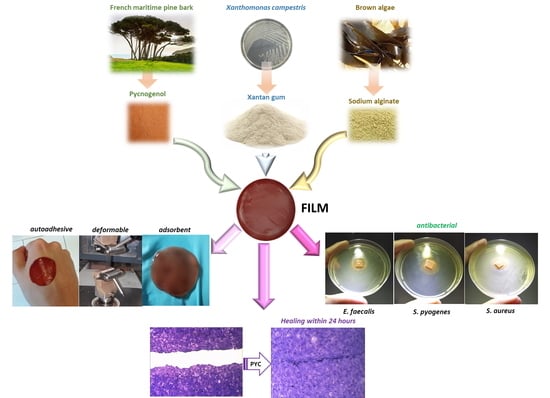
Development and Characterization of Xanthan Gum and Alginate Based Bioadhesive Film for Pycnogenol Topical Use in Wound Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Film P reparation
2.2.2. Film Storage Conditions
- (1)
- CaCl2 (relative humidity, R.H. 40%) at R.T.,
- (2)
- saturated MgCl2 solution at RT (R.H. 33%),
- (3)
- saturated MgCl2 solution at 4.0 °C (R.H. 34%).
2.2.3. Thermogravimetric Analyses
2.2.4. Mechanic Characterization
2.2.5. Morphology and Thickness
2.2.6. Water Content
- (1)
- ventilated oven at 42 °C,
- (2)
- desiccator under CaCl2,
- (3)
- desiccator under P2O5.
2.2.7. Water Holding Studies
2.2.8. Ex Vivo Adhesion Studies
2.2.9. Release Studies
2.2.10. Antimicrobial Activity
2.2.11. Cytotoxicity Assay
2.2.12. In vitro Wound Healing Assay
2.2.13. Statistical Analysis
3. Results and Discussions
3.1. Unloaded Film Preparation and Characterization
3.2. Storage Conditions
3.3. Water Content Measurement
3.4. Mechanical Characterization
3.5. Morphology and Thickness
3.6. Water Holding Studies
3.7. Ex Vivo Adhesion Capacity
3.8. Loaded Film Preparation
3.9. Thermogravimetric Analysis
3.10. Mechanical Characterization
3.11. Ex Vivo Adhesion Capacity
3.12. Release Studies
3.13. Antimicrobial Activity Assay
3.14. Cytotoxicity and Wound Healing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rohdewald, P.J. Pycnogenol®, French maritime pine bark extract. In Encyclopedia of Dietary Supplements; Informa Healthcare: New York, NY, USA, 2004; ISBN 9781482204056. [Google Scholar]
- Ebadi, M. (Ed.) Pharmacodynamic Basis of Herbal Medicine; Taylor & Francis: Boca Raton, FL, USA, 2007; Chapter 54; p. 509. [Google Scholar]
- Sime, S.; Reeve, V.E. Protection from Inflammation, Immunosuppression and Carcinogenesis Induced by UV Radiation in Mice by Topical Pycnogenol®. Photochem. Photobiol. 2004, 79, 193–198. [Google Scholar] [CrossRef]
- Schoenlau, F. Chapter 23-The multifactorial contributions of Pycnogenol® for cognitive function improvement. In Nutraceuticals in Brain Health and Beyond; Elsevier: Amsterdam, The Netherlands, 2021; pp. 335–341. [Google Scholar]
- Ni, Z.; Mu, Y.; Gulati, O. Treatment of melasma with Pycnogenol®. Phyther. Res. 2002, 16, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Lobo, J.M.S. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Blazsó, G.; Gábor, M.; Schönlau, F.; Rohdewald, P. Pycnogenol® accelerates wound healing and reduces scar formation. Phyther. Res. 2004, 18, 579–581. [Google Scholar] [CrossRef]
- Packer, L.; Rimbach, G.; Virgili, F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (pinus maritima) bark, pycnogenol. Free Radic. Biol. Med. 1999, 27, 704–724. [Google Scholar] [CrossRef]
- Canali, R.; Comitato, R.; Schonlau, F.; Virgili, F. The anti-inflammatory pharmacology of Pycnogenol® in humans involves COX-2 and 5-LOX mRNA expression in leukocytes. Int. Immunopharmacol. 2009, 9, 1145–1149. [Google Scholar] [CrossRef]
- Calvo Torras, M.A.; Adelantado Faura, C.; Schönlau, F.; Rohdewald, P. Antimicrobial Activity of Pycnogenol®. Phytother. Res. 2005, 19, 647–648. [Google Scholar] [CrossRef]
- Cetin, E.O.; Yesil-Celiktas, O.; Cavusoglu, T.; Demirel-Sezer, E.; Akdemir, O.; Uyanikgil, Y. Incision wound healing activity of pine bark extract containing topical formulations: A study with histopathological and biochemical analyses in albino rats. Die Pharm. Int. Pharm. Sci. 2013, 68, 75–80. [Google Scholar]
- Okur, M.E.; Ayla, Ş.; Batur, Ş.; Yoltaş, A.; Genç, E.; Pertek, S.; Üstündağ Okur, N. Evaluation of In Situ Gel Containing Pycnogenol for Cutaneous Wound Healing. Medeni. Med. J. 2019, 34, 67–75. [Google Scholar] [CrossRef]
- Rajesh, N. Siddaramaiah Feasibility of xanthan gum-sodium alginate as a transdermal drug delivery system for domperidone. J. Mater. Sci. Mater. Med. 2009, 20, 2085–2089. [Google Scholar] [CrossRef]
- Bombaldi de Souza, R.F.; Bombaldi de Souza, F.C.; Bierhalz, A.C.K.; Pires, A.L.R.; Moraes, Â.M. Biopolymer-based films and membranes as wound dressings. In Biopolymer Membranes and Films; Elsevier: Amsterdam, The Netherlands, 2020; pp. 165–194. [Google Scholar]
- Li, M.; Li, H.; Li, X.; Zhu, H.; Xu, Z.; Liu, L.; Ma, J.; Zhang, M. A Bioinspired Alginate-Gum Arabic Hydrogel with Micro-/Nanoscale Structures for Controlled Drug Release in Chronic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 22160–22175. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.-F.; Jumaat, N. Carboxymethyl cellulose wafers containing antimicrobials: A modern drug delivery system for wound infections. Eur. J. Pharm. Sci. 2014, 51, 173–179. [Google Scholar] [CrossRef]
- Pagano, C.; Ceccarini, M.R.; Calarco, P.; Scuota, S.; Conte, C.; Primavilla, S.; Ricci, M.; Perioli, L. Bioadhesive polymeric films based on usnic acid for burn wound treatment: Antibacterial and cytotoxicity studies. Colloids Surf. B Biointerfaces 2019, 178, 488–499. [Google Scholar] [CrossRef]
- Perioli, L.; Ambrogi, V.; Angelici, F.; Ricci, M.; Giovagnoli, S.; Capuccella, M.; Rossi, C. Development of mucoadhesive patches for buccal administration of ibuprofen. J. Control. Release 2004, 99, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ceccarini, M.R.; Vannini, S.; Cataldi, S.; Moretti, M.; Villarini, M.; Fioretti, B.; Albi, E.; Beccari, T.; Codini, M. In Vitro Protective Effects of Lycium barbarum Berries Cultivated in Umbria (Italy) on Human Hepatocellular Carcinoma Cells. Biomed. Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pagano, C.; Perioli, L.; Latterini, L.; Nocchetti, M.; Ceccarini, M.R.; Marani, M.; Ramella, D.; Ricci, M. Folic acid-layered double hydroxides hybrids in skin formulations: Technological, photochemical and in vitro cytotoxicity on human keratinocytes and fibroblasts. Appl. Clay Sci. 2019, 168, 382–395. [Google Scholar] [CrossRef]
- Hostanska, K.; Rostock, M.; Melzer, J.; Baumgartner, S.; Saller, R. A homeopathic remedy from arnica, marigold, St. John’s wort and comfrey accelerates in vitro wound scratch closure of NIH 3T3 fibroblasts. BMC Complement. Altern. Med. 2012, 12, 100. [Google Scholar] [CrossRef] [Green Version]
- Pagano, C.; Perioli, L.; Baiocchi, C.; Bartoccini, A.; Beccari, T.; Blasi, F.; Calarco, P.; Ceccarini, M.R.; Cossignani, L.; di Michele, A.; et al. Preparation and characterization of polymeric microparticles loaded with Moringa oleifera leaf extract for exuding wound treatment. Int. J. Pharm. 2020, 587, 119700. [Google Scholar] [CrossRef]
- Pereira, R.; Mendes, A.; Bártolo, P. Alginate/Aloe vera hydrogel films for biomedical applications. Procedia CIRP 2013, 5, 210–215. [Google Scholar] [CrossRef]
- Mittal, K.L. (Ed.) Adhesion Aspects of Thin Films, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; Volume 2. [Google Scholar]
- Guest, J.F.; Greener, M.J.; Vowden, K.; Vowden, P. Clinical and economic evidence supporting a transparent Barrier Film dressing in incontinence-associated dermatitis and peri-wound skin protection. J. Wound Care 2011, 76, 78–84. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoon, K.J.; Ko, S.W. Preparation and properties of alginate superabsorbent filament fibers crosslinked with glutaraldehyde. J. Appl. Polym. Sci. 2000, 78, 1797–1804. [Google Scholar] [CrossRef]
- Pagano, C.; Marinozzi, M.; Baiocchi, C.; Beccari, T.; Calarco, P.; Ceccarini, M.R.; Chielli, M.; Orabona, C.; Orecchini, E.; Ortenzi, R.; et al. Bioadhesive Polymeric Films Based on Red Onion Skins Extract for Wound Treatment: An Innovative and Eco-Friendly Formulation. Molecules 2020, 25, 318. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Gu, W.; Peng, C.; Wang, W.; Li, Y.J.; Zong, T.; Tang, Y.; Wu, Z.; Lin, Q.; Ge, M.; et al. A comprehensive study of hygroscopic properties of calcium- and magnesium-containing salts: Implication for hygroscopicity of mineral dust and sea salt aerosols. Atmos. Chem. Phys. 2019, 19, 2115–2133. [Google Scholar] [CrossRef] [Green Version]
- Melero-Tur, S.; García-Morales, S.; Javier Neila-González, F. Design and evaluation of a dehumidifying plaster panel for passive architecture integration. J. Construction 2015, 14, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclet. Quim. 2004, 29, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Siurana, A.; Marcilla, A.; Beltrán, M.; Berenguer, D.; Martínez-Castellanos, I.; Menargues, S. TGA/FTIR study of tobacco and glycerol-tobacco mixtures. Thermochim. Acta 2013, 573, 146–157. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Graft copolymerization of ethylacrylate onto xanthan gum, using potassium peroxydisulfate as an initiator. Int. J. Biol. Macromol. 2011, 49, 527–535. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Alginate–calcium films: Water vapor permeability and mechanical properties as affected by plasticizer and relative humidity. LWT Food Sci. Technol. 2008, 2, 359–366. [Google Scholar] [CrossRef]
- Aarstad, O.; Heggset, E.B.; Pedersen, I.S.; Bjørnøy, S.H.; Syverud, K.; Strand, B.L. Mechanical properties of composite hydrogels of alginate and cellulose nanofibrils. Polymers (Basel) 2017, 9, 378. [Google Scholar] [CrossRef] [Green Version]
- Bellini, M.Z.; de Oliva-Neto, P.; Moraes, A.M. Properties of films obtained from biopolymers of different origins for skin lesions therapy. Braz. Arch. Biol. Technol. 2015, 58, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Hansen, B.; Jemec, G.B. The mechanical properties of skin in osteogenesis imperfecta. Arch. Dermatol. 2002, 138, 909–911. [Google Scholar] [CrossRef] [Green Version]
- Jussila, J.; Leppäniemi, A.; Paronen, M.; Kulomäki, E. Ballistic skin simulant. Forensic. Sci. Int. 2005, 150, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, V.E.; Bartholomai, G.B.; Pilosof, A.M.R. Rheological Properties of Food Gums their Water Binding Capacity and to Interaction as Related to Soy Protein. Lebensm. Wtss. Technol. 1995, 28, 380–385. [Google Scholar] [CrossRef]
- Pagano, C.; Latterini, L.; Di Michele, A.; Luzi, F.; Puglia, D.; Ricci, M.; Iborra, C.A.V.; Perioli, L. Polymeric bioadhesive patch based on ketoprofen-hydrotalcite hybrid for local treatments. Pharmaceutics 2020, 12, 733. [Google Scholar] [CrossRef]
- Luzi, F.; Torre, L.; Puglia, D. Antioxidant Packaging Films Based on Ethylene Vinyl Alcohol Copolymer (EVOH) and Caffeic Acid. Molecules 2020, 25, 3953. [Google Scholar] [CrossRef]
- Mandal, S.M.; Dias, R.O.; Franco, O.L. Phenolic Compounds in Antimicrobial Therapy. J. Med. Food 2017, 20, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; De Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti. Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Schimizzi, A.M.; Del Prete, M.S.; Barchiesi, F.; D’Errico, M.M.; Petrelli, E.; Scalise, G. Epidemiology and microbiology of surgical wound infections. J. Clin. Microbiol. 2000, 38, 918–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef]











| Hydrogel | AL (% wt/wt) | XG (% wt/wt) | Glycerol (% wt/wt) | Bidistilled Water (% wt/wt) |
|---|---|---|---|---|
| A | 2.50 | 7.50 | 10.00 | 80.00 |
| B | 1.50 | 8.50 | 10.00 | 88.00 |
| Film | Storage Conditions | Water Loss (%) |
|---|---|---|
| A | ventilated oven at 42 °C | 4.96 ± 1.58 |
| desiccator under CaCl2 | 6.51 ± 1.58 | |
| desiccator under P2O5 | 6.10 ± 3.35 | |
| B | ventilated oven at 42 °C | 4.33 ± 1.01 |
| desiccator under CaCl2 | 9.77 ± 3.76 | |
| desiccator under P2O5 | 8.30 ± 1.25 |
| Film | AL/XG (Ratio wt/wt) | σmax (MPa) | εat σmax (%) | E (MPa) |
|---|---|---|---|---|
| A | 2.5/7.5 | 0.303 ± 0.077 * | 23 ± 4 ** | 2.823 ± 0.148 *** |
| B | 1.5/8.5 | 0.120 ± 0.010 | 22 ± 4 | 1.278 ± 0.169 |
| Film | AL (% wt/wt) | XG (% wt/wt) | PYC (% wt/wt) | Glycerol (% wt/wt) | Water (% wt/wt) |
|---|---|---|---|---|---|
| A-Loaded | 1.50 | 3.02 | 20.10 | 40.20 | 35.17 |
| B-Loaded | 0.87 | 3.40 | 20.30 | 40.61 | 34.81 |
| σmax (MPa) | εat σmax (%) | E(MPa) | |
|---|---|---|---|
| Film A-Loaded | 0.215 ± 0.007 * | 17 ± 2 ** | 3.070 ± 0.044 *** |
| Film B-Loaded | 0.055 ± 0.005 | 18 ± 1 | 0.620 ± 0.044 |
| Mt/M∞ = kt | Mt/M∞ = kt0.5 | Mt/M∞ = 1-e-kt | |
|---|---|---|---|
| Zero-order Kinetics | Higuchi Kinetics (Release 0–60%) | First Order Kinetics | |
| Film A-loaded | y = 0.0447x + 46.761 R2 = 0.3877 | y = 6.1205x + 0.1606 R2 = 0.9869 | y = −0.0007x − 0.3057 R2 = 0.6355 |
| PYC 10 mg/mL (mm) | PYC 1 mg/mL (mm) | film A-Loaded (mm) | |
|---|---|---|---|
| K. pneumoniae | - | - | - |
| E. coli | - | - | - |
| P. mirabilis | - | - | - |
| S. aureus | 19 | - | 19 |
| S. epidermidis | 20 | - | - |
| E. faecalis | 17 | - | 18 |
| B. subtilis | 17 | - | - |
| S. pyogenes | 21 | - | 24 |
| P. aeruginosa | - | - | - |
| C. albicans | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, C.; Puglia, D.; Luzi, F.; Michele, A.D.; Scuota, S.; Primavilla, S.; Ceccarini, M.R.; Beccari, T.; Iborra, C.A.V.; Ramella, D.; et al. Development and Characterization of Xanthan Gum and Alginate Based Bioadhesive Film for Pycnogenol Topical Use in Wound Treatment. Pharmaceutics 2021, 13, 324. https://doi.org/10.3390/pharmaceutics13030324
Pagano C, Puglia D, Luzi F, Michele AD, Scuota S, Primavilla S, Ceccarini MR, Beccari T, Iborra CAV, Ramella D, et al. Development and Characterization of Xanthan Gum and Alginate Based Bioadhesive Film for Pycnogenol Topical Use in Wound Treatment. Pharmaceutics. 2021; 13(3):324. https://doi.org/10.3390/pharmaceutics13030324
Chicago/Turabian StylePagano, Cinzia, Debora Puglia, Francesca Luzi, Alessandro Di Michele, Stefania Scuota, Sara Primavilla, Maria Rachele Ceccarini, Tommaso Beccari, César Antonio Viseras Iborra, Daniele Ramella, and et al. 2021. "Development and Characterization of Xanthan Gum and Alginate Based Bioadhesive Film for Pycnogenol Topical Use in Wound Treatment" Pharmaceutics 13, no. 3: 324. https://doi.org/10.3390/pharmaceutics13030324