The Effects of Accelerated Temperature-Controlled Stability Systems on the Release Profile of Primary Bile Acid-Based Delivery Microcapsules
Abstract
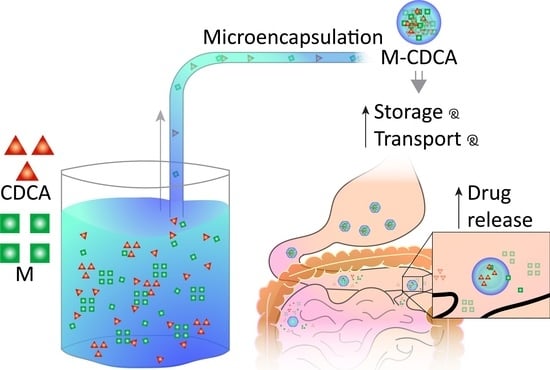
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Drug Preparations
2.3. Preparation of Microcapsules
2.4. Characterization of Loaded Microcapsules
2.4.1. Microcapsule Morphology and Surface Analysis
2.4.2. Microcapsule Resistance and Buoyancy
2.4.3. Swelling Studies
2.4.4. Accelerated Stability Testing
2.4.5. Drug Release Studies and High-Performance Liquid Chromatography (HPLC) Instrumentation and Chromatographic Conditions
2.4.6. Liquid Chromatography Mass Spectrometry (LC-MS) Instrumentation and Chromatographic Conditions
2.5. Statistical Analysis
3. Results and Discussion
3.1. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDXR) Spectroscopy
3.2. Drug and CDCA Contents of Microcapsules
3.3. Mechanical Strength and Buoyancy
3.4. Accelerated Stability Testing
3.5. Swelling Studies
3.6. Drug Release Studies
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mooranian, A.; Raj Wagle, S.; Kovacevic, B.; Takechi, R.; Mamo, J.; Lam, V.; Watts, G.F.; Mikov, M.; Golocorbin-Kon, S.; Stojanovic, G.; et al. Bile acid bio-nanoencapsulation improved drug targeted-delivery and pharmacological effects via cellular flux: 6-months diabetes preclinical study. Sci. Rep. 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Waterman, K.C.; Carella, A.J.; Gumkowski, M.J.; Lukulay, P.; MacDonald, B.C.; Roy, M.C.; Shamblin, S.L. Improved Protocol and Data Analysis for Accelerated Shelf-Life Estimation of Solid Dosage Forms. Pharm. Res. 2007, 24, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Zolnik, B.S.; Leary, P.E.; Burgess, D.J. Elevated temperature accelerated release testing of PLGA microspheres. J. Control. Release 2006, 112, 293–300. [Google Scholar] [CrossRef]
- He, N.; Sun, H.; Dai, M. Evaluation of the influence of humidity and temperature on the drug stability by initial average rate experiment. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014, 39, 501–510. [Google Scholar]
- Zhao, Q.; Zhan, X.C.; Li, L.L.; Li, C.R.; Lin, T.; Yin, X.D.; He, N. Effect of programmed humidification and temperature on drug stability. Yao Xue Xue Bao 2004, 39, 1001–1005. [Google Scholar] [PubMed]
- Yoshioka, S.; Aso, Y.; Takeda, Y. Statistical evaluation of accelerated stability data obtained at a single temperature. II. Estimation of shelf-life from remaining drug content. Chem. Pharm. Bull. 1990, 38, 1760–1762. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yamaji, T.; Takayama, K. Effects of packaging and heat transfer kinetics on drug-product stability during storage under uncontrolled temperature conditions. J. Pharm. Sci. 2013, 102, 1495–1503. [Google Scholar] [CrossRef]
- Kadam, A.N.; Najlah, M.; Wan, K.W.; Ahmed, W.; Crean, S.J.; Phoenix, D.A.; Taylor, K.M.G.; Elhissi, A.M.A. Stability of parenteral nanoemulsions loaded with paclitaxel: The influence of lipid phase composition, drug concentration and storage temperature. Pharm. Dev. Technol. 2014, 19, 999–1004. [Google Scholar] [CrossRef]
- Sakurai, A.; Sako, K.; Maitani, Y. Influence of manufacturing factors on physical stability and solubility of solid dispersions containing a low glass transition temperature drug. Chem. Pharm. Bull. 2012, 60, 1366–1371. [Google Scholar] [CrossRef] [Green Version]
- Beirowski, J.; Inghelbrecht, S.; Arien, A.; Gieseler, H. Freeze drying of nanosuspensions, 2: The role of the critical formulation temperature on stability of drug nanosuspensions and its practical implication on process design. J. Pharm. Sci. 2011, 100, 4471–4481. [Google Scholar] [CrossRef]
- Chen, M.; Lu, J.; Deng, W.; Singh, A.; Mohammed, N.N.; Repka, M.A.; Wu, C. Influence of processing parameters and formulation factors on the bioadhesive, temperature stability and drug release properties of hot-melt extruded films containing miconazole. AAPS PharmSciTech 2014, 15, 522–529. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Levons, J.; Narang, A.S.; Raghavan, K.; Rao, V.M. Reactive Impurities in Excipients: Profiling, Identification and Mitigation of Drug–Excipient Incompatibility. AAPS PharmSciTech 2011, 12, 1248–1263. [Google Scholar] [CrossRef] [Green Version]
- Waterman, K.C.; Gerst, P.; Dai, Z. A generalized relation for solid-state drug stability as a function of excipient dilution: Temperature-independent behavior. J. Pharm. Sci. 2012, 101, 4170–4177. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Hendradi, E.; Erawati, T.; Jannah, E.N.; Febrina, W. Influence of drug-polymer ratio on physical characteristics and release of metformin hydrochloride from metforminalginate microspheres. Trop. J. Pharm. Res. 2018, 17, 1229–1233. [Google Scholar] [CrossRef]
- Gumieniczek, A.; Berecka-Rycerz, A.; Mroczek, T.; Wojtanowski, K. Determination of Chemical Stability of Two Oral Antidiabetics, Metformin and Repaglinide in the Solid State and Solutions Using LC-UV, LC-MS, and FT-IR Methods. Molecules 2019, 24, 4430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pareta, R.A.; Farney, A.C.; Opara, E.C. Design of a bioartificial pancreas. Pathobiol. 2013, 80, 194–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbaspourrad, A.; Carroll, N.J.; Kim, S.-H.; Weitz, D.A. Polymer Microcapsules with Programmable Active Release. J. Am. Chem. Soc. 2013, 135, 7744–7750. [Google Scholar] [CrossRef] [Green Version]
- Franco, P.; De Marco, I. Eudragit: A Novel Carrier for Controlled Drug Delivery in Supercritical Antisolvent Coprecipitation. Polymers 2020, 12, 234. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. Characterization of a novel bile acid-based delivery platform for microencapsulated pancreatic β-cells. Artif. Cells Nanomed. Biotechnol. 2014, 44, 194–200. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Al-Sallami, H.S.; Fang, Z.; Mukkur, T.K.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Watts, G.F.; et al. Microencapsulation as a novel delivery method for the potential antidiabetic drug, Probucol. Drug Des. Dev. Ther. 2014, 8, 1221–1230. [Google Scholar]
- Mooranian, A.; Negrulj, R.; Mathavan, S.; Martinez, J.; Sciarretta, J.; Chen-Tan, N.; Mukkur, T.K.; Mikov, M.; Lalic-Popovic, M.; Stojancevic, M.; et al. Stability and Release Kinetics of an Advanced Gliclazide-Cholic Acid Formulation: The Use of Artificial-Cell Microencapsulation in Slow Release Targeted Oral Delivery of Antidiabetics. J. Pharm. Innov. 2014, 9, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Negrulj, R.; Mathavan, S.; Martinez, J.; Sciarretta, J.; Chen-Tan, N.; Mukkur, T.K.; Mikov, M.; Lalic-Popovic, M.; Stojancevic, M.; et al. An advanced microencapsulated system: A platform for optimized oral delivery of antidiabetic drug-bile acid formulations. Pharm. Dev. Technol. 2015, 20, 702–709. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Electrokinetic potential-stabilization by bile acid-microencapsulating formulation of pancreatic beta-cells cultured in high ratio poly-L-ornithine-gel hydrogel colloidal dispersion: Applications in cell-biomaterials, tissue engineering and biotechnological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1156–1562. [Google Scholar]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. The effect of a tertiary bile acid, taurocholic acid, on the morphology and physical characteristics of microencapsulated probucol: Potential applications in diabetes: A characterization study. Drug Deliv. Transl. Res. 2015, 5, 511–522. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Watts, G.F.; Arfuso, F.; Al-Salami, H. An optimized probucol microencapsulated formulation integrating a secondary bile acid (deoxycholic acid) as a permeation enhancer. Drug Des. Dev. Ther. 2014, 8, 1673–1683. [Google Scholar]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Al-Sallami, H.S.; Fang, Z.; Mukkur, T.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Arfuso, F.M.; et al. Novel artificial cell microencapsulation of a complex gliclazide-deoxycholic bile acid formulation: A characterization study. Drug Des. Dev. Ther. 2014, 8, 1003. [Google Scholar]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Fakhoury, M.; Arfuso, F.; Jones, F.; Al-Salami, H. Advanced bile acid-based multi-compartmental microencapsulated pancreatic ß-cells integrating a polyelectrolyte-bile acid formulation, for diabetes treatment. Artif. Cells Nanomed. Biotechnol. 2016, 44, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Mathavan, S.; Chen-Tan, N.; Arfuso, F.; Al-Salami, H. The role of the bile acid chenodeoxycholic acid in the targeted oral delivery of the anti-diabetic drug gliclazide, and its applications in type 1 diabetes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. Multicompartmental, multilayered probucol microcapsules for diabetes mellitus: Formulation characterization and effects on production of insulin and inflammation in a pancreatic β-cell line. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1642–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Al-Salami, H. Novel nano-encapsulation of probucol in microgels: Scanning electron micrograph characterizations, buoyancy profiling, and antioxidant assay analyses. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S741–S747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooranian, A.; Negrulj, R.; Al-Sallami, H.S.; Fang, Z.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Arfuso, F.; Al-Salami, H. Release and swelling studies of an innovative antidiabetic-bile acid microencapsulated formulation, as a novel targeted therapy for diabetes treatment. J. Microencapsul. 2015, 32, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Al-Sallami, H.S.; Fang, Z.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Watts, G.F.; Matthews, V.; Arfuso, F.; et al. Probucol release from novel multicompartmental microcapsules for the oral targeted delivery in type 2 diabetes. AAPS PharmSciTech 2015, 16, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negrulj, R.; Mooranian, A.; Chen-Tan, N.; Al-Sallami, H.S.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Watts, G.F.; Arfuso, F.; Al-Salami, H. Swelling, mechanical strength, and release properties of probucol microcapsules with and without a bile acid, and their potential oral delivery in diabetes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1290–1297. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Mikov, M.; Golocorbin-Kon, S.; Arfuso, F.; Al-Salami, H. Novel chenodeoxycholic acid–sodium alginate matrix in the microencapsulation of the potential antidiabetic drug, probucol: An in vitro study. J. Microencapsul. 2015, 32, 589–597. [Google Scholar] [CrossRef]
- Mathavan, S.; Chen-Tan, N.; Arfuso, F.; Al-Salami, H. A comprehensive study of novel microcapsules incorporating gliclazide and a permeation enhancing bile acid: Hypoglycemic effect in an animal model of Type-1 diabetes. Drug Deliv. 2016, 23, 2869–2880. [Google Scholar] [CrossRef]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Development and validation of a new analytical HPLC method for simultaneous determination of the antidiabetic drugs, metformin and gliclazide. J. Food Drug Anal. 2019, 27, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Advanced and multifaceted stability profiling of the first-line antidiabetic drugs metformin, gliclazide and glipizide under various controlled stress conditions. Saudi Pharm. J. 2020, 28, 362–368. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Takechi, R.; Al-Sallami, H.; Mikov, M.; Golocorbin-Kon, S.; Kovacevic, B.; Arfuso, F.; Al-Salami, H. Pharmacological effects of nanoencapsulation of human-based dosing of probucol on ratio of secondary to primary bile acids in gut, during induction and progression of type 1 diabetes. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S748–S754. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Mamo, J.; Al-Sallami, H.; Al-Salami, H. The biological effects of the hypolipidaemic drug probucol microcapsules fed daily for 4 weeks, to an insulin-resistant mouse model: Potential hypoglycaemic and anti-inflammatory effects. Drug Deliv. Transl. Res. 2018, 8, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Mathavan, S.; Ionescu, C.M.; Kovacevic, B.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Dass, C.R.; Al-Salami, H. Formulation buoyancy of nanoencapsulated gliclazide using primary, conjugated and deconjugated bile acids. Ther. Deliv. 2019, 10, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Singla, D.; Sakhuja, N. Stability testing of pharmaceutical products. J. Appl. Pharm. Sci. 2012, 2, 129–138. [Google Scholar]
- Mooranian, A.; Negrulj, R.; Al-Salami, H.; Morahan, G.; Jamieson, E. Designing anti-diabetic β-cells microcapsules using polystyrenic sulfonate, polyallylamine, and a tertiary bile acid: Morphology, bioenergetics, and cytokine analysis. Biotechnol. Prog. 2016, 32, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Angadi, S.; Kale, S.; Shelke, S.; Kawade, S.; Kadam, R. Stability indicating UV spectroscopic method for the estimation of metformin hydrochloride in bulk and tablets. Int. J. Life Sci. Rev. 2015, 1, 27–33. [Google Scholar]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Mooranian, A.; Zamani, N.; Takechi, R.; Luna, G.; Mikov, M.; Golocorbin-Kon, S.; Elnashar, M.; Arfuso, F.; Al-Salami, H. An in vivo pharmacological study: Variation in tissue-accumulation for the drug probucol as the result of targeted microtechnology and matrix-acrylic acid optimization and stabilization techniques. PLoS ONE 2019, 14, e0214984. [Google Scholar] [CrossRef] [Green Version]
- Goto, S.; Kawata, M.; Nakamura, M.; Nagatsuma, Y.; Fujinaga, K.; Aoyama, T. Evaluation of the sustained release properties of Eudragit RS, RL and S (acrylic resins) microcapsules containing ketoprofen in beagle dogs. J. Microencapsul. 1988, 5, 343–360. [Google Scholar] [CrossRef]
- Ndesendo, V.M.K.; Meixner, W.; Korsatko, W.; Korsatko-Wabnegg, B. Microencapsulation of chloroquine diphosphate by Eudragit RS100. J. Microencapsul. 1996, 13, 1–8. [Google Scholar] [CrossRef]
- Kaffash, E.; Saremnejad, F.; Abbaspour, M.; Mohajeri, S.A.; Garekani, H.A.; Jafarian, A.H.; Sardo, H.S.; Akhgari, A.; Nokhodchi, A. Statistical optimization of alginate-based oral dosage form of 5-aminosalicylic acid aimed to colonic delivery: In vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2019, 52, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Barakat, N.S.; Shazly, G.A.; Almedany, A.H. Influence of polymer blends on the characterization of gliclazide—Encapsulated into poly (Æ-caprolactone) microparticles. Drug Dev. Ind. Pharm. 2013, 39, 352–362. [Google Scholar] [CrossRef]
- Kaffash, E.; Abbaspour, M.; Afrasiabi Garekani, H.; Jahanian, Z.; Saremnejad, F.; Akhgari, A. The Effect of Thermal-Treating on Drug Release from Sustained Release Alginate-Eudragit RS Matrices. Adv. Pharm. Bull. 2021, 11, 318–326. [Google Scholar]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Al-Salami, H. Eudragit®-based microcapsules of probucol with a gut-bacterial processed secondary bile acid. Ther. Deliv. 2018, 9, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef]
- Song, R. Mechanism of Metformin: A Tale of Two Sites. Diabetes Care 2016, 39, 187–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markowicz-Piasecka, M.; MHuttunen, K.; Mateusiak, L.; Mikiciuk-Olasik, E.; Sikora, J. Is metformin a perfect drug? Updates in pharmacokinetics and pharmacodynamics. Curr. Pharm. Des. 2017, 23, 2532–2550. [Google Scholar] [CrossRef]
- Zhou, K.; Donnelly, L.; Yang, J.; Li, M.; Deshmukh, H.; Van Zuydam, N.; Ahlqvist, E.; Spencer, C.C.; Groop, L.; Wellcome Trust Case Control Consortium 2; et al. Heritability of variation in glycaemic response to metformin: A genome-wide complex trait analysis. Lancet Diabetes Endocrinol. 2014, 2, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Rena, G.; Pearson, E.R.; Sakamoto, K. Molecular mechanism of action of metformin: Old or new insights? Diabetologia 2013, 56, 1898–1906. [Google Scholar] [CrossRef] [Green Version]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, S.; Fonseca, V.; Berner, B.; Cramer, M.; Chiang, Y.-K.; Lewin, A. Efficacy, tolerability, and safety of a novel once-daily extended-release metformin in patients with type 2 diabetes. Diabetes Care 2006, 29, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Role of metformin in various pathologies: State-of-the-art microcapsules for improving its pharmacokinetics. Ther. Deliv. 2020, 11, 733–753. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mooranian, A.; Carey, L.; Ionescu, C.M.; Walker, D.; Jones, M.; Wagle, S.R.; Kovacevic, B.; Foster, T.; Chester, J.; Johnston, E.; et al. The Effects of Accelerated Temperature-Controlled Stability Systems on the Release Profile of Primary Bile Acid-Based Delivery Microcapsules. Pharmaceutics 2021, 13, 1667. https://doi.org/10.3390/pharmaceutics13101667
Mooranian A, Carey L, Ionescu CM, Walker D, Jones M, Wagle SR, Kovacevic B, Foster T, Chester J, Johnston E, et al. The Effects of Accelerated Temperature-Controlled Stability Systems on the Release Profile of Primary Bile Acid-Based Delivery Microcapsules. Pharmaceutics. 2021; 13(10):1667. https://doi.org/10.3390/pharmaceutics13101667
Chicago/Turabian StyleMooranian, Armin, Louise Carey, Corina Mihaela Ionescu, Daniel Walker, Melissa Jones, Susbin Raj Wagle, Bozica Kovacevic, Thomas Foster, Jacqueline Chester, Edan Johnston, and et al. 2021. "The Effects of Accelerated Temperature-Controlled Stability Systems on the Release Profile of Primary Bile Acid-Based Delivery Microcapsules" Pharmaceutics 13, no. 10: 1667. https://doi.org/10.3390/pharmaceutics13101667