Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, In Vivo and Stability Evaluations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Moringa Oleifera Seed Oil (MOO)
Seed Collection and Oil Extraction
2.2.2. Black Seed Oil (BSO)
Seed Collection and Extraction
BSO Standardization
2.2.3. Preparation of the Bio-SNEDDS and Self-Emulsification Assessment
2.2.4. Particle Size and Polydispersity Index Measurement
2.2.5. Transmission Electron Microscopy (TEM)
2.2.6. Equilibrium Solubility Test
2.2.7. UPLC Analysis
2.2.8. APG Precipitation Test
2.2.9. In Vitro Dissolution Test
2.2.10. Antimicrobial Activity Test
Disc Diffusion Assays
2.2.11. In Vivo Oral Bioavailability Study
Animals
Drug Administration
Sample Preparation of Apigenin
UPLC-MS/MS Plasma Analysis
Pharmacokinetic (PK) Data Analysis
2.2.12. Stability Test
2.2.13. Statistical Analysis
3. Results and Discussion
3.1. UPLC and UPLC-MS/MS Analysis of APG
3.2. Characterization of the SNEDDS
3.2.1. Bio-SNEDDS Formulation Design
3.2.2. Assessment of Self-Emulsification Efficiency of the Bio-SNEDDS
3.2.3. Formulation Droplet Size and PDIs Analysis
3.2.4. Transmission Electron Microscopy (TEM)
3.2.5. Effect of Oil on the Droplet Size of the Formulation of APG
3.2.6. Effect of the Surfactants on the Droplet Size of Formulation of APG
3.2.7. APG Solubility in Lipid Formulation
3.2.8. The Best Optimized Formulation
3.3. Dynamic Dispersion Studies
3.4. In Vitro Release Studies
Antimicrobial Activity (Disc Diffusion Assays)
3.5. Stability of Apigenin in the SNEDDS
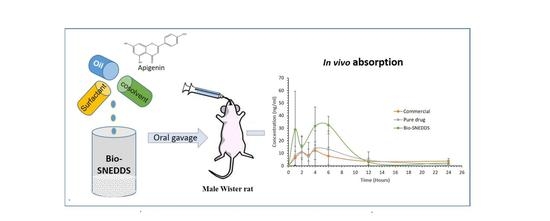
3.6. Pharmacokinetic Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Devraj, R.; Williams, H.D.; Warren, D.B.; Mohsin, K.; Porter, C.J.; Pouton, C.W. In vitro assessment of drug-free and fenofibrate-containing lipid formulations using dispersion and digestion testing gives detailed insights into the likely fate of formulations in the intestine. Eur. J. Pharm. Sci. 2013, 49, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Wang, F.; Zhou, R.; Song, X.; Xie, M. Apigenin: A current review on its beneficial biological activities. J. Food Biochem. 2017, 41, 12376. [Google Scholar] [CrossRef]
- Liang, H.; Sonego, S.; Gyengesi, E.; Rangel, A.; Niedermayer, G.; Karl, T.; Münch, G. OP-25—Anti-Inflammatory and Neuroprotective Effect of Apigenin: Studies in the GFAP-IL6 Mouse Model of Chronic Neuroinflammation. Free Radic. Biol. Med. 2017, 108, 10. [Google Scholar] [CrossRef]
- Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar]
- Panda, S.; Kar, A. Apigenin (4′,5,7-trihydroxyflavone) regulates hyperglycaemia, thyroid dysfunction and lipid peroxidation in alloxan-induced diabetic mice. J. Pharm. Pharmacol. 2007, 59, 1543–1548. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Ding, B.; Chen, H.; Wang, C.; Zhai, Y.; Zhai, G. Preparation and in vitro evaluation of apigenin loaded lipid nanocapsules. J. Nanosci. Nanotechnol. 2013, 13, 6546–6552. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Zu, Y.; Wang, L.; Deng, Y.; Wu, M.; Wang, H. Enhanced Solubility and Bioavailability of Apigenin via Preparation of Solid Dispersions of Mesoporous Silica Nanoparticles. Iran. J. Pharm. Res. 2019, 18, 168–182. [Google Scholar]
- Altamimi, M.A.; Elzayat, E.M.; Alshehri, S.M.; Mohsin, K.; Ibrahim, M.A.; Al Meanazel, O.T.; Shakeel, F.; Alanazi, F.K.; Alsarra, I.A. Utilizing spray drying technique to improve oral bioavailability of apigenin. Adv. Powder Technol. 2018, 29, 1676–1684. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Shakeel, F.; Ibrahim, M.A.; Elzayat, E.M.; Altamimi, M.; Mohsin, K.; Almeanazel, O.T.; Alkholief, M.; Alshetaili, A.; Alsulays, B.; et al. Dissolution and bioavailability improvement of bioactive apigenin using solid dispersions prepared by different techniques. Saudi Pharm. J. 2018. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.M.; Zhang, Z.H.; Song, J.; Cheng, X.D.; Jiang, J.; Jia, X.B. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014, 9, 2327–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Khemtong, C.; Yang, X.; Chang, X.; Gao, J. Nanonization strategies for poorly water-soluble drugs. Drug Discov. Today 2011, 16, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Liu, D.; Gao, Y.; Qian, S. Preparation of apigenin nanocrystals using supercritical antisolvent process for dissolution and bioavailability enhancement. Eur. J. Pharm. Sci. 2013, 48, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, K.; Long, M.A.; Pouton, C.W. Design of lipid-based formulations for oral administration of poorly water-soluble drugs: Precipitation of drug after dispersion of formulations in aqueous solution. J. Pharm. Sci. 2009, 98, 3582–3595. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, K.; Alamri, R.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Hussain, M.D. Development of self-nanoemulsifying drug delivery systems for the enhancement of solubility and oral bioavailability of fenofibrate, a poorly water-soluble drug. Int. J. Nanomed. 2016, 11, 2829–2838. [Google Scholar]
- Kazi, M.; Al-Qarni, H.; Alanazi, F.K. Development of oral solid self-emulsifying lipid formulations of risperidone with improved in vitro dissolution and digestion. Eur. J. Pharm. Biopharm. 2017, 114, 239–249. [Google Scholar] [CrossRef]
- Wu, W.; Zu, Y.; Wang, L.; Wang, H.; Li, Y.; Wu, M.; Zhao, X.; Fu, Y. Preparation, characterization and antitumor activity evaluation of apigenin nanoparticles by the liquid antisolvent precipitation technique. Drug Deliv. 2017, 24, 1713–1720. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.J.; Guo, C.Y.; Hou, J.N.; Zhang, W.D.; Zhai, G.X. Preparation and in vitro characterization of apigemin-loaded nanostructured lipid carriers. J. Chin. Med. Mater. 2011, 34, 962–965. [Google Scholar]
- Fatouros, D.G.; Karpf, D.M.; Nielsen, F.S.; Mullertz, A. Clinical studies with oral lipid based formulations of poorly soluble compounds. Clin. Risk Manag. 2007, 3, 591–604. [Google Scholar]
- Kang, B.K.; Lee, J.S.; Chon, S.K.; Jeong, S.Y.; Yuk, S.H.; Khang, G.; Lee, H.B.; Cho, S.H. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 2004, 274, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Rushmi, Z.T.; Akter, N.; Mow, R.J.; Afroz, M.; Kazi, M.; de Matas, M.; Rahman, M.; Shariare, M.H. The impact of formulation attributes and process parameters on black seed oil loaded liposomes and their performance in animal models of analgesia. Saudi Pharm. J. 2017, 25, 404–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randhawa, M.A.; Alghamdi, M.S. Anticancer activity of Nigella sativa (black seed)—A review. Am. J. Chin. Med. 2011, 39, 1075–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wang, C.; Chow, A.H.; Ren, K.; Gong, T.; Zhang, Z.; Zheng, Y. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: Formulation and bioavailability studies. Int. J. Pharm. 2010, 383, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Shahba, A.A.; Alrashoud, S.; Alwadei, M.; Sherif, A.Y.; Alanazi, F.K. Bioactive Self-Nanoemulsifying Drug Delivery Systems (Bio-SNEDDS) for Combined Oral Delivery of Curcumin and Piperine. Molecules 2020, 25, 1703. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhang, L.; Meng, L.; Wang, J.; Zhai, G. Design and evaluation of a self-microemulsifying drug delivery system for apigenin. Drug Dev. Ind. Pharm. 2013, 39, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Vander Pol, M.; Agle, M.; Zaman, S.; Schneider, C.; Ndegwa, P.; Vaddella, V.K.; Johnson, K.; Shingfield, K.J.; Karnati, S.K. Effect of lauric acid and coconut oil on ruminal fermentation, digestion, ammonia losses from manure, and milk fatty acid composition in lactating cows. J. Dairy Sci. 2009, 92, 5561–5582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, M.N.; Sarmah, B.C.; Tamuli, M.K.; Das, A.; Kalita, D. Effect of dietary sunflower oil and coconut oil on adipose tissue gene expression, fatty acid composition and serum lipid profile of grower pigs. Arch. Anim. Nutr. 2012, 66, 271–282. [Google Scholar] [CrossRef]
- Eid, A.; Elmarzugi, N.; Enshasy, H.E. Development of Avocado oil Nanoemulsion Hydrogel using Sucrose Ester Stearate. J. Appl. Pharm. Sci. 2013, 3, 145–147. [Google Scholar]
- Hosseinzadeh, F.; Salehi, M.; Tanideh, N.; Mehrabani, D.; Sayarifard, A.; Sedighi, A. The Healing Effect of Grape Seed Oil Enema with or without Sesame Oil in Acetic Acid Induced Ulcerative Colitis of Rats. World J. Plast. Surg. 2017, 6, 176–182. [Google Scholar]
- Ismail, A.F.; Salem, A.A.; Eassawy, M.M. Hepatoprotective effect of grape seed oil against carbon tetrachloride induced oxidative stress in liver of gamma-irradiated rat. J. Photochem. Photobiol. B 2016, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, G.; Karabulut, İ.; Topçu, A.; Asiltürk, M.; Kutlu, T. Roasting-Related Changes in Oxidative Stability and Antioxidant Capacity of Apricot Kernel Oil. J. Am. Oil Chem. Soc. 2010, 87, 401–409. [Google Scholar] [CrossRef]
- Cretella, A.B.M.; Soley, B.D.S.; Pawloski, P.L.; Ruziska, R.M.; Scharf, D.R.; Ascari, J.; Cabrini, D.A.; Otuki, M.F. Expanding the anti-inflammatory potential of Moringa oleifera: Topical effect of seed oil on skin inflammation and hyperproliferation. J. Ethnopharmacol. 2020, 254, 112708. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.L.; Hossain, M.S. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int. J. Immunopharmacol. 2000, 22, 729–740. [Google Scholar] [CrossRef]
- Van Way, C.W.; Dunn, E.L.; Hamstra, R.D. The effect of intravenous safflower oil emulsion on the clotting mechanism. Am. Surg. 1983, 49, 460–464. [Google Scholar]
- Alwadei, M.; Kazi, M.; Alanazi, F.K. Novel oral dosage regimen based on self-nanoemulsifying drug delivery systems for codelivery of phytochemicals—Curcumin and thymoquinone. Saudi. Pharm. J. 2019, 27, 866–876. [Google Scholar] [CrossRef]
- Kazi, M.; Shariare, M.H.; Al-bgomi, M.; Hussain, M.D.; Alanazi, F.K. Simultaneous determination of curcumin (Cur) and thymoquinone (THQ) in lipid based self-nanoemulsifying systems and its application to the commercial product using UHPLC-UV-Vis spectrophotometer. Curr. Pharm. Anal. 2018, 14, 277–285. [Google Scholar] [CrossRef]
- Mohsin, K.; Pouton, C.W. The influence of the ratio of lipid to surfactant and the presence of cosolvent on phase behaviour during aqueous dilution of lipid-based drug delivery systems. J. Drug Del. Sci. Tech. 2012, 22, 531–540. [Google Scholar] [CrossRef]
- Shahba, A.A.; Mohsin, K.; Alanazi, F.K. Novel self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of cinnarizine: Design, optimization, and in-vitro assessment. AAPS Pharm. Sci. Tech. 2012, 13, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.; Wunderlich, M.; Stippler, E.; Dressman, J. Development of Dissolution Tests on the Basis of Gastrointestinal Physiology. Pharm. Dissolution Test. 2005, 193–227. [Google Scholar] [CrossRef]
- Aref, H.L.; Karima, B.H.S.; Fekih, A.; Chemli, R.; Mars, M.; Aouni, M.; Chaumon, J.P.; Said, K. Variability in antimicrobial activity of latex from two varieties of Ficus carica. Afr. J. Microbiol. Res. 2011, 5, 1361–1367. [Google Scholar]
- Pouton, C.W.; Porter, C.J. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Alhasani, K.F.; Kazi, M.; Ibrahim, M.A.; Shahba, A.A.; Alanazi, F.K. Self-nanoemulsifying ramipril tablets: A novel delivery system for the enhancement of drug dissolution and stability. Int. J. Nanomed. 2019, 14, 5435–5448. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.; Serrano, D.R.; Mauger, M.; Bolás-Fernández, F.; Dea-Ayuela, M.A.; Lalatsa, A. Orally Bioavailable and Effective Buparvaquone Lipid-Based Nanomedicines for Visceral Leishmaniasis. Mol. Pharm. 2018, 15, 2570–2583. [Google Scholar] [CrossRef]
- Shazly, G.; Mohsin, K. Dissolution improvement of solid self-emulsifying drug delivery systems of fenofibrate using an inorganic high surface adsorption material. Acta Pharm. 2015, 65, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.C.; Britten, N.J.; Olanoff, L.S.; Badalamenti, J.N. Gel-matrixsystems exhibiting bimodal controlled release for oral drug delivery. J. Control. Release 1989, 9, 169–175. [Google Scholar] [CrossRef]









| Name | Chemical Description | Bioactive Characteristics | Supplier |
|---|---|---|---|
| Coconut oil fatty acid (CoFA) | CoFA has a higher content of tocotrienols and tocopherols (forms of vitamin E), sterols (precursors to fat-soluble vitamins and steroid hormones). It has proteins which can adsorb and kill bacteria. | Antioxidant activity; antimicrobial effects [28,29]. | Cremer Oleo GmbH & Co, Hamburg, Germany |
| Avocado oil (AVO) | AVO is high in monounsaturated fatty acids, particularly oleic acid, followed by palmitic and linoleic acids. Avocado oil is naturally low in acidity, has the highest smoke point and contains more than 50% monounsaturated fat, which makes it less prone to oxidation. | Antioxidant activity; promotes the accumulation of HDL cholesterol; anti-inflammatory activity [30]. | Nikkol chemical co, Tokyo, Japan |
| Grape seed oil (GSO) | GSO has a high content of polyunsaturated fatty acids, such as omega-6 and omega 9, in the range of 85–90%. It has a large amount of phenolic compounds, including flavonoids, carotenoids, phenolic acids. | Chemo preventive to control cancer; anti-inflammatory; anticholesterol agent; an immunostimulating effect [31,32]. | Nikkol chemical co, Tokyo, Japan |
| Apricot oil (APO) | APO contains various phytochemicals, such as pro-vitamin A beta-carotene; polyphenols, including catechins and chlorogenic acid; as well as oleic acid and linoleic acid (omega-9). | Antioxidant activity; anti-inflammatory and respiratory support; anticancer potential and antibacterial [33]. | Nikkol chemical co, Tokyo, Japan |
| Moringa Oleifera (MOO) | MOO is rich in oleic acid, tocopherols and sterols. It has proteins which can adsorb and kill bacteria, as well as a high content of β-carotene and vitamin C. | Antioxidant; anti-inflammation; remedy for infectious diseases, hematological diseases, hepatorenal diseases, gastrointestinal disorders, rheumatic arthritis, and cardiovascular diseases [34]. | Cold pressed, Dhaka, Bangladesh |
| Black seed oil | Thymoquinone 20–50% | Anticancer activity; anti-infective; anthelmintic; antimicrobial; anti-viral, antibacterial, antifungal, antiparasitic and antispasmodic activity; anti-inflammatory; antihistaminic and analgesic; antipyretic, and antiulcerative activity [35,36]. | Cold pressed, Dhaka, Bangladesh |
| Safflower oil | Rich in linoleic acid and omega-6 fatty acid. The seeds contain a lignan glycoside known as tracheloside; serotonin derivatives. | Capable of protecting against heart disease; antioxidant; anti-inflammatory and respiratory support [37]. | Nikkol chemical co, Japan |
| Apigenin | Flavonoid, phytopolyphenol. | Anti-inflammatory; antioxidant; antistress; antidiabetic, induces autophagy (cellular waste-recycling system) in leukemia cells; antibacterial agent [5]. | Beijing Mesochem Technology Co. Ltd., Beijing, China |
| F. N. | Bioactive Oil * | Mono- and Di-Glycerides Oil ** | Nonionic Surfactant and Co-Solvent *** | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AVO | GPO | APO | MOO | SFO | BSO | CoFA | CMCM | I988 | TO106V | HCO30 | T85 | T80 | TC | |
| F1 | 35 | 15 | 50 | |||||||||||
| F2 | 35 | 15 | 50 | |||||||||||
| F3 | 35 | 15 | 50 | |||||||||||
| F4 | 35 | 15 | 50 | |||||||||||
| F5 | 35 | 15 | 50 | |||||||||||
| F6 | 35 | 15 | 50 | |||||||||||
| F7 | 35 | 15 | 50 | |||||||||||
| F8 | 35 | 15 | 50 | |||||||||||
| F9 | 35 | 15 | 50 | |||||||||||
| F10 | 35 | 15 | 50 | |||||||||||
| F11 | 30 | 10 | 50 | 10 | ||||||||||
| F12 | 35 | 15 | 50 | |||||||||||
| F13 | 35 | 15 | 50 | |||||||||||
| F14 | 100 | |||||||||||||
| No | Formulation (w/w) | Appearance | Mean Droplet Size (nm) | PDI | Solubility (mg/g) | Bio-SNEDDS |
|---|---|---|---|---|---|---|
| F1 | AVO:I988(7:3)/T80[1/1] | Turbid (Coarse) | 2405 ± 124 | 0.194 | 1.05 ± 0.32 | No |
| F2 | GPO:I988(7:3)/T80[1/1] | Turbid (Coarse) | 2640 ± 59 | 0.163 | ND | No |
| F3 | SFO:I988(7:3)/T80[1/1] | Turbid (Coarse) | 987 ± 112 | 0.781 | 0.39 ± 0.01 | No |
| F4 | APO:I988(7:3)/T80[1/1] | Turbid (Coarse) | 821 ± 135 | 0.366 | 1.20 ± 0.32 | No |
| F5 | MOO:I988(7:3)/T80[1/1] | Bluish (Coarse) | 234.6 ± 24.4 | 0.470 | 2.04 ± 0.19 | No |
| F6 | APO:I988(7:3)/HCO30[1/1] | Bluish (Fine) | 113.6 ± 5.8 | 0.476 | 2.86 ± 0.09 | Yes |
| F7 | AVO:I988(7:3)/HCO30[1/1] | Bluish (Fine) | 104.5 ± 6.3 | 0.579 | 1.90 ± 0.21 | Yes |
| F8 | BSO:I988(7:3)/HCO30[1/1] | Bluish (Fine) | 104.70 ± 21.35 | 0.873 | 1.53 ± 0.05 | Yes |
| F9 | BSO:CMCM(7:3)/HCO30[1/1] | Bluish (Fine) | 97.86 ± 11.25 | 0.636 | 1.66 ± 0.02 | Yes |
| F10 | APO:CMCM(7:3)/HCO30[1/1] | Bluish (Fine) | 62.85 ± 4.68 | 0.407 | 2.42 ± 0.03 | Yes |
| F11 | CoFA:CMCM:TC (3:1:1)/HCO30 [1/1] | Transparent | 57.00 ± 14.80 | 0.419 | 12.50 ± 0.24 | Yes |
| F12 | APO:I988(7:3)/TO106V [1/1] | Turbid | 936.55 ± 58.23 | 0.888 | ND | No |
| F13 | APO:I988(7:3)/T85[1/1] | Turbid (Fine) | 179.10 ± 22.75 | 0.423 | ND | No |
| F14 | TC | Transparent | 21.34 ± 3.13 | 0.356 | 19.02 ± 1.02 | No |
| Sample | Zone of Inhibition against S. Aureus (ATCC25923)/mm | Zone of Inhibition Against E. coli (ATCC25922)/mm | Zone of Inhibition against C. Albicans (ATCC60193)/mm |
|---|---|---|---|
| Bio-SNEDDS | 12 | 9 | 18 |
| Commercial APG | - | - | 9 |
| Ampicillin | 25 | 27 | NT * |
| Kanamycin | 23 | 25 | NT * |
| Nystatin | NT * | NT | 25 |
| DMSO | - | - | - |
| Time (Months) | Drug Content (%) | Particle Size (nm) | Zeta Potential (mV) | Bio-SNEDDS Appearance |
|---|---|---|---|---|
| 0 | 100 | 57.12 ± 11.45 | −14.21 ± 3.12 | Transparent |
| 3 | 96.8 | 79.20 ± 8.23 | −13.90 ± 2.11 | Transparent |
| 6 | 90.8 | 269.93 ± 13.85 | −16.50 ± 1.23 | Hazy |
| Pharmacokinetic Parameters | Pure APG (Mean ± SEM, n = 6) | APG Bio-SNEDDS (Mean ± SEM, n = 6) |
|---|---|---|
| Tmax (h) | 3.60 ± 1.67 | 4.20 ± 2.04 |
| Cmax (ng/mL) | 21.38 ± 14.35 | 43.84 ± 20.13 |
| AUC0–t (ng *h/mL) | 146.54 ± 139.62 * | 280.37 ± 58.62 * |
| AUC0–∞ (ng *h/mL) | 157.59 ± 136.13 | 298.77 ± 55.72 |
| CL/F ((mg)/(ng/mL)/h) | 0.1195 ± 0.0943 *** | 0.0343 ± 0.0057 *** |
| T1/2 (h) | 7.87 ± 5.03 | 4.77 ± 2.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazi, M.; Alhajri, A.; Alshehri, S.M.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.; Altamimi, M.A.; Alanazi, F.K. Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, In Vivo and Stability Evaluations. Pharmaceutics 2020, 12, 749. https://doi.org/10.3390/pharmaceutics12080749
Kazi M, Alhajri A, Alshehri SM, Elzayat EM, Al Meanazel OT, Shakeel F, Noman O, Altamimi MA, Alanazi FK. Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, In Vivo and Stability Evaluations. Pharmaceutics. 2020; 12(8):749. https://doi.org/10.3390/pharmaceutics12080749
Chicago/Turabian StyleKazi, Mohsin, Abdullah Alhajri, Sultan M. Alshehri, Ehab M. Elzayat, Osaid T. Al Meanazel, Faiyaz Shakeel, Omar Noman, Mohammad A. Altamimi, and Fars K. Alanazi. 2020. "Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, In Vivo and Stability Evaluations" Pharmaceutics 12, no. 8: 749. https://doi.org/10.3390/pharmaceutics12080749