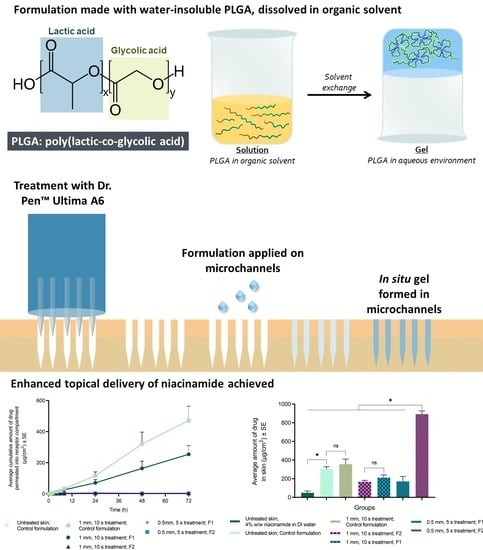
In Situ Gel Formation in Microporated Skin for Enhanced Topical Delivery of Niacinamide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Studies
2.3. Preparation of In Situ Formulation
2.4. In Vitro Permeation Testing
2.4.1. Preparation of Skin
2.4.2. Measurement of Skin Electrical Resistance
2.4.3. Microneedle Treatment of Skin
Dye Binding Studies
Confocal Microscopy
2.4.4. Histological Evaluation
2.4.5. Evaluation of Topical Delivery of Niacinamide
2.5. Analytical Method
2.6. Data Analysis/Statistics
3. Results
3.1. Solubility Study
3.2. Preparation of In Situ Formulation
3.3. Microneedle Treatment
3.4. Histological Evaluation
3.4.1. Topical Delivery of Niacinamide with Maltose MNs
3.4.2. Topical Delivery of Niacinamide with MN Device
3.5. Analytical Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Environmental Working Group Niacinamide. Available online: https://www.ewg.org/skindeep/ingredient/704134/NIACINAMIDE/ (accessed on 13 June 2019).
- Haque, T.; Lane, M.E.; Sil, B.C.; Crowther, J.M.; Moore, D.J. In vitro permeation and disposition of niacinamide in silicone and porcine skin of skin barrier-mimetic formulations. Int. J. Pharm. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehring, W. Nicotinic acid/niacinamide and the skin. J. Cosmet. Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.A. Here’s What Niacinamide Can—and Can’t—Do for Your Skin. Available online: https://www.self.com/story/what-niacinamide-can-do-for-your-skin (accessed on 13 June 2019).
- National Institutes of Health: Office of Dietary Supplements (ODS) Niacin Fact Sheet for Consumers. Available online: https://ods.od.nih.gov/factsheets/Niacin-Consumer/ (accessed on 13 June 2019).
- Bissett, D.L.; Miyamoto, K.; Sun, P.; Li, J.; Berge, C.A. Topical niacinamide reduces yellowing, wrinkling, red blotchiness, and hyperpigmented spots in aging facial skin 1. Int. J. Cosmet. Sci. 2004, 26, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L. Common cosmeceuticals. Clin. Dermatol. 2009, 27, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Crowther, J.M.; Sieg, A.; Blenkiron, P.; Marcott, C.; Matts, P.J.; Kaczvinsky, J.R.; Rawlings, A. V Measuring the effects of topical moisturizers on changes in stratum corneum thickness, water gradients and hydration in vivo. Br. J. Dermatol. 2008, 159, 567–577. [Google Scholar]
- Bagatin, E.; de Freitas, T.H.P.; Machado, M.C.R.; Ribeiro, B.M.; Nunes, S.; Rocha, M.A.D. da Adult female acne: A guide to clinical practice. An. Bras. Dermatol. 2019, 94, 62–75. [Google Scholar] [CrossRef] [Green Version]
- Ramos-e-Silva, M.; Celem, L.R.; Ramos-e-Silva, S.; Fucci-da-Costa, A.P. Anti-aging cosmetics: Facts and controversies. Clin. Dermatol. 2013, 31, 750–758. [Google Scholar] [CrossRef]
- Fitzjarrell, E.A. Topical Spray for Treating Acne Containing Niacinamide and NaPCA. U.S. Patent 5,989,523, 23 November 1999. [Google Scholar]
- Khodaeiani, E.; Fouladi, R.F.; Amirnia, M.; Karimi, E.R. Clinical trial Topical 4 % nicotinamide vs. 1 % clindamycin in moderate inflammatory acne vulgaris. Int. J. Dermatol. 2013, 999–1004. [Google Scholar] [CrossRef]
- Morganti, P.; Berardesca, E.; Guarneri, B.; Guarneri, F.; Fabrizi, G.; Palombo, P.; Palombo, M. Topical clindamycin 1 % vs. linoleic acid-rich phosphatidylcholine and nicotinamide 4 % in the treatment of acne: A multicentre- randomized trial. Int. J. Cosmet. Sci. 2011, 33, 467–476. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Matsubara, A.; Smiles, K. The effect of 2% niacinamide on facial sebum production. J. Cosmet. Laser Ther. 2006, 8, 96–101. [Google Scholar] [CrossRef]
- Panel, C.I.R.E. Final report of the safety assessment of niacinamide and niacin. Int. J. Toxicol. 2005, 24, 1. [Google Scholar]
- Boonme, P.; Boonthongchuay, C.; Wongpoowarak, W.; Amnuaikit, T. Evaluation of nicotinamide microemulsion on the skin penetration enhancement. Pharm. Dev. Technol. 2016, 21, 116–120. [Google Scholar] [CrossRef]
- Tuan-Mahmood, T.M.; McCrudden, M.T.C.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Donnelly, R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Tsai, P.C.; Ramezanli, T.; Michniak-Kohn, B.B. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013. [Google Scholar] [CrossRef] [Green Version]
- Coulman, S.A.; Anstey, A.; Gateley, C.; Morrissey, A.; McLoughlin, P.; Allender, C.; Birchall, J.C. Microneedle mediated delivery of nanoparticles into human skin. Int. J. Pharm. 2009. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.H.; Yamada, M.; Lin, L.L.; Grice, J.E.; Roberts, M.S.; Raphael, A.P.; Benson, H.A.E.; Prow, T.W. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Morrow, D.I.J.; McCarron, P.A.; David Woolfson, A.; Morrissey, A.; Juzenas, P.; Juzeniene, A.; Iani, V.; McCarthy, H.O.; Moan, J. Microneedle arrays permit enhanced intradermal delivery of a preformed photosensitizer. Photochem. Photobiol. 2009. [Google Scholar] [CrossRef]
- Duan, D.; Moeckly, C.; Gysbers, J.; Novak, C.; Prochnow, G.; Siebenaler, K.; Albers, L.; Hansen, K. Enhanced Delivery of Topically-Applied Formulations Following Skin Pre-Treatment with a Hand-Applied, Plastic Microneedle Array. Curr. Drug Deliv. 2011. [Google Scholar] [CrossRef]
- Bal, S.M.; Caussin, J.; Pavel, S.; Bouwstra, J.A. In vivo assessment of safety of microneedle arrays in human skin. Eur. J. Pharm. Sci. 2008. [Google Scholar] [CrossRef]
- Scott, J.A.; Banga, A.K. Cosmetic devices based on active transdermal technologies. Ther. Deliv. 2015, 6, 1089–1099. [Google Scholar] [CrossRef]
- Iriarte, C.; Awosika, O.; Rengifo-Pardo, M.; Ehrlich, A. Review of applications of microneedling in dermatology. Clin. Cosmet. Investig. Dermatol. 2017, 10, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banga, A.K. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781439805107. [Google Scholar]
- Sharad, J. Combination of microneedling and glycolic acid peels for the treatment of acne scars in dark skin. J. Cosmet. Dermatol. 2011, 10, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, B.M.; Zarnitsyn, V.; Prausnitz, M.R.; Anstey, A.; Gateley, C.; Birchall, J.C.; Coulman, S.A. Pocketed microneedles for rapid delivery of a liquid-state botulinum toxin A formulation into human skin. J. Control. Release 2013, 165, 146–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, S.; Gupta, P.B. Automated microneedling device-a new tool in dermatologist’s kit-a review. J. Pakistan Assoc. Dermatol. 2012, 22. [Google Scholar]
- Dr. Pen USA. Available online: https://www.drpenusa.com/ (accessed on 13 June 2019).
- McCrudden, M.T.C.; McAlister, E.; Courtenay, A.J.; González-Vázquez, P.; Raj Singh, T.R.; Donnelly, R.F. Microneedle applications in improving skin appearance. Exp. Dermatol. 2015, 24, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. V An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Parent, M.; Nouvel, C.; Koerber, M.; Sapin, A.; Maincent, P.; Boudier, A. PLGA in situ implants formed by phase inversion: Critical physicochemical parameters to modulate drug release. J. Control. Release 2013, 172, 292–304. [Google Scholar] [CrossRef]
- Bode, C.; Kranz, H.; Siepmann, F.; Siepmann, J. In-situ forming PLGA implants for intraocular dexamethasone delivery. Int. J. Pharm. 2018, 548, 337–348. [Google Scholar] [CrossRef]
- Yeo, Y.; Park, K. Characterization of reservoir-type microcapsules made by the solvent exchange method. Aaps Pharmscitech 2004, 5, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Yeo, Y.; Chen, A.U.; Basaran, O.A.; Park, K. Microencapsulation of Drugs by Solvent Exchange. U.S. Patent 6,599,627, 29 July 2003. [Google Scholar]
- Kwon, H.-Y.; Lee, J.-Y.; Choi, S.-W.; Jang, Y.; Kim, J.-H. Preparation of PLGA nanoparticles containing estrogen by emulsification–diffusion method. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 182, 123–130. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Inactive Ingredient Search for Approved Drug Products; FDA Database: Silver Spring, MD, USA, 2017.
- Dasht Bozorg, B.; Junaid, M.S.A.; Ganti, S.; Banga, A. Enhanced Topical Delivery by a Microcurrent Generating Device. In Proceedings of the 2019 AAPS PharmSci 360, San Antonio, TX, USA, 3–6 November 2019. [Google Scholar]
- Zakeri-Milani, P.; Islambulchilar, Z.; Majidpour, F.; Jannatabadi, E.; Lotfipour, F.; Valizadeh, H. A study on enhanced intestinal permeability of clarithromycin nanoparticles. Brazilian J. Pharm. Sci. 2014, 50, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Kranz, H.; Bodmeier, R. A novel in situ forming drug delivery system for controlled parenteral drug delivery. Int. J. Pharm. 2007. [Google Scholar] [CrossRef] [PubMed]
- Kranz, H.; Bodmeier, R. Structure formation and characterization of injectable drug loaded biodegradable devices: In situ implants versus in situ microparticles. Eur. J. Pharm. Sci. 2008. [Google Scholar] [CrossRef] [PubMed]
- Schoenhammer, K.; Petersen, H.; Guethlein, F.; Goepferich, A. Injectable in situ forming depot systems: PEG-DAE as novel solvent for improved PLGA storage stability. Int. J. Pharm. 2009. [Google Scholar] [CrossRef]
- Madan, M.; Bajaj, A.; Lewis, S.; Udupa, N.; Baig, J.A. In situ forming polymeric drug delivery systems. Indian J. Pharm. Sci. 2009, 71, 242. [Google Scholar] [CrossRef] [Green Version]
- Kempe, S.; Mäder, K. In situ forming implants - An attractive formulation principle for parenteral depot formulations. J. Control. Release 2012. [Google Scholar] [CrossRef]
- Khan, S.; Minhas, M.U.; Tekko, I.A.; Donnelly, R.F.; Thakur, R.R.S. Evaluation of microneedles-assisted in situ depot forming poloxamer gels for sustained transdermal drug delivery. Drug Deliv. Transl. Res. 2019, 9, 764–782. [Google Scholar] [CrossRef] [Green Version]
- Shirwaiker, R.A.; Purser, M.F.; Wysk, R.A. Scaffolding hydrogels for rapid prototyping based tissue engineering. In Rapid Prototyping of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2014; pp. 176–200. [Google Scholar]
- Bruschi, M.L.; Borghi-Pangoni, F.B.; Junqueira, M.V.; de Souza Ferreira, S.B. Nanostructured therapeutic systems with bioadhesive and thermoresponsive properties. In Nanostructures for Novel Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 313–342. [Google Scholar]
- Bermudez, J.M.; Grau, R. Thermosensitive poloxamer-based injectables as controlled drug release platforms for veterinary use: Development and in-vitro evaluation. Int. Res. J. Pharm. Pharmacol. 2011, 1, 109–118. [Google Scholar]
- Ahmed, T. Approaches to develop PLGA based in situ gelling system with low initial burst. Pak. J. Pharm. Sci. 2015, 28. [Google Scholar]
- Lin, X.; Yang, S.; Gou, J.; Zhao, M.; Zhang, Y.; Qi, N.; He, H.; Cai, C.; Tang, X.; Guo, P. A novel risperidone-loaded SAIB–PLGA mixture matrix depot with a reduced burst release: Effects of solvents and PLGA on drug release behaviors in vitro/in vivo. J. Mater. Sci. Mater. Med. 2012, 23, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Lambert, W.J.; Peck, K.D. Development of an in situ forming biodegradable poly-lactide-coglycolide system for the controlled release of proteins. J. Control. Release 1995, 33, 189–195. [Google Scholar] [CrossRef]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing poly (lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, E.V.; Collins, S.D.; Isseroff, R.R.; Smith, R.L. Microneedle array for transdermal biological fluid extraction and in situ analysis. Sensors Actuators A Phys. 2004, 114, 267–275. [Google Scholar] [CrossRef]
- Kalluri, H.; Kolli, C.S.; Banga, A.K. Characterization of microchannels created by metal microneedles: Formation and closure. AAPS J. 2011, 13, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.K.; Jiang, S.J.; Hwang, S.M.; Choi, E.H.; Lee, J.S.; Lee, S.H. Functional and structural changes of the epidermal barrier induced by various types of insults in hairless mice. Arch. Dermatol. Res. 2001, 293, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Kennish, L.; Reidenberg, B. A review of the effect of occlusive dressings on lamellar bodies in the stratum corneum and relevance to transdermal absorption. Dermatol. Online J. 2005, 11, 7. [Google Scholar]
- Jiang, S.; Koo, S.-W.; Lee, S.H. The morphologic changes in lamellar bodies and intercorneocyte lipids after tape stripping and occlusion with a water vapor-impermeable membrane. Arch. Dermatol. Res. 1998, 290, 145–151. [Google Scholar] [CrossRef]
- Menon, G.K.; Feingold, K.R.; Elias, P.M. Lamellar body secretory response to barrier disruption. J. Invest. Dermatol. 1992, 98, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, H.; Banga, A.K. Formation and closure of microchannels in skin following microporation. Pharm. Res. 2011, 28, 82–94. [Google Scholar] [CrossRef]








| Formulations Prepared 1 | Components (% w/w) | |||
|---|---|---|---|---|
| EXPANSORB® DLG 50-2A | EXPANSORB® DLG 50-8A | DMSO | PEG 400 | |
| Control | - | - | 12.50 | 87.50 |
| F1 (“Low” MW PLGA 2 formulation) | 20.00 | - | 10.00 | 70.00 |
| F2 (“High” MW PLGA 3 formulation) | - | 20.00 | 10.00 | 70.00 |
| Treatment 1 | Formulation Applied 2 | |||||
|---|---|---|---|---|---|---|
| Control | F1 | F2 | Solution in Deionized Water | |||
| No treatment | ✓ | ✕ | ✕ | ✓ | ||
| Dr. Pen™ Ultima A6 | 1 mm needle length | 10 s application | ✓ | ✓ | ✓ | ✕ |
| 0.5 mm needle length | 5 s application | ✕ | ✓ | ✓ | ✕ | |
| Maltose microneedle | 0.5 mm needle length | 2 min application | ✕ | ✓ | ✕ | ✓ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattaccharjee, S.; Beck-Broichsitter, M.; Banga, A.K. In Situ Gel Formation in Microporated Skin for Enhanced Topical Delivery of Niacinamide. Pharmaceutics 2020, 12, 472. https://doi.org/10.3390/pharmaceutics12050472
Bhattaccharjee S, Beck-Broichsitter M, Banga AK. In Situ Gel Formation in Microporated Skin for Enhanced Topical Delivery of Niacinamide. Pharmaceutics. 2020; 12(5):472. https://doi.org/10.3390/pharmaceutics12050472
Chicago/Turabian StyleBhattaccharjee, Sonalika, Moritz Beck-Broichsitter, and Ajay K. Banga. 2020. "In Situ Gel Formation in Microporated Skin for Enhanced Topical Delivery of Niacinamide" Pharmaceutics 12, no. 5: 472. https://doi.org/10.3390/pharmaceutics12050472