An Investigation into the Relationship between Xanthan Gum Film Coating Materials and Predicted Oro-Esophageal Gliding Performance for Solid Oral Dosage Forms
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Aqueous Coating Formulations
2.3. Preparation of Film Coated Discs
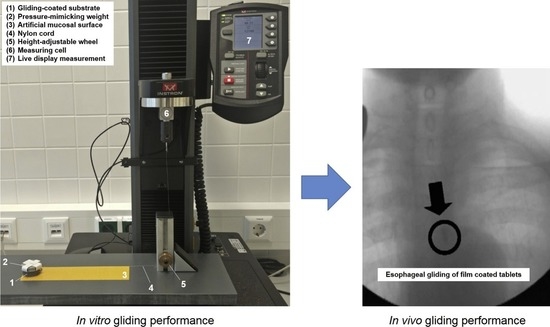
2.4. Evaluation of the Gliding Performance
2.5. Multivariate Analysis
3. Results
3.1. Gliding Performance Assessments
3.2. Multivariate Analysis
3.2.1. SF as Input for Predicted Gliding Performance
3.2.2. DF as Input for Predicted Gliding Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Batchelor, H.K.; Marriott, J.F. Formulations for children: Problems and solutions. Br. J. Clin. Pharmacol. 2015, 79, 405–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnaby-Mann, G.; Crary, M. PIll swallowing by adults with dysphagia. Arch. Otolaryngol. Neck Surg. 2005, 131, 970–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiele, J.; Quinzler, R.; Klimm, H.; Pruszydlo, M.; Haefeli, W. Difficulties swallowing solid oral dosage forms in a general practice population: Prevalence, causes, and relationship to dosage forms. Eur. J. Clin. Pharmacol. 2013, 69, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Schiele, J.T.; Penner, H.; Schneider, H.; Quinzler, R.; Reich, G.; Wezler, N.; Micol, W.; Oster, P.; Haefeli, W.E. Swallowing Tablets and Capsules Increases the Risk of Penetration and Aspiration in Patients with Stroke-Induced Dysphagia. Dysphagia 2015, 30, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, S.; Gosch, M.; Breitkreutz, J. Swallowing dysfunction and dysphagia is an unrecognized challenge for oral drug therapy. Int. J. Pharm. 2012, 430, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Fusco, S.; Cariati, D.; Schepisi, R.; Ganzetti, R.; Sestili, M. Management of oral drug therapy in elderly patients with dysphagia. J. Gerontol. Geriatr. 2016, 64, 9–20. [Google Scholar]
- Kelly, J.; D’Cruz, G.; Wright, D. Patients with dysphagia: Experiences of taking medication. J. Adv. Nurs. 2010, 66, 82–91. [Google Scholar] [CrossRef]
- Kirkevold, Ø.; Engedal, K. What is the matter with crushing pills and opening capsules? Int. J. Nurs. Pract. 2010, 16, 81–85. [Google Scholar] [CrossRef]
- Kikendall, J.; Friedman, A.; Oyewole, M.; Fleischer, D.; Johnson, L. Pill-induced esophageal injury. Dig. Dis. Sci. 1983, 28, 174–182. [Google Scholar] [CrossRef]
- Teplick, J.G.; Teplick, S.K.; Ominsky, S.H.; Haskin, M.E. Esophagitis caused by oral medication. Radiology 1980, 134, 23–25. [Google Scholar] [CrossRef]
- Channer, K.S.; Virjee, J.P. The effect of formulation on oesophageal transit. J. Pharm. Pharmacol. 1985, 37, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Marvola, M.; Rajaniemi, M.; Marttila, E.; Vahervuo, K.; Sothmann, A. Effect of dosage form and formulation factors on the adherence of drugs to the esophagus. J. Pharm. Sci. 1983, 72, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Ghosh, A.; Datta, S.; Das, S.; Mohanraj, P.; Deb, J.; Bhanoji Rao, M.E. Effects of plasticizers and surfactants on the film forming properties of hydroxypropyl methylcellulose for the coating of diclofenac sodium tablets. Saudi Pharm. J. 2009, 17, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Drumond, N.; Stegemann, S. Polymer adhesion predictions for oral dosage forms to enhance drug administration safety. Part 1: In vitro approach using particle interaction methods. Colloids Surf. B Biointerfaces 2018, 165, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Drumond, N.; Stegemann, S. Polymer adhesion predictions for oral dosage forms to enhance drug administration safety. Part 2: In vitro approach using mechanical force methods. Colloids Surf. B Biointerfaces 2018, 166, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, D.; Wahlgren, M. Comparison of in vitro methods of measuring mucoadhesion: Ellipsometry, tensile strength and rheological measurements. Colloids Surf. B Biointerfaces 2012, 92, 353–359. [Google Scholar] [CrossRef]
- Woertz, C.; Preis, M.; Breitkreutz, J.; Kleinebudde, P. Assessment of test methods evaluating mucoadhesive polymers and dosage forms: An overview. Eur. J. Pharm. Biopharm. 2013, 85, 843–853. [Google Scholar] [CrossRef]
- Drumond, N.; Stegemann, S. Polymer adhesion predictions for oral dosage forms to enhance drug administration safety. Part 3: Review of in vitro and in vivo methods used to predict esophageal adhesion and transit time. Colloids Surf. B Biointerfaces 2018, 165, 303–314. [Google Scholar] [CrossRef]
- Smart, J.D.; Dunkley, S.; Tsibouklis, J.; Young, S. An evaluation of the adhesion of solid oral dosage form coatings to the oesophagus. Int. J. Pharm. 2015, 496, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Smart, J.D.; Dunkley, S.; Tsibouklis, J.; Young, S. An in vitro model for the evaluation of the adhesion of solid oral dosage forms to the oesophagus. Int. J. Pharm. 2013, 447, 199–203. [Google Scholar] [CrossRef]
- Drumond, N.; Stegemann, S. An evaluation of the gliding performance of solid oral dosage form film coatings using an artificial mucous layer. Colloids Surf. B Biointerfaces 2019, 177, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.M.Z.; Felfel, R.M.; Ogbilikana, P.S.; Thakker, D.; Grant, D.M.; Scotchford, C.A.; Ahmed, I. Single Solvent-Based Film Casting Method for the Production of Porous Polymer Films. Macromol. Mater. Eng. 2018, 303, 1700628. [Google Scholar] [CrossRef]
- Siemann, U. Solvent cast technology-A versatile tool for thin film production. Prog. Colloid Polym. Sci. 2005, 130, 1–14. [Google Scholar] [CrossRef]
- Otsuka, T.; Iwao, Y.; Miyagishima, A.; Itai, S. Application of principal component analysis enables to effectively find important physical variables for optimization of fluid bed granulator conditions. Int. J. Pharm. 2011, 409, 81–88. [Google Scholar] [CrossRef]
- Van Snick, B.; Dhondt, J.; Pandelaere, K.; Bertels, J.; Mertens, R.; Klingeleers, D.; Di Pretoro, G.; Remon, J.P.; Vervaet, C.; De Beer, T.; et al. A multivariate raw material property database to facilitate drug product development and enable in-silico design of pharmaceutical dry powder processes. Int. J. Pharm. 2018, 549, 415–435. [Google Scholar] [CrossRef]
- Mahdi, Z.H.; Maraie, N.K. New Easily Swallowed Tablets with Slippery Coating for the Antihypertensive Drug Valsartan. UK J. Pharm. Biosci. 2015, 3, 9–19. [Google Scholar] [CrossRef]
- Hanna, P.; Gad, S.; Ghonaim, H.; Ghorab, M. Optimization of Gabapentin Release and Targeting Absorption, Through Incorporation into Alginate Beads. Br. J. Pharm. Res. 2013, 3, 597–616. [Google Scholar] [CrossRef]
- Ito, I.; Ito, A.; Unezaki, S. Investigation of Oral Preparation That Is Expected to Improve Medication Administration: Preparation and Evaluation of Oral Gelling Tablet Using Sodium Alginate. Yakugaku Zasshi 2017, 137, 969–977. [Google Scholar] [CrossRef] [Green Version]
- Drumond, N.; van Riet-Nales, D.A.; Karapinar-Çarkit, F.; Stegemann, S. Patients’ appropriateness, acceptability, usability and preferences for pharmaceutical preparations: Results from a literature review on clinical evidence. Int. J. Pharm. 2017, 521, 294–305. [Google Scholar] [CrossRef]
- Drumond, N. Future Perspectives for Patient-Centric Pharmaceutical Drug Product Design with Regard to Solid Oral Dosage Forms. J. Pharm. Innov. 2019. [Google Scholar] [CrossRef] [Green Version]
- Weitschies, W.; Karaus, M.; Cordini, D.; Trahms, L.; Breitkreutz, J.; Semmler, W. Magnetic marker monitoring of disintegrating capsules. Eur. J. Pharm. Sci. 2001, 13, 411–416. [Google Scholar] [CrossRef]
- Okabe, H.; Suzuki, E.; Sugiura, Y.; Yanagimoto, K.; Takanashi, Y.; Hoshi, M.; Nogami, E.; Nakahara, K.; Sekiguchi, T.; Baba, M.; et al. Development of an easily swallowed film formulation. Int. J. Pharm. 2008, 355, 62–66. [Google Scholar] [CrossRef] [PubMed]




| Coating | Film-Forming Agents | Slippery-Inducing Agents |
|---|---|---|
| F84–F86 | XG 0.40–0.60%, PEG 1.0% | CW 0.10–0.5%, SLS 0.20–0.60% |
| F87–F89 | XG 0.40–0.60%, PEG 1.0% | LE 0.30–1.10%, SLS 0.20–0.60% |
| F90–F92 | XG 0.40–0.60%, PEG 1.0% | CA 0.30–1.10%, SLS 0.20–0.60% |
| F93–F95 | XG 0.40–0.60%, PEG 1.0% | GG 0.10–0.20%, SLS 0.20–0.60% |
| F96–F98 | XG 0.40–0.60%, PEG 1.0% | SA 0.10–0.50%, SLS 0.20–0.60% |
| Coating | ML (N) | PWad (mJ) | m | FL (N) | Gwad (mJ) | TWad (mJ) | SF | DF |
|---|---|---|---|---|---|---|---|---|
| F84 | 0.39 ± 0.10 | 1.94 ± 0.14 | 0.01 ± 0.00 | 0.05 ± 0.00 | 14.12 ± 2.65 | 16.06 ± 2.69 | 0.0063 | 0.0015 |
| F85 | 0.26 ± 0.09 | 0.94 ± 0.03 | 0.00 ± 0.00 | 0.05 ± 0.00 | 7.43 ± 1.05 | 8.37 ± 0.54 | 0.0042 | 0.0008 |
| F86 | 0.40 ± 0.02 | 2.37 ± 0.55 | 0.00 ± 0.00 | 0.05 ± 0.00 | 11.08 ± 0.99 | 13.45 ± 0.99 | 0.0065 | 0.0012 |
| F87 | 0.43 ± 0.04 | 2.99 ± 0.47 | 0.01 ± 0.00 | 0.04 ± 0.00 | 16.14 ± 0.84 | 19.13 ± 1.00 | 0.0069 | 0.0017 |
| F88 | 0.32 ± 0.02 | 1.65 ± 0.22 | 0.01 ± 0.00 | 0.08 ± 0.00 | 21.04 ± 3.65 | 22.69 ± 1.47 | 0.0052 | 0.0021 |
| F89 | 0.42 ± 0.03 | 3.21 ± 0.36 | 0.01 ± 0.00 | 0.05 ± 0.01 | 14.66 ± 2.54 | 17.69 ± 1.02 | 0.0068 | 0.0015 |
| F90 | 0.67 ± 0.09 | 5.18 ± 1.21 | 0.02 ± 0.01 | 0.05 ± 0.00 | 22.91 ± 4.65 | 28.09 ± 2.36 | 0.0092 | 0.0024 |
| F91 | 0.58 ± 0.01 | 5.06 ± 1.33 | 0.01 ± 0.00 | 0.20 ± 0.00 | 37.66 ± 3.65 | 42.79 ± 8.47 | 0.0094 | 0.0048 |
| F92 | 0.59 ± 0.05 | 3.64 ± 0.55 | 0.01 ± 0.00 | 0.15 ± 0.00 | 24.94 ± 2.22 | 28.58 ± 4.65 | 0.0095 | 0.0026 |
| F93 | 0.51 ± 0.03 | 3.17 ± 0.74 | 0.01 ± 0.00 | 0.10 ± 0.00 | 19.09 ± 4.56 | 22.26 ± 6.87 | 0.0082 | 0.0021 |
| F94 | 0.45 ± 0.02 | 2.47 ± 0.88 | 0.00 ± 0.00 | 0.11 ± 0.00 | 21.84 ± 1.85 | 24.31 ± 4.54 | 0.0073 | 0.0023 |
| F95 | 0.45 ± 0.01 | 3.74 ± 0.33 | 0.00 ± 0.00 | 0.13 ± 0.00 | 20.81 ± 3.65 | 24.55 ± 4.52 | 0.0073 | 0.0023 |
| F96 | 0.59 ± 0.06 | 6.53 ± 0.21 | 0.01 ± 0.00 | 0.06 ± 0.00 | 16.16 ± 0.58 | 22.69 ± 3.99 | 0.0095 | 0.0018 |
| F97 | 0.53 ± 0.05 | 4.59 ± 0.11 | 0.02 ± 0.00 | 0.06 ± 0.00 | 18.31 ± 0.97 | 22.91 ± 3.54 | 0.0085 | 0.0020 |
| F98 | 0.55 ± 0.04 | 4.84 ± 0.14 | 0.02 ± 0.01 | 0.11 ± 0.01 | 25.87 ± 2.54 | 30.70 ± 4.44 | 0.0089 | 0.0027 |
| F1 | 1.71 ± 0.15 | 8.34 ± 1.36 | 0.03 ± 0.10 | 0.07 ± 0.01 | 25.43 ± 4.25 | 37.99 ± 1.58 | 0.0276 | 0.0030 |
| F0 | 0.83 ± 0.12 | 4.02 ± 0.58 | 0.02 ± 0.00 | 0.00 ± 0.00 | 7.35 ± 1.36 | 11.37 ± 0.25 | 0.0134 | 0.0010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drumond, N.; Stegemann, S. An Investigation into the Relationship between Xanthan Gum Film Coating Materials and Predicted Oro-Esophageal Gliding Performance for Solid Oral Dosage Forms. Pharmaceutics 2020, 12, 1241. https://doi.org/10.3390/pharmaceutics12121241
Drumond N, Stegemann S. An Investigation into the Relationship between Xanthan Gum Film Coating Materials and Predicted Oro-Esophageal Gliding Performance for Solid Oral Dosage Forms. Pharmaceutics. 2020; 12(12):1241. https://doi.org/10.3390/pharmaceutics12121241
Chicago/Turabian StyleDrumond, Nélio, and Sven Stegemann. 2020. "An Investigation into the Relationship between Xanthan Gum Film Coating Materials and Predicted Oro-Esophageal Gliding Performance for Solid Oral Dosage Forms" Pharmaceutics 12, no. 12: 1241. https://doi.org/10.3390/pharmaceutics12121241