near infrared spectroscopy. Eur. J. Pharm. Biopharm. 2019, 141, 90–99. [Google Scholar] [CrossRef]
- Verstraeten, M.; Van Hauwermeiren, D.; Hellings, M.; Hermans, E.; Geens, J.; Vervaet, C.; Nopens, I.; De Beer, T. Model-based NIR spectroscopy implementation for in-line assay monitoring during a pharmaceutical suspension manufacturing process. Int. J. Pharm. 2018, 546, 247–254. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Vervaet, C. Recent progress in continuous manufacturing of oral solid dosage forms. Int. J. Pharm. 2020, 579, 119194. [Google Scholar] [CrossRef]
- Portier, C.; De Vriendt, C.; Vigh, T.; Di Pretoro, G.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Continuous twin screw granulation: Robustness of lactose/MCC-based formulations. Int. J. Pharm. 2020, 588, 119756. [Google Scholar] [CrossRef]
- Byrn, S.; Futran, M.; Thomas, H.; Jayjock, E.; Maron, N.; Meyer, R.F.; Myerson, A.S.; Thien, M.P.; Trout, B.L. Achieving continuous manufacturing for final dosage formation: Challenges and how to meet them 20–21 May, 2014 continuous manufacturing symposium. J. Pharm. Sci. 2015, 104, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Fonteyne, M.; Wickström, H.; Peeters, E.; Vercruysse, J.; Ehlers, H.; Peters, B.H.; Remon, J.P.; Vervaet, C.; Ketolainen, J.; Sandler, N.; et al. Influence of raw material properties upon critical quality attributes of continuously produced granules and tablets. Eur. J. Pharm. Biopharm. 2014, 87, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ricart, B.; Stanton, C.; Smith-Goettler, B.; Verdi, L.; O’Connor, T.; Lee, S.; Yoon, S. Design space determination and process optimization in at-scale continuous twin screw wet granulation. Comput. Chem. Eng. 2019, 125, 271–286. [Google Scholar] [CrossRef]
- Stauffer, F.; Vanhoorne, V.; Pilcer, G.; Chavez, P.F.; Rome, S.; Schubert, M.A.; Aerts, L.; De Beer, T. Raw material variability of an active pharmaceutical ingredient and its relevance for processability in secondary continuous pharmaceutical manufacturing. Eur. J. Pharm. Biopharm. 2018, 127, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gernaey, K.V.; De Beer, T.; Nopens, I. Model-based analysis of high shear wet granulation from batch to continuous processes in pharmaceutical production—A critical review. Eur. J. Pharm. Biopharm. 2013, 85, 814–832. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Bindra, D.S.; Gour, S.; Trinh, J.; Buckley, D.; Badawy, S. Excipient–Process Interactions and their Impact on Tablet Compaction and Film Coating. J. Pharm. Sci. 2014, 103, 3666–3674. [Google Scholar] [CrossRef] [PubMed]
- Van Snick, B.; Holman, J.; Vanhoorne, V.; Kumar, A.; De Beer, T.; Remon, J.P.; Vervaet, C. Development of a continuous direct compression platform for low-dose drug products. Int. J. Pharm. 2017, 529, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Leane, M.; Pitt, K.; Reynolds, G.K.; Dawson, N.; Ziegler, I.; Szepes, A.; Crean, A.M.; Dall Agnol, R. Manufacturing classification system in the real world: Factors influencing manufacturing process choices for filed commercial oral solid dosage formulations, case studies from industry and considerations for continuous processing. Pharm. Dev. Technol. 2018, 23, 964–977. [Google Scholar] [CrossRef]
- Ryckaert, A.; Ghijs, M.; Portier, C.; Djuric, D.; Funke, A.; Vervaet, C.; De Beer, T. The influence of equipment design and process parameters on granule breakage in a semi-continuous fluid bed dryer after continuous twin-screw wet granulation. Pharmaceutics 2021, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Thommes, M.; Rasenack, N.; Krumme, M.; Moll, K.P.; Kleinebudde, P. Simplified formulations with high drug loads for continuous twin-screw granulation. Int. J. Pharm. 2015, 496, 12–23. [Google Scholar] [CrossRef]
- Shirazian, S.; Ismail, H.Y.; Singh, M.; Shaikh, R.; Croker, D.M.; Walker, G.M. Multi-dimensional population balance modelling of pharmaceutical formulations for continuous twin-screw wet granulation: Determination of liquid distribution. Int. J. Pharm. 2019, 566, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Pishnamazi, M.; Casilagan, S.; Clancy, C.; Shirazian, S.; Iqbal, J.; Egan, D.; Edlin, C.; Croker, D.M.; Walker, G.M.; Collins, M.N. Microcrystalline cellulose, lactose and lignin blends: Process mapping of dry granulation via roll compaction. Powder Technol. 2019, 341, 38–50. [Google Scholar] [CrossRef]
- Fülöp, G.; Domokos, A.; Galata, D.; Szabó, E.; Gyürkés, M.; Szabó, B.; Farkas, A.; Madarász, L.; Démuth, B.; Lendér, T.; et al. Integrated twin-screw wet granulation, continuous vibrational fluid drying and milling: A fully continuous powder to granule line. Int. J. Pharm. 2021, 594, 120126. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.; Wilson, D.; Huang, Z.; De Matas, M. Transfer from high-shear batch to continuous twin screw wet granulation: A case study in understanding the relationship between process parameters and product quality attributes. J. Pharm. Sci. 2014, 103, 3075–3082. [Google Scholar] [CrossRef]
- Megarry, A.; Taylor, A.; Gholami, A.; Wikström, H.; Tajarobi, P. Twin-screw granulation and high-shear granulation: The influence of mannitol grade on granule and tablet properties. Int. J. Pharm. 2020, 590, 119890. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Ingram, A.; Rowson, N.A. Comparison of granule properties produced using Twin Screw Extruder and High Shear Mixer: A step towards understanding the mechanism of twin screw wet granulation. Powder Technol. 2013, 238, 91–98. [Google Scholar] [CrossRef]
- Kyttä, K.M.; Lakio, S.; Wikström, H.; Sulemanji, A.; Fransson, M.; Ketolainen, J.; Tajarobi, P. Comparison between twin-screw and high-shear granulation—The effect of filler and active pharmaceutical ingredient on the granule and tablet properties. Powder Technol. 2020, 376, 187–198. [Google Scholar] [CrossRef]
- Matsui, Y.; Watano, S. Evaluation of Properties of Granules and Tablets Prepared by Twin-screw Continuous Granulation and Comparison of Their Properties with Those by Batch Fluidized-bed and High Shear Granulations. J. Soc. Powder Technol. Japan 2018, 55, 86–94. [Google Scholar] [CrossRef]
- Vervaet, C.; Remon, J.P. Continuous granulation in the pharmaceutical industry. Chem. Eng. Sci. 2005, 60, 3949–3957. [Google Scholar] [CrossRef]
- Thompson, M.R. Twin screw granulation-review of current progress. Drug Dev. Ind. Pharm. 2015, 41, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Schuettgut Portal Investing in the Future: Bohle Technology Center to Be Opened. Available online: https://www.schuettgut-portal.com/newsitem/6085/investitionen-in-die-zukunft--bohle-technology-center-wird-i.html (accessed on 12 October 2020).
- Syntegon Xelum Continuous Manufacturing. Available online: https://www.syntegon.com/products/xelum-continuous-manufacturing (accessed on 12 October 2020).
- Yu, L.X. Continuous Manufacturing Has a Strong Impact on Drug Quality. Available online: https://blogs.fda.gov/fdavoice/index.php/2016/04/continuous-manufacturing-has-a-strong-impact-on-drug-quality (accessed on 10 April 2021).
- U.S. Food and Drug Administration. FDA Approves Lorlatinib for Second- or Third-Line Treatment of ALK-Positive Metastatic NSCLC. Available online: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm625027.htm (accessed on 28 January 2019).
- U.S. Food and Drug Administration. FDA Approves Abemaciclib as Initial Therapy for HR-Positive, HER2-Negative Metastatic Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-initial-therapy-hr-positive-her2-negative-metastatic-breast-cancer (accessed on 10 April 2021).
- U.S. Food and Drug Administration. FDA Approves New Treatment for Patients with Acute Myeloid Leukemia. Available online: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm626443.htm (accessed on 28 January 2019).
- Badman, C.; Cooney, C.L.; Florence, A.; Konstantinov, K.; Krumme, M.; Mascia, S.; Nasr, M.; Trout, B.L. Why We Need Continuous Pharmaceutical Manufacturing and How to Make It Happen. J. Pharm. Sci. 2019, 108, 3521–3523. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidance for Industry PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance; U.S. Food and Drug Administration: Rockville, MD, USA, 2004.
- JRS Pharma. Benefits of Using High-Functionality Excipients in a Continuous Manufacturing Process; JRS Pharma: Rosenberg, Germany, 2019. [Google Scholar]
- Manufacturing Chemist the Rise of Independent Excipients. Available online: https://www.manufacturingchemist.com/news/article_page/The_rise_of_independent_excipients/147856 (accessed on 12 October 2020).
- Challener, C. Key Ingredients Needed to Drive the Success of Continuous Manufacturing | Pharmaceutical Technology. Available online: https://www.pharmtech.com/view/key-ingredients-needed-to-drive-the-success-of-continuous-manufacturing (accessed on 12 October 2020).
- DFE Pharma Continuous Manufacturing. Available online: https://www.dfepharma.com.br/Excipients/Certified-Knowledge/Knowledge-Base/ContinuousManufacturing (accessed on 12 October 2020).
- Vandevivere, L.; Portier, C.; Vanhoorne, V.; Häusler, O.; Simon, D.; De Beer, T.; Vervaet, C. Native starch as in situ binder for continuous twin screw wet granulation. Int. J. Pharm. 2019, 571, 118760. [Google Scholar] [CrossRef] [PubMed]
- Vandevivere, L.; Denduyver, P.; Portier, C.; Häusler, O.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Influence of binder attributes on binder effectiveness in a continuous twin screw wet granulation process via wet and dry binder addition. Int. J. Pharm. 2020, 119466. [Google Scholar] [CrossRef] [PubMed]
- Vanhoorne, V.; Bekaert, B.; Peeters, E.; De Beer, T.; Remon, J.P.; Vervaet, C. Improved tabletability after a polymorphic transition of delta-mannitol during twin screw granulation. Int. J. Pharm. 2016, 506, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Alakarjula, M.; Vanhoorne, V.; Toiviainen, M.; De Leersnyder, F.; Vercruysse, J.; Juuti, M.; Ketolainen, J.; Vervaet, C.; Remon, J.P.; et al. Linking granulation performance with residence time and granulation liquid distributions in twin-screw granulation: An experimental investigation. Eur. J. Pharm. Sci. 2016, 90, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Moll, K.P.; Krumme, M.; Kleinebudde, P. Impact of fill-level in twin-screw granulation on critical quality attributes of granules and tablets. Eur. J. Pharm. Biopharm. 2017, 115, 102–112. [Google Scholar] [CrossRef]
- Fonteyne, M.; Correia, A.; De Plecker, S.; Vercruysse, J.; Ilić, I.; Zhou, Q.; Vervaet, C.; Remon, J.P.; Onofre, F.; Bulone, V.; et al. Impact of microcrystalline cellulose material attributes: A case study on continuous twin screw granulation. Int. J. Pharm. 2015, 478, 705–717. [Google Scholar] [CrossRef]
- Vercruysse, J.; Córdoba Díaz, D.; Peeters, E.; Fonteyne, M.; Delaet, U.; Van Assche, I.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous twin screw granulation: Influence of process variables on granule and tablet quality. Eur. J. Pharm. Biopharm. 2012, 82, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Vercruysse, J.; Burggraeve, A.; Fonteyne, M.; Cappuyns, P.; Delaet, U.; Van Assche, I.; De Beer, T.; Remon, J.P.; Vervaet, C. Impact of screw configuration on the particle size distribution of granules produced by twin screw granulation. Int. J. Pharm. 2015, 479, 171–180. [Google Scholar] [CrossRef]
- Djuric, D.; Kleinebudde, P. Impact of screw elements on continuous granulation with a twin-screw extruder. J. Pharm. Sci. 2008, 97, 4934–4942. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Thompson, M.R.; O’Donnell, K.P. Understanding wet granulation in the kneading block of twin screw extruders. Chem. Eng. Sci. 2014, 113, 11–21. [Google Scholar] [CrossRef]
- Willecke, N.; Szepes, A.; Wunderlich, M.; Remon, J.P.; Vervaet, C.; De Beer, T. Identifying overarching excipient properties towards an in-depth understanding of process and product performance for continuous twin-screw wet granulation. Int. J. Pharm. 2017, 522, 234–247. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.R.; O’Donnell, K.P. “Rolling” phenomenon in twin screw granulation with controlled-release excipients. Drug Dev. Ind. Pharm. 2015, 41, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Portier, C.; Pandelaere, K.; Delaet, U.; Vigh, T.; Di Pretoro, G.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Continuous twin screw granulation: A complex interplay between formulation properties, process settings and screw design. Int. J. Pharm. 2020, 576, 119004. [Google Scholar] [CrossRef]
- Vandevivere, L.; Vangampelaere, M.; Portier, C.; de Backere, C.; Häusler, O.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Identifying critical binder attributes to facilitate binder selection for efficient formulation development in a continuous twin screw wet granulation process. Pharmaceutics 2021, 13, 210. [Google Scholar] [CrossRef]
- Portier, C.; Vigh, T.; Di Pretoro, G.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Continuous twin screw granulation: Impact of binder addition method and surfactants on granulation of a high-dosed, poorly soluble API. Int. J. Pharm. 2020, 577, 119068. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Almey, R.; De Beer, T.; Vervaet, C. Delta-mannitol to enable continuous twin-screw granulation of a highly dosed, poorly compactable formulation. Int. J. Pharm. 2020, 583, 119374. [Google Scholar] [CrossRef]
- Willecke, N.; Szepes, A.; Wunderlich, M.; Remon, J.P.; Vervaet, C.; De Beer, T. A novel approach to support formulation design on twin screw wet granulation technology: Understanding the impact of overarching excipient properties on drug product quality attributes. Int. J. Pharm. 2018, 545, 128–143. [Google Scholar] [CrossRef] [Green Version]
- Harting, J.; Kleinebudde, P. Development of an in-line Raman spectroscopic method for continuous API quantification during twin-screw wet granulation. Eur. J. Pharm. Biopharm. 2018, 125, 169–181. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; RPS Publishing: London, UK, 2009; ISBN 9780853697923. [Google Scholar]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Ridgway, C.; Bawuah, P.; Markl, D.; Zeitler, J.A.; Ketolainen, J.; Peiponen, K.E.; Gane, P. On the role of API in determining porosity, pore structure and bulk modulus of the skeletal material in pharmaceutical tablets formed with MCC as sole excipient. Int. J. Pharm. 2017, 526, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Thompson, M.R.; O’Donnell, K.P. Examining drug hydrophobicity in continuous wet granulation within a twin screw extruder. Int. J. Pharm. 2015, 496, 3–11. [Google Scholar] [CrossRef]
- Kashani Rahimi, S.; Paul, S.; Sun, C.C.; Zhang, F. The role of the screw profile on granular structure and mixing efficiency of a high-dose hydrophobic drug formulation during twin screw wet granulation. Int. J. Pharm. 2020, 575, 118958. [Google Scholar] [CrossRef]
- Verstraeten, M.; Van Hauwermeiren, D.; Lee, K.; Turnbull, N.; Wilsdon, D.; am Ende, M.; Doshi, P.; Vervaet, C.; Brouckaert, D.; Mortier, S.T.F.C.; et al. In-depth experimental analysis of pharmaceutical twin-screw wet granulation in view of detailed process understanding. Int. J. Pharm. 2017, 529, 678–693. [Google Scholar] [CrossRef]
- Arndt, O.R.; Baggio, R.; Adam, A.K.; Harting, J.; Franceschinis, E.; Kleinebudde, P. Impact of Different Dry and Wet Granulation Techniques on Granule and Tablet Properties: A Comparative Study. J. Pharm. Sci. 2018, 107, 3143–3152. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Lenhart, V.; Kleinebudde, P. Switch of tablet manufacturing from high shear granulation to twin-screw granulation using quality by design approach. Int. J. Pharm. 2020, 579, 119139. [Google Scholar] [CrossRef]
- Portier, C.; Vigh, T.; Di Pretoro, G.; Leys, J.; Klingeleers, D.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Continuous twin screw granulation: Impact of microcrystalline cellulose batch-to-batch variability during granulation and drying—A QbD approach. Int. J. Pharm. X 2021, 100077. [Google Scholar] [CrossRef] [PubMed]
- Harting, J.; Kleinebudde, P. Optimisation of an in-line Raman spectroscopic method for continuous API quantification during twin-screw wet granulation and its application for process characterisation. Eur. J. Pharm. Biopharm. 2019, 137, 77–85. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Janssens, L.; Vercruysse, J.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous twin screw granulation of controlled release formulations with various HPMC grades. Int. J. Pharm. 2016, 511, 1048–1057. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; de Waard, H.; Moll, K.P.; Kleinebudde, P.; Krumme, M. Simplified end-to-end continuous manufacturing by feeding API suspensions in twin-screw wet granulation. Eur. J. Pharm. Biopharm. 2018, 133, 224–231. [Google Scholar] [CrossRef]
- El Hagrasy, A.S.; Hennenkamp, J.R.; Burke, M.D.; Cartwright, J.J.; Litster, J.D. Twin screw wet granulation: Influence of formulation parameters on granule properties and growth behavior. Powder Technol. 2013, 238, 108–115. [Google Scholar] [CrossRef]
- Hwang, K.-M.; Cho, C.-H.; Yoo, S.-D.; Cha, K.-I.; Park, E.-S. Continuous twin screw granulation: Impact of the starting material properties and various process parameters. Powder Technol. 2019, 356, 847–857. [Google Scholar] [CrossRef]
- Paul, S.; Chang, S.Y.; Dun, J.; Sun, W.J.; Wang, K.; Tajarobi, P.; Boissier, C.; Sun, C.C. Comparative analyses of flow and compaction properties of diverse mannitol and lactose grades. Int. J. Pharm. 2018, 546, 39–49. [Google Scholar] [CrossRef]
- Stauffer, F.; Vanhoorne, V.; Pilcer, G.; Chavez, P.F.; Vervaet, C.; De Beer, T. Managing API raw material variability during continuous twin-screw wet granulation. Int. J. Pharm. 2019, 561, 265–273. [Google Scholar] [CrossRef]
- Stauffer, F.; Vanhoorne, V.; Pilcer, G.; Chavez, P.F.; Vervaet, C.; De Beer, T. Managing API raw material variability in a continuous manufacturing line—Prediction of process robustness. Int. J. Pharm. 2019, 569, 118525. [Google Scholar] [CrossRef]
- Ritala, M.; Jungersen, O.; Holm, P.; Schæfer, T.; Kristensen, H.G. A comparison between binders in the wet phase of granulation in a high shear mixer. Drug Dev. Ind. Pharm. 1986, 12, 1685–1700. [Google Scholar] [CrossRef]
- Wu, Y.; Levons, J.; Narang, A.S.; Raghavan, K.; Rao, V.M. Reactive impurities in excipients: Profiling, identification and mitigation of drug-excipient incompatibility. AAPS PharmSciTech 2011, 12, 1248–1263. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Kleinebudde, P. Influence of granulation temperature on particle size distribution of granules in twin-screw granulation (TSG). Pharm. Dev. Technol. 2019, 24, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Vanhoorne, V.; Vanbillemont, B.; Vercruysse, J.; De Leersnyder, F.; Gomes, P.; De Beer, T.; Remon, J.P.; Vervaet, C. Development of a controlled release formulation by continuous twin screw granulation: Influence of process and formulation parameters. Int. J. Pharm. 2016, 505, 61–68. [Google Scholar] [CrossRef]
- Roggo, Y.; Pauli, V.; Jelsch, M.; Pellegatti, L.; Elbaz, F.; Ensslin, S.; Kleinebudde, P.; Krumme, M. Continuous manufacturing process monitoring of pharmaceutical solid dosage form: A case study. J. Pharm. Biomed. Anal. 2020, 179, 112971. [Google Scholar] [CrossRef]
- Dhenge, R.M.; Cartwright, J.J.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Effects of properties of granulation liquid. Powder Technol. 2012, 229, 126–136. [Google Scholar] [CrossRef]
- Cao, Q.R.; Choi, Y.W.; Cui, J.H.; Lee, B.J. Formulation, release characteristics and bioavailability of novel monolithic hydroxypropylmethylcellulose matrix tablets containing acetaminophen. J. Control. Release 2005, 108, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, M. Surfactants and Polymers in Drug Delivery; Marcel Dekker: New York, NY, USA, 2002; ISBN 9780824743758. [Google Scholar]
- Tadros, T.F. Applied Surfactants: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2005; ISBN 3527306293. [Google Scholar]
- Thompson, M.R.; Weatherley, S.; Pukadyil, R.N.; Sheskey, P.J. Foam granulation: New developments in pharmaceutical solid oral dosage forms using twin screw extrusion machinery. Drug Dev. Ind. Pharm. 2012, 38, 771–784. [Google Scholar] [CrossRef]
- Thompson, M.R.; Mu, B.; Sheskey, P.J. Aspects of foam stability influencing foam granulation in a twin screw extruder. Powder Technol. 2012, 228, 339–348. [Google Scholar] [CrossRef]
- Rocca, K.E.; Weatherley, S.; Sheskey, P.J.; Thompson, M.R. Influence of filler selection on twin screw foam granulation. Drug Dev. Ind. Pharm. 2015, 41, 35–42. [Google Scholar] [CrossRef]
- Li, H.; Thompson, M.R.; O’Donnell, K.P. Progression of wet granulation in a twin screw extruder comparing two binder delivery methods. AIChE J. 2015, 61, 780–791. [Google Scholar] [CrossRef]
- Weatherley, S.; Thompson, M.R.; Sheskey, P.J. A study of foam granulation and wet granulation in a twin screw extruder. Can. J. Chem. Eng. 2013, 91, 725–730. [Google Scholar] [CrossRef]
- Seem, T.C.; Rowson, N.A.; Ingram, A.; Huang, Z.; Yu, S.; de Matas, M.; Gabbott, I.; Reynolds, G.K. Twin screw granulation—A literature review. Powder Technol. 2015, 276, 89–102. [Google Scholar] [CrossRef]
- Keleb, E.I.; Vermeire, A.; Vervaet, C.; Remon, J.P. Twin screw granulation as a simple and efficient tool for continuous wet granulation. Int. J. Pharm. 2004, 273, 183–194. [Google Scholar] [CrossRef]
- Schmidt, A.; de Waard, H.; Moll, K.-P.; Krumme, M.; Kleinebudde, P. Quantitative Assessment of Mass Flow Boundaries in Continuous Twin-screw Granulation. Chimia 2016, 70, 604–609. [Google Scholar] [CrossRef]
- Dhenge, R.M.; Fyles, R.S.; Cartwright, J.J.; Doughty, D.G.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Granule properties. Chem. Eng. J. 2010, 164, 322–329. [Google Scholar] [CrossRef]
- Djuric, D.; Van Melkebeke, B.; Kleinebudde, P.; Remon, J.P.; Vervaet, C. Comparison of two twin-screw extruders for continuous granulation. Eur. J. Pharm. Biopharm. 2009, 71, 155–160. [Google Scholar] [CrossRef]
- Thompson, M.R.; Sun, J. Wet granulation in a twin-screw extruder: Implications of screw design. J. Pharm. Sci. 2010, 99, 2090–2103. [Google Scholar] [CrossRef] [PubMed]
- Dhenge, R.M.; Cartwright, J.J.; Doughty, D.G.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Effect of powder feed rate. Adv. Powder Technol. 2011, 22, 162–166. [Google Scholar] [CrossRef]
- Djuric, D.; Kleinebudde, P. Continuous granulation with a twin-screw extruder: Impact of material throughput. Pharm. Dev. Technol. 2010, 15, 518–525. [Google Scholar] [CrossRef]
- Fonteyne, M.; Vercruysse, J.; Díaz, D.C.; Gildemyn, D.; Vervaet, C.; Remon, J.P.; Beer, T. De Real-time assessment of critical quality attributes of a continuous granulation process. Pharm. Dev. Technol. 2013, 18, 85–97. [Google Scholar] [CrossRef]
- Li, J.; Pradhan, S.U.; Wassgren, C.R. Granule transformation in a twin screw granulator: Effects of conveying, kneading, and distributive mixing elements. Powder Technol. 2019, 346, 363–372. [Google Scholar] [CrossRef]
- Bandari, S.; Nyavanandi, D.; Kallakunta, V.R.; Janga, K.Y.; Sarabu, S.; Butreddy, A.; Repka, M.A. Continuous twin screw granulation—An advanced alternative granulation technology for use in the pharmaceutical industry. Int. J. Pharm. 2020, 580, 119215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, T.; Kashani-Rahimi, S.; Zhang, F. A review of twin screw wet granulation mechanisms in relation to granule attributes. Drug Dev. Ind. Pharm. 2021, 1–12. [Google Scholar] [CrossRef]
- Meier, R.; Moll, K.-P.; Krumme, M.; Kleinebudde, P. Simplified, High Drug-Loaded Formulations Containing Hydrochlorothiazide for Twin-Screw Granulation. Chemie Ing. Tech. 2017, 89, 1025–1033. [Google Scholar] [CrossRef]
- Van Snick, B.; Kumar, A.; Verstraeten, M.; Pandelaere, K.; Dhondt, J.; Di Pretoro, G.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Impact of material properties and process variables on the residence time distribution in twin screw feeding equipment. Int. J. Pharm. 2019, 556, 200–216. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicine. European Pharmacopoeia 10.0: 5.4 Residual Solvents; Council Of Europe: Strasbourg, France, 2018. [Google Scholar]
- Démuth, B.; Fülöp, G.; Kovács, M.; Madarász, L.; Ficzere, M.; Köte, Á.; Szabó, B.; Nagy, B.; Balogh, A.; Csorba, K.; et al. Continuous manufacturing of homogeneous ultralow-dose granules by twin-screw wet granulation. Period. Polytech. Chem. Eng. 2020, 64, 391–400. [Google Scholar] [CrossRef]
- De Leersnyder, F.; Vanhoorne, V.; Bekaert, H.; Vercruysse, J.; Ghijs, M.; Bostijn, N.; Verstraeten, M.; Cappuyns, P.; Van Assche, I.; Vander Heyden, Y.; et al. Breakage and drying behaviour of granules in a continuous fluid bed dryer: Influence of process parameters and wet granule transfer. Eur. J. Pharm. Sci. 2018, 115, 223–232. [Google Scholar] [CrossRef]
- Dahlgren, G.; Tajarobi, P.; Simone, E.; Ricart, B.; Melnick, J.; Puri, V.; Stanton, C.; Bajwa, G. Continuous Twin Screw Wet Granulation and Drying—Control Strategy for Drug Product Manufacturing. J. Pharm. Sci. 2019, 108, 3502–3514. [Google Scholar] [CrossRef] [PubMed]
- Rehrl, J.; Karttunen, A.P.; Nicolaï, N.; Hörmann, T.; Horn, M.; Korhonen, O.; Nopens, I.; De Beer, T.; Khinast, J.G. Control of three different continuous pharmaceutical manufacturing processes: Use of soft sensors. Int. J. Pharm. 2018, 543, 60–72. [Google Scholar] [CrossRef] [PubMed]




| Water Soluble Filler (Grade) | Ratio Water Soluble Filler/MCC | API | Granular API Content | Reference(s) |
|---|---|---|---|---|
| Lactose (Pharmatose 200M) | 1 | Acetaminophen | 5% | [12] |
| Lactose (Flowlac 100) | 1 | Acetaminophen | 15% | [66] |
| Lactose (Pharmatose 200M) | 2.33 | Albendazole | 50% | [67] |
| Lactose (Flowlac 100) | 1 | Caffeine | 15% | [66] |
| Lactose (Flowlac 100) | 1 | Griseofulvin | 15% | [66] |
| Lactose (Pharmatose 200M) | 1 | Hydrochlorothiazide | 60% | [68] |
| Lactose (Flowlac 100) | 1 | Ibuprofen | 15% | [66] |
| Lactose (Granulac 70) | 1.33 | Ibuprofen | 30% | [69] |
| Lactose (Granulac 200) | 1.4 | Ibuprofen | 51.5% | [70] |
| Lactose (Pharmatose 200M) | 1 | Mebendazole | 5% | [57] |
| Lactose (Pharmatose 200M) | 1 | Mebendazole | 10% | [12] |
| Lactose (Pharmatose 200M) | 1 | Mebendazole | 50% | [57,59] |
| Lactose (Pharmatose 200M) | 1 | Metformin.HCl | 5% | [57,71] |
| Lactose (Pharmatose 200M) | 1 | Metformin.HCl | 10% | [12] |
| Lactose (Pharmatose 200M) | 1 | Metformin.HCl | 50% | [57] |
| Lactose (Pharmatose 200M) | 1 | Theophylline | 5% | [12] |
| Water Soluble Filler (Grade) | Ratio Water Soluble Filler/MCC | API | Granular API Content | Reference(s) |
|---|---|---|---|---|
| Mannitol (Pearlitol 160C) | 1 | Acetaminophen | 25% | [29] |
| Mannitol (Pearlitol 160C) | 1 | Allopurinol | 25% | [27,29] |
| Mannitol (Pearlitol 200SD) | 1 | Allopurinol | 25% | [27] |
| Mannitol (Pearlitol 160C) | 1 | Metformin.HCl | 25% | [29] |
| Mannitol (Pearlitol 160C) | 2.08 | Not disclosed | 22% | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portier, C.; Vervaet, C.; Vanhoorne, V. Continuous Twin Screw Granulation: A Review of Recent Progress and Opportunities in Formulation and Equipment Design. Pharmaceutics 2021, 13, 668. https://doi.org/10.3390/pharmaceutics13050668
Portier C, Vervaet C, Vanhoorne V. Continuous Twin Screw Granulation: A Review of Recent Progress and Opportunities in Formulation and Equipment Design. Pharmaceutics. 2021; 13(5):668. https://doi.org/10.3390/pharmaceutics13050668
Chicago/Turabian StylePortier, Christoph, Chris Vervaet, and Valérie Vanhoorne. 2021. "Continuous Twin Screw Granulation: A Review of Recent Progress and Opportunities in Formulation and Equipment Design" Pharmaceutics 13, no. 5: 668. https://doi.org/10.3390/pharmaceutics13050668