Preparation and Evaluation of Azelaic Acid Topical Microemulsion Formulation: In Vitro and In Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Azelaic Acid-Loaded Microemulsions
2.3. Mean Droplet Size Determination
2.4. Determination of Viscosity
2.5. In Vitro Skin Permeation Study
2.6. Condition of HPLC Analysis of Azelaic Acid
2.7. Pharmacodynamic Evaluation
2.8. Skin Irritation Determination
2.9. Stability of Azelaic Acid-Loaded Formulation Determination
2.10. Data Analysis
3. Results and Discussion
3.1. Physicohemical Characterization
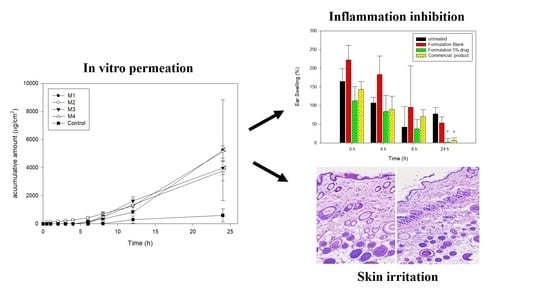
3.2. In Vitro Permeation Study
3.3. Pharmacodynamic Evaluation
3.4. Skin Irritation
3.5. Stability of the Azelaic Acid-Loaded Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leeming, J.P.; Holland, K.T.; Bojar, R.A. The in vitro antimicrobial effect of azelaic acid. Br. J. Dermatol. 1986, 115, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Schulte, B.C.; Wu, W.; Rosen, T. Azelaic Acid: Evidence-based Update on Mechanism of Action and Clinical Application. J. Drugs Dermatol. 2015, 14, 964–968. [Google Scholar] [PubMed]
- NDA Approvals for Calender Year 2002. Available online: http://www.fda.gov/cder/rdmt/ndaaps02cy.htm (accessed on 14 October 2003).
- Hashim, P.W.; Chen, T.; Harper, J.C.; Kircik, L.H. The Efficacy and Safety of Azelaic Acid 15% Foam in the Treatment of Facial Acne Vulgaris. J. Drugs Dermatol. 2018, 17, 641–645. [Google Scholar] [PubMed]
- Apriani, E.F.; Rosana, Y.; Iskandarsyah, I. Formulation, characterization, and in vitro testing of azelaic acid ethosome-based cream against Propionibacterium acnes for the treatment of acne. J. Adv. Pharm. Technol. Res. 2019, 10, 75–80. [Google Scholar] [PubMed]
- Tomic, I.; Juretic, M.; Jug, M.; Pepic, I.; Cetina Cizmek, B.; Filipovic-Grcic, J. Preparation of in situ hydrogels loaded with azelaic acid nanocrystals and their dermal application performance study. Int. J. Pharm. 2019, 563, 249–258. [Google Scholar] [CrossRef]
- Burchacka, E.; Potaczek, P.; Paduszynski, P.; Karlowicz-Bodalska, K.; Han, T.; Han, S. New effective azelaic acid liposomal gel formulation of enhanced pharmaceutical bioavailability. Biomed. Pharmacother. 2016, 83, 771–775. [Google Scholar] [CrossRef]
- Aytekin, M.; Gursoy, R.N.; Ide, S.; Soylu, E.H.; Hekimoglu, S. Formulation and characterization of liquid crystal systems containing azelaic acid for topical delivery. Drug Dev. Ind. Pharm. 2013, 39, 228–239. [Google Scholar] [CrossRef]
- Shinde, U.A.; Modani, S.H.; Singh, K.H. Design and Development of Repaglinide Microemulsion Gel for Transdermal Delivery. AAPS PharmSciTech 2017, 19, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Tsai, M.J.; Fang, Y.P.; Fu, Y.S.; Huang, Y.B.; Wu, P.C. Microemulsion formulation design and evaluation for hydrophobic compound: Catechin topical application. Colloids Surf. B Biointerfaces 2017, 161, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Gaseo, M.R.; Gallarate, M.; Pattarino, F. In vitro permeation of azelaic acid from viscosized microemulsions. Int. J. Pharm. 1991, 69, 193–196. [Google Scholar]
- Yu, M.; Ma, H.X.; Lei, M.Z.; Li, N.; Tan, F.P. In vitro/in vivo characterization of nanoemulsion formulation of metronidazole with improved skin targeting and anti-rosacea properties. Eur. J. Pharm. Biopharm. 2014, 88, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.S.; Choi, J.G.; Park, E.S.; Chi, S.C. Transdermal delivery of ketoprofen using microemulsions. Int. J. Pharm. 2001, 228, 161–170. [Google Scholar] [CrossRef]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion components screening and selection: A technical note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Azeem, A.; Khan, Z.I.; Aqil, M.; Ahmad, F.J.; Khar, R.K.; Talegaonkar, S. Microemulsions as a surrogate carrier for dermal drug delivery. Drug Dev. Ind. Pharm. 2009, 35, 525–547. [Google Scholar] [CrossRef]
- Kibbe, A.H. Handbook of Pharmaceutical Excipients, 3rd ed.; American Pharmaceutical Association and Pharmaceutical Press: Washington, DC, USA, 2000. [Google Scholar]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol(R)-Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.A.; Park, P.J.; Kim, S.B.; Bin, B.H.; Jang, D.J.; Kim, S.T. Topical Delivery of Coenzyme Q10-Loaded Microemulsion for Skin Regeneration. Pharmaceutics 2020, 12, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ustundag Okur, N.; Caglar, E.S.; Siafaka, P.I. Novel Ocular Drug Delivery Systems: An Update on Microemulsions. J. Ocul. Pharmacol. Ther. 2020, 36, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Aydogmus, Z. Highly sensitive and selective spectrophotometric and spectrofluorimetric methods for the determination of ropinirole hydrochloride in tablets. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 70, 69–78. [Google Scholar] [CrossRef]
- Garbacki, N.; Gloaguen, V.; Damas, J.; Hoffmann, L.; Tits, M.; Angenot, L. Inhibition of croton oil-induced oedema in mice ear skin by capsular polysaccharides from cyanobacteria. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 361, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yu, M.; Tan, F.; Li, N. Improved percutaneous delivery of azelaic acid employing microemulsion as nanocarrier: Formulation optimization, in vitro and in vivo evaluation. RSC Adv. 2015, 5, 28985–28995. [Google Scholar] [CrossRef]
- Azeem, A.; Talegaonkar, S.; Negi, L.M.; Ahmad, F.J.; Khar, R.K.; Iqbal, Z. Oil based nanocarrier system for transdermal delivery of ropinirole: A mechanistic, pharmacokinetic and biochemical investigation. Int. J. Pharm. 2012, 422, 436–444. [Google Scholar] [CrossRef]
- Tsai, M.J.; Huang, Y.B.; Fang, J.W.; Fu, Y.S.; Wu, P.C. Preparation and evaluation of submicron-carriers for naringenin topical application. Int. J. Pharm. 2015, 481, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, S.; Udupa, N. Glibenclamide transdermal patches: Physicochemical, pharmacodynamic, and pharmacokinetic evaluations. J. Pharm. Sci. 2004, 93, 1577–1594. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Update on rosacea pathogenesis and correlation with medical therapeutic agents. Cutis 2006, 78, 97–100. [Google Scholar] [PubMed]
- Ngan, C.L.; Basri, M.; Tripathy, M.; Abedi Karjiban, R.; Abdul-Malek, E. Physicochemical characterization and thermodynamic studies of nanoemulsion-based transdermal delivery system for fullerene. Sci. World J. 2014, 2014, 219035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagavifar, M.; Herath, S.; Weerasooriya, U.P.; Sepehrnoori, K.; Pope, G. Measurement of Microemulsion Viscosity and Its Implications for Chemical Enhanced Oil Recovery. SPE J. 2018, 23, 66–83. [Google Scholar] [CrossRef]
- Teichmann, A.; Heuschkel, S.; Jacobi, U.; Presse, G.; Neubert, R.H.; Sterry, W.; Lademann, J. Comparison of stratum corneum penetration and localization of a lipophilic model drug applied in an o/w microemulsion and an amphiphilic cream. Eur. J. Pharm. Biopharm. 2007, 67, 699–706. [Google Scholar] [CrossRef]
- Schoemaker, J.H.; Bousema, M.T.; Zijlstra, H.; van der Horst, F.A. Treatment of erythropoietic protoporphyria with hydroxyethylrutosides. Dermatology 1995, 191, 36–38. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Lee, K.F.; Huang, Y.B.; Huang, C.T.; Wu, P.C. In vitro permeation and in vivo whitening effect of topical hesperetin microemulsion delivery system. Int. J. Pharm. 2010, 388, 257–262. [Google Scholar] [CrossRef]
- Azeem, A.; Ahmad, F.J.; Khar, R.K.; Talegaonkar, S. Nanocarrier for the transdermal delivery of an antiparkinsonian drug. AAPS PharmSciTech 2009, 10, 1093–1103. [Google Scholar] [CrossRef]



| Formulation | M1 | M2 | M3 | M4 |
|---|---|---|---|---|
| IPM | 10 | 10 | 10 | 10 |
| Cremophor EL | 20 | 20 | 20 | 20 |
| Transcutol | 10 | 10 | 10 | 10 |
| PEG | 26 | 26 | 26 | - |
| PG | - | - | - | 26 |
| Ethanol | - | 5 | 10 | 10 |
| Water | 34 | 29 | 24 | 24 |
| Azelaic | 5 | 5 | 5 | 5 |
| Q24h(μg/cm2) | 5239.5 ± 3590.9 | 5131.1 ± 435.4 | 3974.9 ± 479.8 | 3764.8 ± 707.8 |
| Droplet size (nm) | 220.7 ± 4.6 | 243.2 ± 1.3 | 700.7 ± 13.6 | 8674.6 ± 475.9 |
| PDI | 0.27 ± 0.01 | 0.28 ± 0.00 | 0.24 ± 0.02 | 3.28 ± 0.31 |
| Viscosity (cps) | 247.4 ± 24.1 | 187.5 ± 10.7 | 145.4 ± 5.0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, W.-H.; Chen, P.-K.; Fang, C.-W.; Lin, Y.-C.; Wu, P.-C. Preparation and Evaluation of Azelaic Acid Topical Microemulsion Formulation: In Vitro and In Vivo Study. Pharmaceutics 2021, 13, 410. https://doi.org/10.3390/pharmaceutics13030410
Hung W-H, Chen P-K, Fang C-W, Lin Y-C, Wu P-C. Preparation and Evaluation of Azelaic Acid Topical Microemulsion Formulation: In Vitro and In Vivo Study. Pharmaceutics. 2021; 13(3):410. https://doi.org/10.3390/pharmaceutics13030410
Chicago/Turabian StyleHung, Wan-Hsuan, Ping-Kang Chen, Chih-Wun Fang, Ying-Chi Lin, and Pao-Chu Wu. 2021. "Preparation and Evaluation of Azelaic Acid Topical Microemulsion Formulation: In Vitro and In Vivo Study" Pharmaceutics 13, no. 3: 410. https://doi.org/10.3390/pharmaceutics13030410