Current Insights into 3D Bioprinting: An Advanced Approach for Eye Tissue Regeneration
Abstract
:1. Introduction
2. 3D Bioprinting for Eye Tissue Engineering
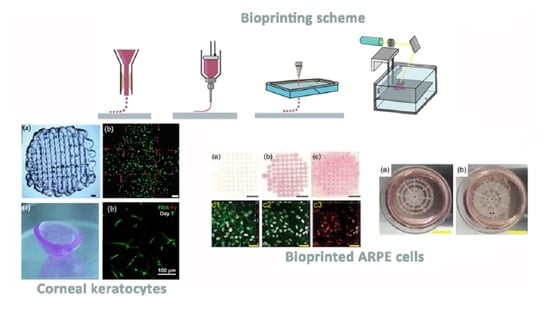
2.1. Cornea

2.2. Retina
2.3. Conjunctiva
3. 3D Printing for Ocular Drug Delivery
4. Ethical Issues and Commercialization Regulatory Aspects
5. Current Challenges and Future Perspectives of Ocular 3D Bioprinting
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Corsi, A.; de Souza, F.F.; Pagani, R.N.; Kovaleski, J.L. Big Data Analytics as a Tool for Fighting Pandemics: A Systematic Review of Literature. J. Ambient. Intell. Hum. Comput. 2020, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Warty, R.R.; Sursas, J.A.; Payne, O.; Nair, A.; Krishnan, S.; da Silva Costa, F.; Wallace, E.M.; Vollenhoven, B. The Effectiveness of Virtual Reality in Managing Acute Pain and Anxiety for Medical Inpatients: Systematic Review. J. Med. Internet Res. 2020, 22, e17980. [Google Scholar] [CrossRef] [PubMed]
- Bova, L.; Billi, F.; Cimetta, E. Mini-Review: Advances in 3D Bioprinting of Vascularized Constructs. Biol. Direct 2020, 15, 1–5. [Google Scholar] [CrossRef]
- Loai, S.; Kingston, B.; Wang, Z.; Philpott, D.; Tao, M.; Cheng, H. Clinical Perspectives on 3D Bioprinting Paradigms for Regenerative Medicine. Regen. Med. Front. 2019, 1, e190004. [Google Scholar]
- Tan, C.T.; Liang, K.; Ngo, Z.H.; Dube, C.T.; Lim, C.Y. Application of 3D Bioprinting Technologies to the Management and Treatment of Diabetic Foot Ulcers. Biomedicines 2020, 8, 441. [Google Scholar] [CrossRef] [PubMed]
- Di Marzio, N.; Eglin, D.; Serra, T.; Moroni, L. Bio-Fabrication: Convergence of 3D Bioprinting and Nano-Biomaterials in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 326. [Google Scholar] [CrossRef]
- Poomathi, N.; Singh, S.; Prakash, C.; Patil, R.V.; Perumal, P.T.; Barathi, V.A.; Balasubramanian, K.K.; Ramakrishna, S.; Maheshwari, N.U. Bioprinting in Ophthalmology: Current Advances and Future Pathways. Rapid Prototyp. J. 2019, 25, 496–514. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Melchels, F.P.W.; Ferreira, M.J.S.; Moxon, S.R.; Potjewyd, G.; Dargaville, T.R.; Kimber, S.J.; Domingos, M. Emulating Human Tissues and Organs: A Bioprinting Perspective Toward Personalized Medicine. Chem. Rev. 2020, 120, 11128–11174. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink Properties before, during and After 3D Bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent Trends in Bioinks for 3D Printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Song, X.; Li, X.; Chen, Z.; Zhou, C.; Zhou, Q.; Chen, Y. Recent Progress in Biomimetic Additive Manufacturing Technology: From Materials to Functional Structures. Adv. Mater. 2018, 30, 1706539. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. ‘Printability’ of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Adv. Healthc. Mater. 2017, 6. Epub 30 May 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent Advances in Bioprinting Techniques: Approaches, Applications and Future Prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, Z.; Liu, X.; Coates, P.T.; Wallace, G.G. Advances in Printing Biomaterials and Living Cells: Implications for Islet Cell Transplantation. Curr. Opin. Organ. Transplant. 2016, 21, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The Upcoming 3D-Printing Revolution in Microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, J.; Melchels, F.P.W.; Weinans, H.; Kruyt, M.C.; Malda, J. Applications of 3D Printing in Medicine; 5 Years Later. Ned. Tijdschr. Geneeskd. 2019, 163, D3683. [Google Scholar] [PubMed]
- Sommer, A.C.; Blumenthal, E.Z. Implementations of 3D Printing in Ophthalmology. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xue, Q.; Li, J.; Ma, L.; Yao, Y.; Ye, H.; Cui, Z.; Yang, H. 3D Bioprinting for Artificial Cornea: Challenges and Perspectives. Med. Eng. Phys. 2019, 71, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, L.; Maram, J.; Fonn, D.; Woods, C.; Simpson, T. Metrics of the Normal Cornea: Anterior Segment Imaging with the Visante OCT. Clin. Exp. Optom. 2010, 93, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J. The Blood-Ocular Barriers. Surv. Ophthalmol. 1979, 23, 279–296. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, P.; Edgar, T.Y.S.; Yeong, W.Y.; Laude, A. Hybrid Three-Dimensional (3D) Bioprinting of Retina Equivalent for Ocular Research. Int. J. Bioprint 2017, 3, 008. [Google Scholar] [CrossRef] [Green Version]
- Cubo-Mateo, N.; Podhajsky, S.; Knickmann, D.; Slenzka, K.; Ghidini, T.; Gelinsky, M. Can 3D Bioprinting be a Key for Exploratory Missions and Human Settlements on the Moon and Mars? Biofabrication 2020, 12, 043001. [Google Scholar] [CrossRef]
- Nayak, K.; Misra, M. A Review on Recent Drug Delivery Systems for Posterior Segment of Eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef] [PubMed]
- Fenton, O.S.; Paolini, M.; Andresen, J.L.; Müller, F.J.; Langer, R. Outlooks on Three-Dimensional Printing for Ocular Biomaterials Research. J. Ocul. Pharmacol. Ther. 2020, 36, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willoughby, C.E.; Ponzin, D.; Ferrari, S.; Lobo, A.; Landau, K.; Omidi, Y. Anatomy and Physiology of the Human Eye: Effects of Mucopolysaccharidoses Disease on Structure and Function—A Review. Clin. Experiment. Ophthalmol. 2010, 38, 2–11. [Google Scholar] [CrossRef]
- Ludwig, P.E.; Huff, T.J.; Zuniga, J.M. The Potential Role of Bioengineering and Three-Dimensional Printing in Curing Global Corneal Blindness. J. Tissue Eng. 2018, 9, 2041731418769863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Xue, Q.; Hu, H.; Yu, M.; Gao, L.; Luo, Y.; Li, Y.; Li, J.; Ma, L.; Yao, Y.; et al. Integrated 3D Bioprinting-Based Geometry-Control Strategy for Fabricating Corneal Substitutes. J. Zhejiang Univ. B Sci. 2019, 20, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Shiju, T.M.; Carlos de Oliveira, R.; Wilson, S.E. 3D in Vitro Corneal Models: A Review of Current Technologies. Exp. Eye Res. 2020, 200, 108213. [Google Scholar] [CrossRef]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human Stem Cell Based Corneal Tissue Mimicking Structures using Laser-Assisted 3D Bioprinting and Functional Bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef]
- McKay, T.B.; Hutcheon, A.E.K.; Guo, X.; Zieske, J.D.; Karamichos, D. Modeling the Cornea in 3-Dimensions: Current and Future Perspectives. Exp. Eye Res. 2020, 197, 108127. [Google Scholar] [CrossRef] [PubMed]
- Gibney, R.; Matthyssen, S.; Patterson, J.; Ferraris, E.; Zakaria, N. The Human Cornea as a Model Tissue for Additive Biomanufacturing: A Review. Procedia Cirp. 2017, 65, 56–63. [Google Scholar] [CrossRef]
- Prina, E.; Mistry, P.; Sidney, L.E.; Yang, J.; Wildman, R.D.; Bertolin, M.; Breda, C.; Ferrari, B.; Barbaro, V.; Hopkinson, A.; et al. 3D Microfabricated Scaffolds and Microfluidic Devices for Ocular Surface Replacement: A Review. Stem Cell Rev. Rep. 2017, 13, 430–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuest, M.; Yam, G.H.; Mehta, J.S.; Duarte Campos, D.F. Prospects and Challenges of Translational Corneal Bioprinting. Bioengineering 2020, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Duarte Campos, D.F.; Rohde, M.; Ross, M.; Anvari, P.; Blaeser, A.; Vogt, M.; Panfil, C.; Yam, G.H.; Mehta, J.S.; Fischer, H.; et al. Corneal Bioprinting Utilizing Collagen-based Bioinks and Primary Human Keratocytes. J. Biomed. Mater. Res. Part A 2019, 107, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.; Coulson-Thomas, V. Cell Therapy of Corneal Diseases. Cornea 2016, 35 (Suppl. 1), S9–S19. [Google Scholar] [CrossRef] [Green Version]
- Meller, D.; Pauklin, M.; Thomasen, H.; Westekemper, H.; Steuhl, K. Amniotic Membrane Transplantation in the Human Eye. Dtsch. Ärzteblatt Int. 2011, 108, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.B.; McKelvie, J.; Green, C.R.; Misra, S.L. Corneal Curvature: The Influence of Corneal Accommodation and Biomechanics on Corneal Shape. Transl. Vis. Sci. Technol. 2019, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Iyamu, E.; Iyamu, J.; Obiakor, C.I. The Role of Axial Length-Corneal Radius of Curvature Ratio in Refractive State Categorization in a Nigerian Population. ISRN Ophthalmol. 2011, 2011, 138941–138946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D Bioprinting of a Corneal Stroma Equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.; Park, J.; Lee, K.; Lee, S.; Lee, D.; Kim, K.H.; Kim, H.K.; Cho, D. Shear-Induced Alignment of Collagen Fibrils using 3D Cell Printing for Corneal Stroma Tissue Engineering. Biofabrication 2019, 11, 035017. [Google Scholar] [CrossRef] [PubMed]
- Kilic Bektas, C.; Hasirci, V. Cell Loaded 3D Bioprinted GelMA Hydrogels for Corneal Stroma Engineering. Biomater. Sci. 2020, 8, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting Three-Dimensional Cell-Laden Tissue Constructs with Controllable Degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.W.; Lee, S.J.; Park, S.H.; Kim, J.C. Ex Vivo Functionality of 3D Bioprinted Corneal Endothelium Engineered with Ribonuclease 5-Overexpressing Human Corneal Endothelial Cells. Adv. Healthc. Mater. 2018, 7, 1800398. [Google Scholar] [CrossRef]
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-Organizing Optic-Cup Morphogenesis in Three-Dimensional Culture. Nature 2011, 472, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; Geisert, E.E.; Nickerson, J.M. Chapter Twenty-Two—Introduction to the Retina. In Progress in Molecular Biology and Translational Science; Hejtmancik, J.F., Nickerson, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 134, pp. 383–396. [Google Scholar]
- Thoreson, W.B.; Dacey, D.M. Diverse Cell Types, Circuits, and Mechanisms for Color Vision in the Vertebrate Retina. Physiol. Rev. 2019, 99, 1527–1573. [Google Scholar] [CrossRef]
- Holmes, D. Reconstructing the Retina. Nature 2018, 561, S2–S3. [Google Scholar] [CrossRef] [Green Version]
- Ao, J.; Wood, J.P.; Chidlow, G.; Gillies, M.C.; Casson, R.J. Retinal Pigment Epithelium in the Pathogenesis of Age-Related Macular Degeneration and Photobiomodulation as a Potential Therapy? Clin. Exp. Ophthalmol. 2018, 46, 670–686. [Google Scholar] [CrossRef] [Green Version]
- Yvon, C.; Ramsden, C.M.; Lane, A.; Powner, M.B.; da Cruz, L.; Coffey, P.J.; Carr, A.F. Using Stem Cells to Model Diseases of the Outer Retina. Comput. Struct. Biotechnol. J. 2015, 13, 382–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorber, B.; Hsiao, W.; Martin, K.R. Three-Dimensional Printing of the Retina. Curr. Opin. Ophthalmol. 2016, 27, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Chen, P.P. Glaucoma. Am. Fam. Physician 2016, 93, 668–674. [Google Scholar] [PubMed]
- Tsang, S.H.; Sharma, T. Retinitis Pigmentosa (Non-Syndromic). Adv. Exp. Med. Biol. 2018, 1085, 125–130. [Google Scholar]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-Related Macular Degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Worthington, K.S.; Wiley, L.A.; Kaalberg, E.E.; Collins, M.M.; Mullins, R.F.; Stone, E.M.; Tucker, B.A. Two-Photon Polymerization for Production of Human iPSC-Derived Retinal Cell Grafts. Acta Biomater. 2017, 55, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Hertz, J.; Qu, B.; Hu, Y.; Patel, R.D.; Valenzuela, D.A.; Goldberg, J.L. Survival and Integration of Developing and Progenitor-Derived Retinal Ganglion Cells Following Transplantation. Cell Transplant. 2014, 23, 855–872. [Google Scholar] [CrossRef]
- Singhal, S.; Bhatia, B.; Jayaram, H.; Becker, S.; Jones, M.F.; Cottrill, P.B.; Khaw, P.T.; Salt, T.E.; Limb, G.A. Human Müller Glia with Stem Cell Characteristics Differentiate into Retinal Ganglion Cell (RGC) Precursors in Vitro and Partially Restore RGC Function in Vivo Following Transplantation. Stem Cells Transl. Med. 2012, 1, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Kador, K.E.; Grogan, S.P.; Dorthé, E.W.; Venugopalan, P.; Malek, M.F.; Goldberg, J.L.; D’lima, D.D. Control of Retinal Ganglion Cell Positioning and Neurite Growth: Combining 3D Printing with Radial Electrospun Scaffolds. Tissue Eng. Part A 2016, 22, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, A.C.; Hippert, C.; Duran, Y.; West, E.L.; Bainbridge, J.W.B.; Warre-Cornish, K.; Luhmann, U.F.O.; Lakowski, J.; Sowden, J.C.; Ali, R.R.; et al. Repair of the Degenerate Retina by Photoreceptor Transplantation. Proc. Natl. Acad. Sci. USA 2013, 110, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Ballios, B.G.; Cooke, M.J.; Donaldson, L.; Coles, B.L.K.; Morshead, C.M.; van der Kooy, D.; Shoichet, M.S. A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny Following Transplantation. Stem Cell Rep. 2015, 4, 1031–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLaren, R.E.; Pearson, R.A.; MacNeil, A.; Douglas, R.H.; Salt, T.E.; Akimoto, M.; Swaroop, A.; Sowden, J.C.; Ali, R.R. Retinal Repair by Transplantation of Photoreceptor Precursors. Nature 2006, 444, 203–207. [Google Scholar] [CrossRef]
- Hynes, S.R.; Lavik, E.B. A Tissue-Engineered Approach towards Retinal Repair: Scaffolds for Cell Transplantation to the Subretinal Space. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Tao, S.; Young, M. Synthetic Polymer Scaffolds for Stem Cell Transplantation in Retinal Tissue Engineering. Polymers 2011, 3, 899–914. [Google Scholar] [CrossRef]
- Treharne, A.J.; Grossel, M.C.; Lotery, A.J.; Thomson, H.A. The Chemistry of Retinal Transplantation: The Influence of Polymer Scaffold Properties on Retinal Cell Adhesion and Control. Br. J. Ophthalmol. 2011, 95, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Sorkio, A.E.; Vuorimaa-Laukkanen, E.; Hakola, H.M.; Liang, H.; Ujula, T.A.; Valle-Delgado, J.; Österberg, M.; Yliperttula, M.L.; Skottman, H. Biomimetic Collagen I and IV Double Layer Langmuir-Schaefer Films as Microenvironment for Human Pluripotent Stem Cell Derived Retinal Pigment Epithelial Cells. Biomaterials 2015, 51, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Baranov, P.; Michaelson, A.; Kundu, J.; Carrier, R.L.; Young, M. Interphotoreceptor Matrix-Poly(Ε-Caprolactone) Composite Scaffolds for Human Photoreceptor Differentiation. J. Tissue Eng. 2014, 5, 2041731414554139. [Google Scholar] [CrossRef] [PubMed]
- Munaz, A.; Vadivelu, R.K.; St. John, J.; Barton, M.; Kamble, H.; Nguyen, N. Three-Dimensional Printing of Biological Matters. J. Sci. Adv. Mater. Devices 2016, 1, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Chui, T.Y.P.; Song, H.; Clark, C.A.; Papay, J.A.; Burns, S.A.; Elsner, A.E. Cone Photoreceptor Packing Density and the Outer Nuclear Layer Thickness in Healthy Subjects. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3545–3553. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Chui, T.Y.P.; Zhong, Z.; Elsner, A.E.; Burns, S.A. Variation of Cone Photoreceptor Packing Density with Retinal Eccentricity and Age. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7376–7384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worthington, K.S.; Wiley, L.A.; Bartlett, A.M.; Stone, E.M.; Mullins, R.F.; Salem, A.K.; Guymon, C.A.; Tucker, B.A. Mechanical Properties of Murine and Porcine Ocular Tissues in Compression. Exp. Eye Res. 2014, 121, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Hertz, J.; Robinson, R.; Valenzuela, D.A.; Lavik, E.B.; Goldberg, J.L. A Tunable Synthetic Hydrogel System for Culture of Retinal Ganglion Cells and Amacrine Cells. Acta Biomater. 2013, 9, 7622–7629. [Google Scholar] [CrossRef] [Green Version]
- Lorber, B.; Hsiao, W.; Hutchings, I.M.; Martin, K.R. Adult Rat Retinal Ganglion Cells and Glia can be Printed by Piezoelectric Inkjet Printing. Biofabrication 2014, 6, 015001. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Tan, Y.S.E.; Yeong, W.Y.; Li, H.Y.; Laude, A. A Bilayer Photoreceptor-Retinal Tissue Model with Gradient Cell Density Design: A Study of Microvalve-Based Bioprinting. J. Tissue Eng. Regen. Med. 2018, 12, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, X.; Zhu, W.; Zhong, Z.; Moran, A.; Wang, W.; Zhang, K.; Chen, S. 3D Bioprinting of Hydrogels for Retina Cell Culturing. Bioprinting 2018, 12, e00029. [Google Scholar] [CrossRef]
- Taurone, S.; Spoletini, M.; Ralli, M.; Gobbi, P.; Artico, M.; Imre, L.; Czakò, C.; Kovàcs, I.; Greco, A.; Micera, A. Ocular Mucous Membrane Pemphigoid: A Review. Immunol. Res. 2019, 67, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Witt, J.; Mertsch, S.; Borrelli, M.; Dietrich, J.; Geerling, G.; Schrader, S.; Spaniol, K. Decellularised Conjunctiva for Ocular Surface Reconstruction. Acta Biomater. 2018, 67, 259–269. [Google Scholar] [CrossRef]
- Yamaguchi, T. Inflammatory Response in Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES192–DES199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, J.; de Paiva, C.S.; Pflugfelder, S.C. Immune—Goblet Cell Interaction in the Conjunctiva. Ocul. Surf. 2020, 18, 326–334. [Google Scholar] [CrossRef]
- Dehghani, S.; Rasoulianboroujeni, M.; Ghasemi, H.; Keshel, S.H.; Nozarian, Z.; Hashemian, M.N.; Zarei-Ghanavati, M.; Latifi, G.; Ghaffari, R.; Cui, Z.; et al. 3D-Printed Membrane as an Alternative to Amniotic Membrane for Ocular Surface/Conjunctival Defect Reconstruction: An in Vitro & in Vivo Study. Biomaterials 2018, 174, 95–112. [Google Scholar] [PubMed]
- Mathew, E.; Pitzanti, G.; Larrañeta, E.; Lamprou, D.A. 3D Printing of Pharmaceuticals and Drug Delivery Devices. Pharmaceutics 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.; Datta, P.; Ayan, B.; Ozbolat, V.; Sosnoski, D.; Ozbolat, I.T. 3D Bioprinting for Drug Discovery and Development in Pharmaceutics. Acta Biomater. 2017, 57, 26–46. [Google Scholar] [CrossRef]
- Won, J.Y.; Kim, J.; Gao, G.; Kim, J.; Jang, J.; Park, Y.; Cho, D. 3D Printing of Drug-Loaded Multi-Shell Rods for Local Delivery of Bevacizumab and Dexamethasone: A Synergetic Therapy for Retinal Vascular Diseases. Acta Biomater. 2020, 116, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.A.; Mollica, P.A.; Johnson, G.D.; Ogle, R.C.; Bruno, R.D.; Sachs, P.C. Accessible Bioprinting: Adaptation of a Low-Cost 3D-Printer for Precise Cell Placement and Stem Cell Differentiation. Biofabrication 2016, 8, 025017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco Fernandez, R. The Philosophical Basis of Eugenesia. Medicina 1960, 40, 2. [Google Scholar]
- Pashkov, V.; Harkusha, A. 3-D Bioprinting Law Regulation Perspectives. Wiad. Lek. 2017, 70, 480–482. [Google Scholar] [PubMed]
- Gilbert, F.; O’Connell, C.D.; Mladenovska, T.; Dodds, S. Print Me an Organ? Ethical and Regulatory Issues Emerging from 3D Bioprinting in Medicine. Sci. Eng. Ethics 2018, 24, 73–91. [Google Scholar] [CrossRef]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.A.; Liu, W.; Jimenez, A.; Yang, J.; Akpek, A.; Liu, X.; Pi, Q.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, e1805510. [Google Scholar] [CrossRef] [PubMed]







| 3D Bioprinting Technique | Materials of the Bio-Inks and Inks | Cells | Scaffold Function/Study Objective | In Vivo | Most Relevant Results | Ref. |
|---|---|---|---|---|---|---|
| Extrusion 3D bioprinting | Sodium alginate and methacrylated type I collagen | Human corneal keratocytes | Tissue replication. Corneal stroma structure | No |
| [42] |
| Extrusion 3D bioprinting | Methacrylated gelatin (GelMA) | Human corneal keratocytes | Tissue replication. Corneal stroma structure | No |
| [44] |
| Extrusion 3D bioprinting | Decellularized corneal extracellular matrix based bio-ink | Human corneal keratocytes differentiated from human turbinate derived mesenchymal stem cells | Tissue replication. Corneal stroma structure | New Zealand white rabbits |
| [43] |
| Drop-on-demand inkjet bioprinting | Type I collagen and agarose | Human corneal keratocytes | Tissue replication. Corneal stroma structure | No |
| [37] |
| Extrusion 3D bioprinting | Sodium alginate, gelatin and type I collagen | Human corneal epithelial cells | Tissue replication. Corneal epithelium structure | No |
| [45] |
| Combination of digital light processing (DLP) and extrusion 3D bioprinting | Methacrylated gelatin (GelMA) for DLP Sodium alginate and gelatin for extrusion 3D-bioprinting | Human corneal epithelial cells | Tissue replication. Development of supportive structure with DLP technique in order to bio-print corneal epithelium structure on it | No |
| [30] |
| Laser-assisted 3D bioprinting | 2 Types: Human recombinant laminin and Hyaluronic acid sodium Human collagen type I and Human blood plasma + Thrombin | Human embryonic stem cells (hESC) Human adipose derived stem cells (hASC) | Tissue replication. Cornea epithelium structure Corneal stroma structure | No Explanted porcine corneas |
| [32] |
| Extrusion 3D bioprinting | Gelatin based bio-ink | Human corneal endothelial cells genetically modified to express ribonuclease (R5) | Tissue replication. Corneal endothelium structure. | New Zealand white rabbits. Descemet’s membrane-denuded corneal disorder model. |
| [46] |
| 3D Bioprinting Technique | Materials of the Bio-Inks and Inks | Cells | Scaffold Function/Study Objective | In Vivo | Most Relevant Results | Ref. |
|---|---|---|---|---|---|---|
| Laser assisted 3D bioprinting | HA-GM (hyaluronic acid with methacrylation by glycidyl-hydroxyl reaction) and PEG-RGDS (Arg-Gly-Asp-Ser peptide) | Retinal pigment epithelial cells (RPE) Human fetal retinal progenitor cells (fRPCs) | Tissue equivalent replication. Retina made up of two layers | No |
| [76] |
| Piezoelectric inkjet bioprinting | DMEM (Dubelcco’s Modified Eagle’s Medium) (not structural function) | Retinal ganglion cell (RGCs) neurons Retinal glial cells. | Study the effect of piezoelectric inkjet bioprinting in the viability of the printed cells. | No |
| [74] |
| Microvalve-based inkjet bioprinting | DMEM:F12 (not structural function) Alginate and Pluronic | Human retinal pigmented epithelial cell line (ARPE-19) Human retinoblastoma cell line (Y79) | Tissue replication. Retina made up of two layers. | No |
| [24] |
| Two-photon lithography | Indium tin oxide (ITO)-coated glass | Human induced pluripotent stem cell (iPSC) | Development of scaffolds to deliver correctly oriented retinal progenitor cells | No |
| [57] |
| Thermal inkjet 3D bioprinting combined with electrospinning | Alginate and culture Medium for 3D bioprinting Polylactic acid (PLA) dissolved in 1,1,1,3,3,3 hexafluoro-isopropanol (HFIP) and matrigel for electrospinning | Retinal ganglion Cells (rgcS) | Development of scaffolds to deliver correctly oriented retinal progenitor cells | No |
| [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Alonso, S.; Villate-Beitia, I.; Gallego, I.; Lafuente-Merchan, M.; Puras, G.; Saenz-del-Burgo, L.; Pedraz, J.L. Current Insights into 3D Bioprinting: An Advanced Approach for Eye Tissue Regeneration. Pharmaceutics 2021, 13, 308. https://doi.org/10.3390/pharmaceutics13030308
Ruiz-Alonso S, Villate-Beitia I, Gallego I, Lafuente-Merchan M, Puras G, Saenz-del-Burgo L, Pedraz JL. Current Insights into 3D Bioprinting: An Advanced Approach for Eye Tissue Regeneration. Pharmaceutics. 2021; 13(3):308. https://doi.org/10.3390/pharmaceutics13030308
Chicago/Turabian StyleRuiz-Alonso, Sandra, Ilia Villate-Beitia, Idoia Gallego, Markel Lafuente-Merchan, Gustavo Puras, Laura Saenz-del-Burgo, and José Luis Pedraz. 2021. "Current Insights into 3D Bioprinting: An Advanced Approach for Eye Tissue Regeneration" Pharmaceutics 13, no. 3: 308. https://doi.org/10.3390/pharmaceutics13030308