Electrospun Orodispersible Films of Isoniazid for Pediatric Tuberculosis Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solutions for Electrospinning
2.3. Characterization of the Solutions
2.3.1. Electrical Conductivity of the Spinning Solutions
2.3.2. Rheological Properties of the Spinning Solutions
2.4. Electrospinning Process
2.5. Characterization of the Orodispersible Films (ODFs)
2.5.1. Morphological Assessment of the ODFs
2.5.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.5.3. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
2.6. High Performance Liquid Chromatography (HPLC)
2.7. Drug Content Quantification
2.8. Disintegration Time of the ODFs in Simulated Salivary Fluid (SSF)
2.9. In Vitro Drug Release in Simulated Salivary Fluid
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of the Spinning Solutions
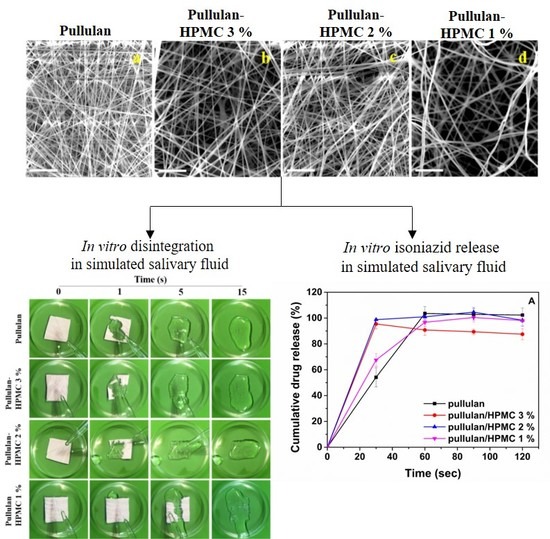
3.2. Fiber Morphology
3.3. Thermal Properties of the ODFs
3.4. Thermogravimetric Analysis
3.5. FTIR Analysis
3.6. Disintegration Test
3.7. In Vitro Drug Release in Simulated Salivary Fluid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Childhood TB Training Toolkit. Available online: https://apps.who.int/iris/bitstream/handle/10665/134387/9789241507783_eng.pdf?sequence=1 (accessed on 7 April 2020).
- Weismuller, M.M.; Graham, S.M.; Claessens, N.J.M.; Meijnen, S.; Salaniponi, F.M.; Harries, A.D. Diagnosis of childhood tuberculosis in Malawi: An audit of hospital practice. Int. J. Tuberc. Lung Dis. 2002, 6, 432–438. [Google Scholar] [PubMed]
- WHO. Recommendations for Investigating Contacts of Persons with Infectious Tuberculosis in Low- and Middle-Income Countries. Available online: http://apps.who.int/iris/bitstream/handle/10665/77741/9789241504492_eng.pdf (accessed on 7 April 2020).
- WHO. Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management. Available online: http://apps.who.int/iris/bitstream/handle/10665/260233/9789241550239-eng.pdf?sequence=1 (accessed on 8 April 2020).
- Churchyard, G.J.; Scano, F.; Grant, A.D.; Chaisson, R.E. Tuberculosis preventive therapy in the era of HIV infection: Overview and research priorities. J. Infect. Dis. 2007, 196, S52–S62. [Google Scholar] [CrossRef] [PubMed]
- Birungi, F.M.; Graham, S.M.; Uwimana, J.; Musabimana, A.; van Wyk, B. Adherence to isoniazid preventive therapy among child contacts in Rwanda: A mixed-methods study. PLoS ONE 2019, 14, e0211934. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, S.S.; Reid, A.J.; Mandalakas, A.M.; Enarson, D.A.; Beyers, N.; Morrison, J.; Hesseling, A.C. Operational challenges in managing Isoniazid Preventive Therapy in child contacts: A high-burden setting perspective. BMC Public Health 2011, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ranmal, S.; Batchelor, H.K.; Orlu-Gul, M.; Ernest, T.B.; Thomas, I.W.; Flanagan, T.; Kendall, R.; Tuleu, C. Formulation factors affecting acceptability of oral medicines in children. Int. J. Pharm. 2015, 492, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Orubu, E.S.; Tuleu, C. Medicines for children: Flexible solid oral formulations. Bull. World Health Organ. 2017, 95, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Slavkova, M.; Breitkreutz, J. Orodispersible drug formulations for children and elderly. Eur. J. Pharm. Sci. 2015, 75, 2–9. [Google Scholar] [CrossRef]
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef]
- Visser, J.C.; Woerdenbag, H.J.; Hanff, L.M.; Frijlink, H.W. Personalized Medicine in Pediatrics: The Clinical Potential of Orodispersible Films. AAPS PharmSciTech 2017, 18, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Visser, J.C.; Woerdenbag, H.J.; Crediet, S.; Gerrits, E.; Lesschen, M.A.; Hinrichs, W.L.J.; Breitkreutz, J.; Frijlink, H.W. Orodispersible films in individualized pharmacotherapy: The development of a formulation for pharmacy preparations. Int. J. Pharm. 2015, 478, 155–163. [Google Scholar] [CrossRef]
- Orlu, M.; Ranmal, S.R.; Sheng, Y.; Tuleu, C.; Seddon, P. Acceptability of orodispersible films for delivery of medicines to infants and preschool children. Drug Deliv. 2017, 24, 1243–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thabet, Y.; Lunter, D.; Breitkreutz, J. Continuous manufacturing and analytical characterization of fixed-dose, multilayer orodispersible films. Eur. J. Pharm. Sci. 2018, 117, 236–244. [Google Scholar] [CrossRef] [PubMed]
- El Meshad, A.N.; El Hagrasy, A.S. Characterization and optimization of orodispersible mosapride film formulations. AAPS PharmSciTech 2011, 12, 1384–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, H.; Liu, M.; Qu, W.; Hu, Z.; Brunson, E.; Johnson, J.; Almoazen, H. Evaluation of Chlorpheniramine Maleate microparticles in orally disintegrating film and orally disintegrating tablet for pediatrics. Drug Dev. Ind. Pharm. 2014, 40, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Pein, M.; Breitkreutz, J. Development of a taste-masked orodispersible film containing dimenhydrinate. Pharmaceutics 2012, 4, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Senta-Loys, Z.; Bourgeois, S.; Pailler-Mattei, C.; Agusti, G.; Briançon, S.; Fessi, H. Formulation of orodispersible films for paediatric therapy: Investigation of feasibility and stability for tetrabenazine as drug model. J. Pharm. Pharmacol. 2017, 69, 582–592. [Google Scholar] [CrossRef]
- Ghosal, K.; Chandra, A.; Praveen, G.; Snigdha, S.; Sudeep, R.; Agatemor, C.; Thomas, S.; Provaznik, I. Electrospinning over Solvent Casting: Tuning of Mechanical Properties of Membranes. Sci. Rep. 2018, 8, 5058. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Orlu, M.; Woerdenbag, H.J.; Scarpa, M.; Kiefer, O.; Kottke, D.; Sjöholm, E.; Öblom, H.; Sandler, N.; Hinrichs, W.L.J.; et al. Oromucosal films: From patient centricity to production by printing techniques. Expert Opin. Drug Deliv. 2019, 16, 981–993. [Google Scholar] [CrossRef] [Green Version]
- Casian, T.; Borbás, E.; Ilyés, K.; Démuth, B.; Farkas, A.; Rapi, Z.; Bogdan, C.; Iurian, S.; Toma, V.; Știufiuc, R.; et al. Electrospun amorphous solid dispersions of meloxicam: Influence of polymer type and downstream processing to orodispersible dosage forms. Int. J. Pharm. 2019, 569, 118593. [Google Scholar] [CrossRef]
- Song, Q.; Guo, X.; Sun, Y.; Yang, M. Anti-solvent Precipitation Method Coupled Electrospinning Process to Produce Poorly Water-Soluble Drug-Loaded Orodispersible Films. AAPS PharmSciTech 2019, 20, 273. [Google Scholar] [CrossRef]
- Domokos, A.; Balogh, A.; Dénes, D.; Nyerges, G.; Ződi, L.; Farkas, B.; Marosi, G.; Nagy, Z.K. Continuous manufacturing of orally dissolving webs containing a poorly soluble drug via electrospinning. Eur. J. Pharm. Sci. 2019, 130, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Rustemkyzy, C.; Belton, P.; Qi, S. Preparation and Characterization of Ultra rapidly Dissolving Orodispersible Films for Treating and Preventing Iodine Deficiency in the Pediatric Population. J. Agric. Food Chem. 2015, 63, 9831–9838. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.-Y.; Jia, X.-W.; Liu, Q.; Kong, B.-H.; Wang, H. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, S.; Cuq, B.; Gontard, N. Recent innovations in edible and/or biodegradable packaging materials. Food Addit Contam. 1997, 14, 741–751. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Garsuch, V.; Breitkreutz, J. Comparative investigations on different polymers for the preparation of fast-dissolving oral films. J. Pharm. Pharmacol. 2010, 62, 539–545. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Liu, S.-C.; Li, R.; Tomasula, P.M.; Sousa, A.M.M.; Liu, L. Electrospun Food-Grade Ultrafine Fibers from Pectin and Pullulan Blends. Food Nutr. Sci. 2016, 7, 636–646. [Google Scholar] [CrossRef] [Green Version]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Tomasula, P.M.; Sousa, A.M.M.; Liou, S.-C.; Li, R.; Bonnaillie, L.M.; Liu, L.S. Electrospinning of casein/pullulan blends for food-grade applications. J. Dairy Sci. 2016, 99, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Alborzi, S.; Lim, L.-T.; Kakuda, Y. Electrospinning of Sodium Alginate-Pectin Ultrafine Fibers. J. Food Sci. 2010, 75, C100–C107. [Google Scholar] [CrossRef] [PubMed]
- Nazari, K.; Kontogiannidou, E.; Ahmad, R.H.; Gratsani, A.; Rasekh, M.; Arshad, M.S.; Sunar, B.S.; Armitage, D.; Bouropoulos, N.; Chang, M.-W.; et al. Development and characterization of cellulose based electrospun mats for buccal delivery of non-steroidal anti-inflammatory drug (NSAID). Eur. J. Pharm. Sci. 2017, 102, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bromberg, L.; Hatton, T.A.; Rutledge, G.C. Electrospun cellulose acetate fibers containing chlorhexidine as a bactericide. Polymer 2008, 49, 1266–1275. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Tan, S.-H.; Inai, R.; Kotaki, M.; Ramakrishna, S. Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer 2005, 46, 6128–6134. [Google Scholar] [CrossRef]
- Yu, D.-G.; Yang, J.-M.; Branford-White, C.; Lu, P.; Zhang, L.; Zhu, L.-M. Third generation solid dispersions of ferulic acid in electrospun composite nanofibers. Int. J. Pharm. 2010, 400, 158–164. [Google Scholar] [CrossRef]
- Xiao, Q.; Lim, L.-T. Pullulan-alginate fibers produced using free surface electrospinning. Int. J. Biol. Macromol. 2018, 112, 809–817. [Google Scholar] [CrossRef]
- Li, X.-G.; Huang, M.-R.; Bai, H. Thermal decomposition of cellulose ethers. J. Appl. Polym. Sci. 1999, 73, 2927–2936. [Google Scholar] [CrossRef]
- Almeida, E.A.M.S.; Facchi, S.P.; Martins, A.F.; Nocchi, S.; Schuquel, I.T.A.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Synthesis and characterization of pectin derivative with antitumor property against Caco-2 colon cancer cells. Carbohydr. Polym. 2015, 115, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.P.; Peltzer, M.A.; Garrigós, M.C.; Jiménez, A. Structure and mechanical properties of sodium and calcium caseinate edible active films with carvacrol. J. Food Eng. 2013, 114, 486–494. [Google Scholar] [CrossRef] [Green Version]
- El Zowalaty, M.; Hussein, M.Z.; Arulselvan, P.; Fakurazi, S.; Webster, T.; Geilich, B.; Saifullah, B. Synthesis, characterization, and efficacy of antituberculosis isoniazid zinc aluminum-layered double hydroxide-based nanocomposites. Int. J. Nanomedicine 2016, 11, 3225–3237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urias-Orona, V.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Carvajal-Millán, E.; Gardea, A.A.; Ramírez-Wong, B. A novel pectin material: Extraction, characterization and gelling properties. Int. J. Mol. Sci. 2010, 11, 3686–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighatpanah, N.; Mirzaee, H.; Khodaiyan, F.; Kennedy, J.F.; Aghakhani, A.; Hosseini, S.S.; Jahanbin, K. Optimization and characterization of pullulan produced by a newly identified strain of Aureobasidium pullulans. Int. J. Biol. Macromol. 2020, 152, 305–313. [Google Scholar] [CrossRef]
- Shingel, K.I. Determination of structural peculiarities of dextran, pullulan and γ-irradiated pullulan by Fourier-transform IR spectroscopy. Carbohydr. Res. 2002, 337, 1445–1451. [Google Scholar] [CrossRef]
- Guidance for Industry Orally Disintegrating Tablets. Available online: http://academy.gmp-compliance.org/guidemgr/files/Orally-Disintegrating_Tablets_Final_FDA_8528fnl.pdf (accessed on 11 March 2020).
- Leathers, T.D. Biotechnological production and applications of pullulan. Appl. Microbiol. Biotechnol. 2003, 62, 468–473. [Google Scholar] [CrossRef]







| Formulation | Shear Viscosity (Pa·s) | Conductivity (μS/cm) |
|---|---|---|
| Pullulan | 2.09 | 65 ± 0.1 |
| Pullulan/HPMC 3% | 11.00 | 91 ± 0.2 |
| Pullulan/HPMC 2% | 5.63 | 85 ± 0.1 |
| Pullulan/HPMC 1% | 2.08 | 77.5 ± 0.5 |
| Pullulan/pectin 3% | 4.85 | 498 ± 1.0 |
| Pullulan/pectin 2% | 3.94 | 343 ± 0.6 |
| Pullulan/pectin 1% | 2.81 | 248 ± 2.0 |
| Pullulan/NaCas 3% | 1.39 | 385 ± 0.6 |
| Pullulan/NaCas 2% | 1.66 | 239 ± 0.6 |
| Pullulan/NaCas 1% | 2.01 | 192 ± 0.6 |
| Formulation | Loading Capacity (%) (± SD) | Encapsulation Efficiency (%) (± SD) |
|---|---|---|
| Pullulan-ISO | 10.85 ± 0.57 | 90.42 ± 4.78 |
| Pullulan/HPMC 3%-ISO | 11.77 ± 0.13 | 98.11 ± 1.12 |
| Pullulan/HPMC 2%-ISO | 11.46 ± 0.20 | 95.52 ± 1.71 |
| Pullulan/HPMC 1%-ISO | 11.25 ± 0.79 | 93.80 ± 6.63 |
| Pullulan/pectin 3%-ISO | 11.45 ± 0.26 | 95.14 ± 2.2 |
| Pullulan/pectin 2%-ISO | 11.66 ± 0.32 | 97.20 ± 2.64 |
| Pullulan/pectin 1%-ISO | 11.28 ± 0.94 | 93.97 ± 7.84 |
| Pullulan/NaCas 3%-ISO | 11.40 ± 0.43 | 95.05 ± 3.59 |
| Pullulan/NaCas 2%-ISO | 11.06 ± 0.68 | 93.00 ± 5.66 |
| Pullulan/NaCas 1%-ISO | 11.13 ± 0.71 | 92.79 ± 5.94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chachlioutaki, K.; Tzimtzimis, E.K.; Tzetzis, D.; Chang, M.-W.; Ahmad, Z.; Karavasili, C.; Fatouros, D.G. Electrospun Orodispersible Films of Isoniazid for Pediatric Tuberculosis Treatment. Pharmaceutics 2020, 12, 470. https://doi.org/10.3390/pharmaceutics12050470
Chachlioutaki K, Tzimtzimis EK, Tzetzis D, Chang M-W, Ahmad Z, Karavasili C, Fatouros DG. Electrospun Orodispersible Films of Isoniazid for Pediatric Tuberculosis Treatment. Pharmaceutics. 2020; 12(5):470. https://doi.org/10.3390/pharmaceutics12050470
Chicago/Turabian StyleChachlioutaki, Konstantina, Emmanouil K. Tzimtzimis, Dimitrios Tzetzis, Ming-Wei Chang, Zeeshan Ahmad, Christina Karavasili, and Dimitrios G. Fatouros. 2020. "Electrospun Orodispersible Films of Isoniazid for Pediatric Tuberculosis Treatment" Pharmaceutics 12, no. 5: 470. https://doi.org/10.3390/pharmaceutics12050470